Abstract
Insulin-like growth factor receptor-1 (IGF-1R) inhibition could be a relevant therapeutic approach in small cell lung cancer (SCLC) given the importance of an IGF-1R autocrine loop and its role in DNA damage repair processes. We assessed IGF-1R and pAkt protein expression in 83 SCLC human specimens. The efficacy of R1507 (a monoclonal antibody directed against IGF-1R) alone or combined with cisplatin or ionizing radiation (IR) was evaluated in H69, H146 and H526 cells in vitro and in vivo. Innovative genomic and functional approaches were conducted to analyze the molecular behavior under the different treatment conditions. A total of 53% and 37% of human specimens expressed IGF-1R and pAkt, respectively. R1507 demonstrated single agent activity in H146 and H526 cells but not in H69 cells. R1507 exhibited synergistic effects with both Cisplatin and IR in vitro. The triple combination R1507-Cisplatin-IR led to a dramatic delay in tumor growth compared to Cisplatin-IR in H526 cells. Analyzing the apparent absence of antitumoral effect of R1507 alone in vivo, we observed a transient reduction of IGF-1R staining intensity in vivo, concomitant to the activation of multiple cell surface receptors and intracellular proteins involved in proliferation, angiogenesis and survival. Finally, we identified that the nucleotide excision repair pathway (NER) was mediated after exposure to R1507-CDDP and R1507-IR in vitro and in vivo. In conclusion, adding R1507 to the current standard Cisplatin-IR doublet reveals remarkable chemo- and radiosensitizing effects in selected SCLC models and warrants to be investigated in the clinical setting.
Introduction
Small cell lung cancer (SCLC) accounts for 15% of all lung cancers and is the cause of death in 90–95% of affected individuals within 5 years(1). Over the past 20 years, progress in treatment has been limited and the standard of care is still therapy combining cisplatin plus ionizing radiation (IR) and cisplatin-based polychemotherapy for locally advanced and extended disease, respectively(2). New strategies based on a better understanding of SCLC biology are urgently needed.
The presence of an Insulin Growth factor-1 (IGF1)/Insulin Growth factor-1 Receptor (IGF-1R) autocrine loop is thought to be instrumental in driving the aggressive course of SCLC(3). This receptor is a well-recognized promoter of malignant transformation, proliferation and survival through the transduction of Phosphoinositide 3-kinase (PI3K)/Akt and Mitogen-activated protein kinase (MAPK) pathways(4). Furthermore, the IGF-1R pathway has been implicated in resistance to DNA damaging agents, suggesting its role in DNA damage repair(5–8). Previous studies on in vitro models of SCLC suggest that inhibition of the IGF-IR pathway might enhance the sensitivity of SCLC to chemotherapy through the inhibition of PI3K-Akt activation(9,10). However, these studies did not include in vivo validation and comprehensive molecular analyses on the interaction between chemotherapy and IGF-1R inhibitors. As a result, there is little rationale to support the four phase I/II studies that are currently investigating agents targeting the IGF-1R/IGF axis in patients with SCLC: OSI-906 vs. Topotecan (NCT01387386 & NCT01533181, phase II trials), Cisplatin Etoposide and Cixutumumab (NCT00887159, phase II trial), Pasireotide and Topotecan (NCT01417806, phase II trial).
The aim of the present study was to investigate the efficacy of R1507 (an IgG1 monoclonal antibody directed against the IGF-1R) both in vitro and in vivo in a context mimicking the clinical course of patients treated for SCLC (IR combined with chemotherapy). We first evaluated the expression of the phosphorylated forms of IGF-1R and of Akt in 83 human SCLC specimens. We then assessed the efficacy of R1507 against selected SCLC cell lines and examined the consequences on IGF-1R downstream signaling. We also investigated whether R1507 could potentiate the effects of cisplatin and IR in vitro and in vivo. Finally, we conducted an integrated genomic and functional analysis to explore the adaptive mechanisms occurring (i) after long-term versus short-term R1507 exposure and (ii) with Cisplatin- and IR-R1507 combinations.
Materials and Methods
Human established SCLC cell lines NCI-H146, NCI-H69 and NCI-H526 were obtained from the American Type Culture Collection (ATCC-LGC Standards, authentication by ATCC using short tandem repeat profiling). No further authentication was performed on these cell lines: they were passaged within the six months of the purchase. All cell lines cultured in the following medium: RPMI 1640 (GIBCO® - Invitrogen) supplemented with 1% GlutaMAX, 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells growing in floating aggregates were maintained in plastic flasks and incubated at 37°C in 5% CO2 humidified atmosphere.
Reagents
R1507 (RO4858696; Robatumumab, Roche), is a fully humanized IgG1 monoclonal antibody directed against the extracellular portion of IGF-1R. R1507 was provided by Roche Diagnostics and stored at −80°C. It binds with high selectivity to the extracellular domain of IGF-1R (and not to insulin receptor), leading to displacement of IGF-1 binding and loss of protein at the cell surface due to receptor internalization and degradation. Its chemical name is Immunoglobulin G1-kappa, anti-[Homo sapiens insulin-like growth factor 1 receptor (IGF1R, IGF-1R, IGF-1 receptor, CD221)], Homo sapiens monoclonal antibody; gamma1 heavy chain (1-448) [Homo sapiens VH (IGHV3-33*01 (91.80%) -(IGHD)- IGHJ2*01) [8.8.11] (1-118) –IGHG1*01 (119-448)], (221-215′)-disulfide with kappa light chain (1′-215′) [Homo sapiens V-KAPPA (IGKV3-11*01 (97.90%) –IGKJ1*01) [6.3.10] (1′-108′) -IGKC*01 (109′-215′)]; (227-227″:230-230″)-bisdisulfide dimer. The molecular formula of R1507 is C6476H10012N1748O2000S40 and its molecular weight is 145.6 kDa. Its 3-dimensional structure has been reported elsewhere (11). R1507 does not cross react with mouse IGF-1R(11). Toxicity in the clinical setting has already been described elsewhere(12). Cisplatin was purchased from Mylan Pharmaceuticals and stored at room temperature. Its chemical formula is Cis-PtCl2(NH3)2 and its molar mass is 301.1 g/mol.
