Abstract
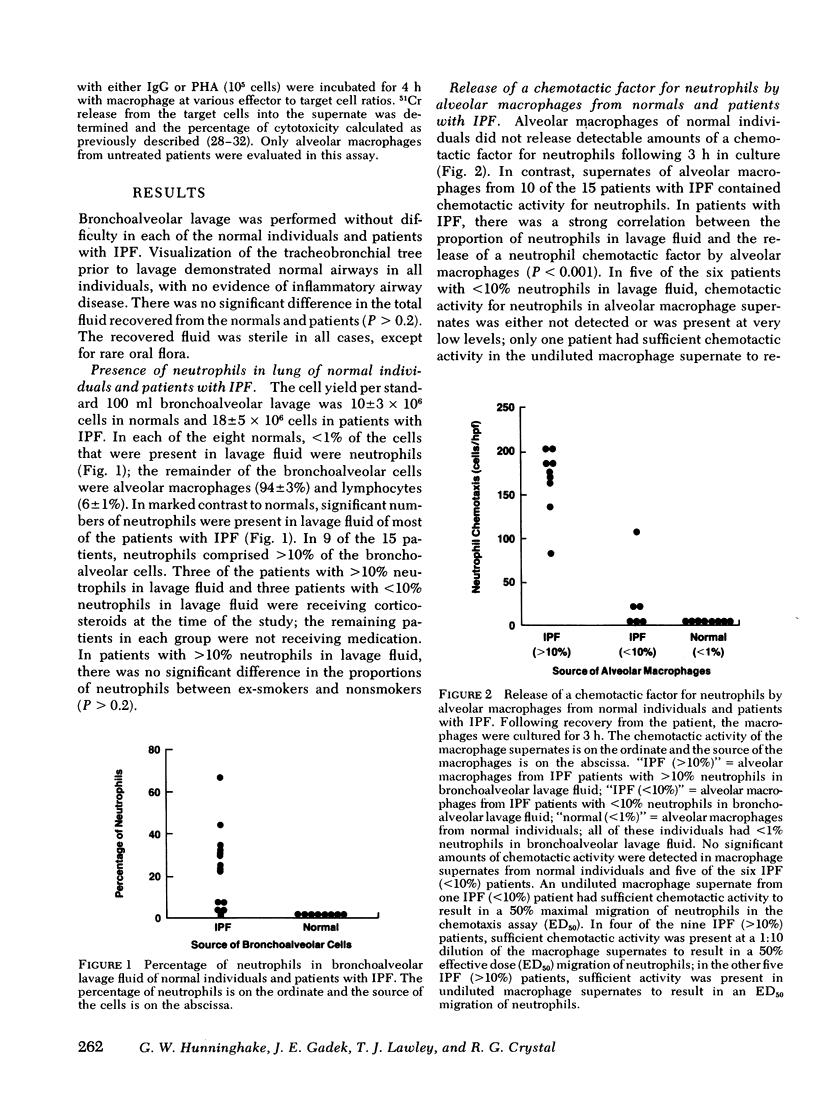
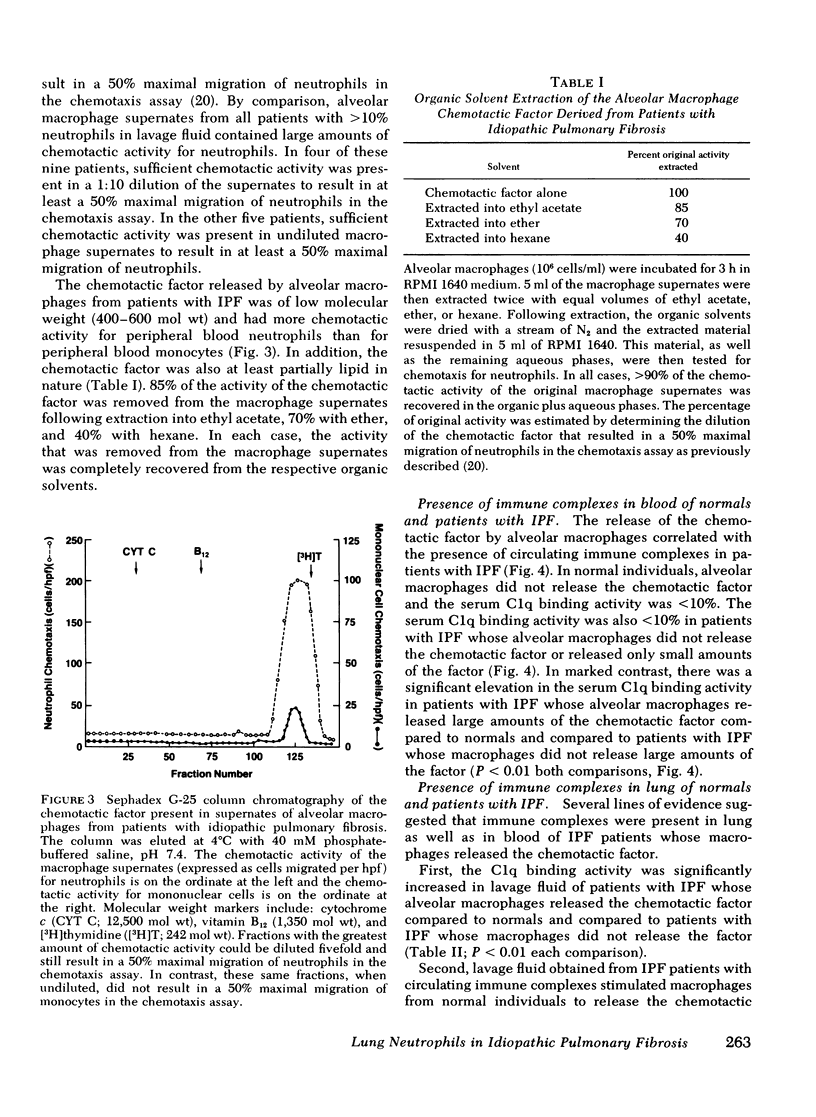
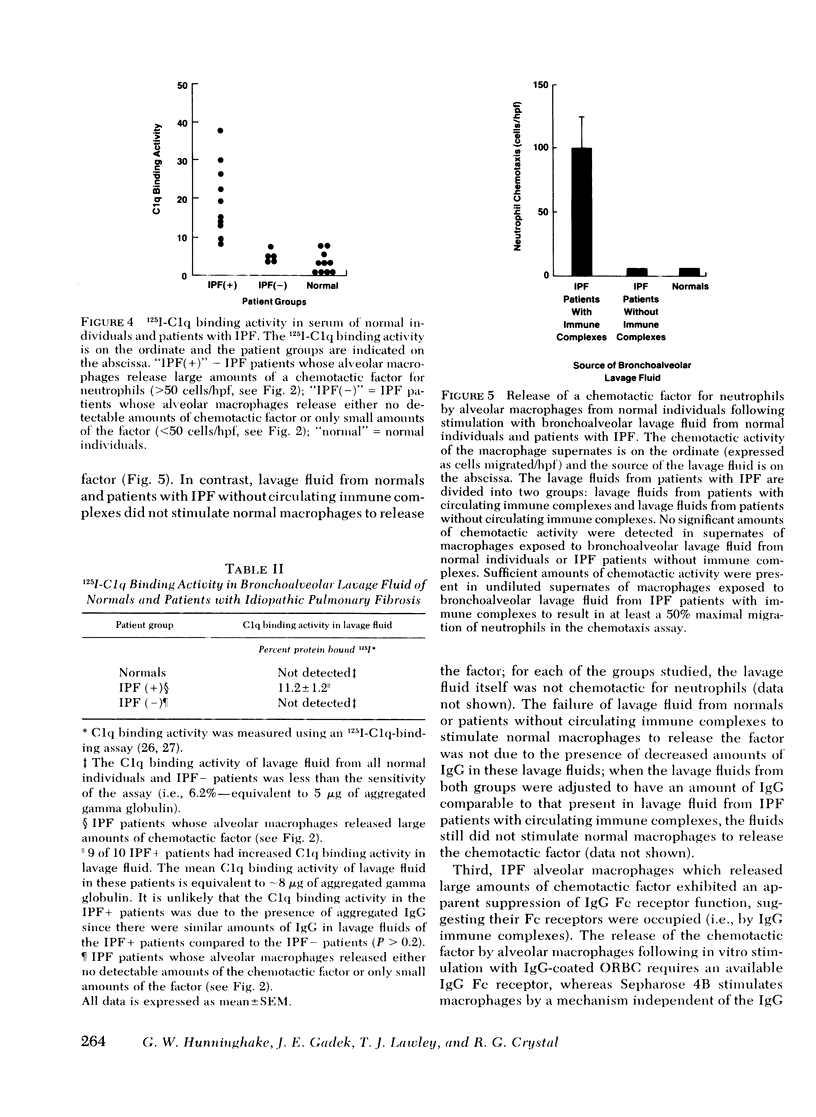
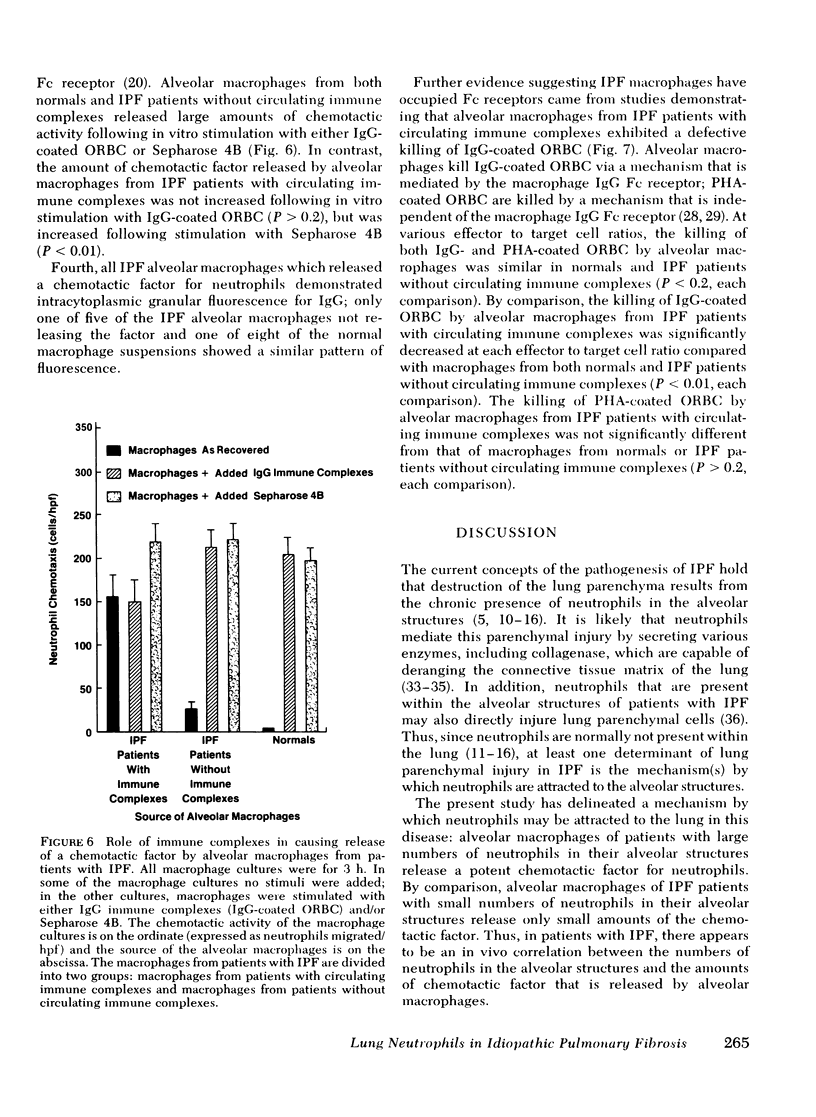
Neutrophils are a characteristic feature of the alveolitis of idiopathic pulmonary fibrosis (IPF). a chronic disorder limited to lung. One mechanism by which neutrophils may be selectively attracted to lung and not other tissues is via the secretion of the neutrophil-specific chemotactic factor by alveolar macrophages. To evaluate the role of alveolar macrophages in modulating the migration of neutrophils to he lung in IPF, alveolar macrophages, obtained by bronchoalveolar lavage of patients with IPF, were evaluated for their ability to release a chemotactic factor for neutrophils. Unstimulated alveolar macrophages from normal individuals did not release the factor. In patients with IPF, there was a significant correlation between the proportions of neutrophils in lavage fluid and the release of a chemotactic factor for neutrophils by alveolar macrophages (p less than 0.001). The chemotactic factor released by IPF alveolar macrophages was of low molecular weight (400-600), at least partially lipid in nature, and preferentially attracted neutrophils compared with monocytes. Several lines of evidence suggested that immune complexes in the lung stimulated alveolar macrophages of patients with IPF to release the chemotactic factor. First, immune complexes stimulated normal macrophages to release the factor.Second, there was a significant correlation between the release of the chemotactic factor by IPF alveolar macrophages and the levels of immune complexes in bronchoalveolar lavage fluid. Third, bronchoalveolar lavage fluid containing immune complexes stimulated normal macrophages to release the factor. Fourth, IPF alveolar macrophages that released large amounts of the chemotactic factor had an apparent suppression of their immunoglobulin (Ig)G Fc receptor function, suggesting that immune complexes were bound to their surface. In contrast, the IgG Fc receptor function of IPF alveolar macrophages that released only small amounts of the factor was similar to that of normal macrophages. These studies suggest that neutrophils are attracted to the lung in patients with IPF by a potent chemotactic factor released by alveolar macrophages that have been stimulated, in vivo, via their IgG Fc receptor by immune complexes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody A. R., Craighead J. E. Interstitial associations of cells lining air spaces in human pulmonary fibrosis. Virchows Arch A Pathol Anat Histol. 1976 Nov 22;372(1):39–49. doi: 10.1007/BF00429715. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Dreisin R. B., Schwarz M. I., Theofilopoulos A. N., Stanford R. E. Circulating immune complexes in the idiopathic interstitial pneumonias. N Engl J Med. 1978 Feb 16;298(7):353–357. doi: 10.1056/NEJM197802162980701. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Frank M. M., Hamburger M. I., Lawley T. J., Kimberly R. P., Plotz P. H. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979 Mar 8;300(10):518–523. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Hunninghake G. W., Zimmerman R. L., Crystal R. G. Regulation of the release of alveolar macrophage-derived neutrophil chemotactic factor. Am Rev Respir Dis. 1980 Apr;121(4):723–733. doi: 10.1164/arrd.1980.121.4.723. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Kelman J. A., Fells G., Weinberger S. E., Horwitz A. L., Reynolds H. Y., Fulmer J. D., Crystal R. G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979 Oct 4;301(14):737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Frank M. M. Kinetic analysis of chemotactic factor generation in human serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1975 Jan;3(3):334–346. doi: 10.1016/0090-1229(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr Effects of soluble immune complexes on Fc receptor- and C3b receptor-mediated phagocytosis by macrophages. J Exp Med. 1980 Oct 1;152(4):905–919. doi: 10.1084/jem.152.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Crystal R. G. The connective tissue of lung. Am Rev Respir Dis. 1975 Nov;112(5):657–711. doi: 10.1164/arrd.1975.112.5.657. [DOI] [PubMed] [Google Scholar]
- Henson P. M., McCarthy K., Larsen G. L., Webster R. O., Giclas P. C., Dreisin R. B., King T. E., Shaw J. O. Complement fragments, alveolar macrophages, and alveolitis. Am J Pathol. 1979 Oct;97(1):93–110. [PMC free article] [PubMed] [Google Scholar]
- Hinson K. F. Diffuse pulmonary fibrosis. Hum Pathol. 1970 Jun;1(2):275–288. doi: 10.1016/s0046-8177(70)80040-5. [DOI] [PubMed] [Google Scholar]
- Horwitz A. L., Hance A. J., Crystal R. G. Granulocyte collagenase: selective digestion of type I relative to type III collagen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):897–901. doi: 10.1073/pnas.74.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Divergent effects of cyclophosphamide administration on mononuclear killer cells: quantitative depletion of cell numbers versus qualitative suppression of functional capabilities. J Immunol. 1976 Jul;117(1):337–342. [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunologic reactivity of the lung. III. Effects of corticosteroids on alveolar macrophage cytotoxic effector cell function. J Immunol. 1977 Jan;118(1):146–150. [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Lymphocyte-mediated cytotoxicity against human allogeneic and autologous lymphoid targets after concanavalin A-activation of cytotoxic effector cells. J Immunol. 1977 Sep;119(3):1122–1128. [PubMed] [Google Scholar]
- Hunninghake G. W., Fulmer J. D., Young R. C., Jr, Gadek J. E., Crystal R. G. Localization of the immune response in sarcoidosis. Am Rev Respir Dis. 1979 Jul;120(1):49–57. doi: 10.1164/arrd.1979.120.1.49. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Kawanami O., Ferrans V. J., Young R. C., Jr, Roberts W. C., Crystal R. G. Characterization of the inflammatory and immune effector cells in the lung parenchyma of patients with interstitial lung disease. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):407–412. doi: 10.1164/arrd.1981.123.4.407. [DOI] [PubMed] [Google Scholar]
- Hunninghake G., Gadek J., Weinberger S., Kelman J., Elson N., Young R., Fulmer J., Crystal R. G. Comparison of the alveolitis of sarcoidosis and idiopathic pulmonary fibrosis. Chest. 1979 Feb;75(2 Suppl):266–267. doi: 10.1378/chest.75.2.266. [DOI] [PubMed] [Google Scholar]
- Jaffe C. J., Vierling J. M., Jones E. A., Lawley T. J., Frank M. M. Receptor specific clearance by the reticuloendothelial system in chronic liver diseases. Demonstration of defective C3b-specific clearance in primary biliary cirrhosis. J Clin Invest. 1978 Nov;62(5):1069–1077. doi: 10.1172/JCI109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierowski J. A., Gallin J. I., Reynolds H. Y. Mechanism for the inflammatory response in primate lungs. Demonstration and partial characterization of an alveolar macrophage-derived chemotactic factor with preferential activity for polymorphonuclear leukocytes. J Clin Invest. 1977 Feb;59(2):273–281. doi: 10.1172/JCI108638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T. J., Moutsopoulos H. M., Katz S. I., Theofilopoulos A. N., Chused T. M., Frank M. M. Demonstration of circulating immune complexes in Sjögren's syndrome. J Immunol. 1979 Sep;123(3):1382–1387. [PubMed] [Google Scholar]
- Lawrence E. C., Martin R. R., Blaese R. M., Teague R. B., Awe R. J., Wilson R. K., Deaton W. J., Bloom K., Greenberg S. D., Stevens P. M. Increased bronchoalveolar IgG-secreting cells in interstitial lung diseases. N Engl J Med. 1980 May 22;302(21):1186–1188. doi: 10.1056/NEJM198005223022106. [DOI] [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Matthay R. A., Reynolds H. Y. Alveolar macrophage-derived chemotactic factor: kinetics of in vitro production and partial characterization. J Clin Invest. 1980 Feb;65(2):268–276. doi: 10.1172/JCI109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadding J. G. Diffuse pulmonary alveolar fibrosis. Thorax. 1974 May;29(3):271–281. doi: 10.1136/thx.29.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadding J. G., Hinson K. F. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax. 1967 Jul;22(4):291–304. doi: 10.1136/thx.22.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Gewurz H., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide. Generation of a factor chemotactic for polymorphonuclear leukocytes. J Exp Med. 1968 Aug 1;128(2):259–275. doi: 10.1084/jem.128.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Phillips J., Mergenhagen S. E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and Guinea pig serum treated with immune complexes: evidence for c5a as the major chemotactic factor. Infect Immun. 1970 Jun;1(6):521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H. Interstitial pneumonia. Annu Rev Med. 1967;18:423–442. doi: 10.1146/annurev.me.18.020167.002231. [DOI] [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G., MUELLER-EBERHARD H. J. THE ROLE OF SERUM COMPLEMENT IN CHEMOTAXIS OF LEUKOCYTES IN VITRO. J Exp Med. 1965 Aug 1;122:327–346. doi: 10.1084/jem.122.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. Immune complex injury of the lung. Am J Pathol. 1979 Oct;97(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Weinberger S. E., Kelman J. A., Elson N. A., Young R. C., Jr, Reynolds H. Y., Fulmer J. D., Crystal R. G. Bronchoalveolar lavage in interstitial lung disease. Ann Intern Med. 1978 Oct;89(4):459–466. doi: 10.7326/0003-4819-89-4-459. [DOI] [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]