Clonogenic survival assay
Clonogenic survival assays was performed as previously described(13). A total irradiation dose of 2 Gy was delivered using a linear accelerator (200-kV X-ray irradiator, dose rate = 0.96 Gy min-1) within the hour after seeding. Colonies consisting of at least 50 viable cells were estimated 21 days post treatment. The surviving fraction was normalized to the corresponding untreated control. Each experiment was repeated three times.
Western Blot (WB)
WB were performed, as previously described (13). Signals were detected using the Super Signal® West Pico chemoluminescent system (Pierce Biotechnology). IGF-I Receptor β rabbit antibody (1:1000 dilution), pan-Akt rabbit mAb (1:1000 dilution), Phospho-Akt (Ser473) rabbit mAb (1:1000 dilution), p44/42 MAPK rabbit mAb (1:1000 dilution), Phospho-p44/42 MAPK (Thyr202/Thyr204) rabbit mAb (1:1000 dilution), Phospho-MEK 1/2 (Ser 217/221) rabbit mAb (1:1000 dilution) and Cleaved Caspase-9 (Asp330) rabbit mAb (1: 1000 dilution) were purchased from Cell Signaling Technology®. The antibodies ERCC1 mouse mAb (8F1) (1:500 dilution), XPA mouse mAb (1:1000 dilution), Beta-Actin mouse mAb (1:5000 dilution) were purchased from Abcam Inc., Thermo Scientific Pierce Products and Sigma Aldrich, respectively. All primary antibodies were purchased from Cell Signaling Technology®. The following secondary antibodies were used: goat anti-rabbit IgG horseradish peroxidase-conjugated antibodies (Invitrogen) (1:3000 dilution), and goat anti-mouse antibodies (Alexa Fluor® 488) at (1:1000 dilution).
Immunohistochemical (IHC) analysis
The SCLC human specimens were handled according to standardized methods in the department of Pathology at Institut Guystave Roussy using the Discovery XT automated platform (Ventana Medical Systems, Tucson, AZ). After deparrafinization, slides were incubated in pIGF-1R rabbit monoclonal antibody (CONFIRM anti-IGF-1R (G11) antibody, Ventana Medical Systems) directed against the cytoplasmic domain of the phosphorylated form of the pIGF-1R. pAKT expression was also assessed with a rabbit monoclonal antibody (1:50 dilution, Cell Signaling). A section of normal human breast was used as a negative control whereas an invasive ductal carcinoma that was positive for IGF1R protein expression was included as a negative control. The scoring system used to interpret immunohistochemical staining was based on the distribution and the intensity of staining: no staining or slight staining in < 10% cells (0), slight staining in > 10% cells (‘1+’), marked staining in 10–50% cells (‘2+’) and strong staining in > 50% cells (‘3+’) as previously described(14). All the IHC analyses were centrally performed in a blinded manner by a senior pathologist (J.C.).
IGF1R gene copy number in the three selected cell lines was analyzed by fluorescent in situ hybridization (FISH) according to the Vysis® protocol [unmodified] (Abbott Molecular ®). BlueFISH® probes (Bluegnome) were used. The BAC clone RP11-14C10 (labeling with SpectrumOrange™) is located on 15q26.3 and contains IGF-1R. The BAC clone RP-263I19 (labeling with SpectrumGreen™) located on 15q15.3 was used as a control probe. At least 50 nuclei were examined to score the number of signals from each probe per cell and the IGF1R/control ratio.
Gene Expression analysis
All samples were obtained from H526 xenografts in nude mice. Total RNA was isolated from tissues using the RNAlater protocol. Affymetrix human genome U133 Plus 2.0 arrays were employed for expression profiling and the information concerning each probe on the array was extracted from the image data according to the manufacturer’s instructions. The raw intensity values from the gene expression files were imported into the R statistical Software which is used for all data input, diagnostic plots, normalization (RMA), and quality checking steps of the analysis process. Generalized linear models were computed (Limma, Bioconductor) to determine the differentially expressed probe sets (> 2 fold change, adjusted p values were computed for multiple testing (Benjamini and Hochberg’s method). Fisher’s exact tests were computed to test the overlap with gene sets from selected molecular signature databases (Gene Ontology gene sets and Canonical Pathways (KEGG, Biocarta and Reactome)). Only overlaps with a p value of <0.05 significance were retained for further analysis. The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE45626). The RMA normalized gene expression data were uploaded to the Synapse public portal (https://synapse.sagebase.org) for convenience under the following entity: “IGF1R targeting in SCLC (R1507)” (synapse ID syn1588571). Extensive information on Synapse can be found at: https://sagebionetworks.jira.com/wiki/display/SYND/. Further information regarding the use of the Synapse R Client is available on the synapse wiki: https://sagebionetworks.jira.com/wiki/display/SYNR/R+Synapse+Client+Vignette.
Phospho-protein arrays
Human Phospho-RTK arrays were processed according to the manufacturer’s instructions (2010 Cell Signaling Technology, Inc., Pathscan® RTK signaling antibody array kit #7982). Briefly, 50mg of protein lysates were incubated with blocked membranes that were subsequently washed and exposed to a chemiluminescent reagent and to an X-ray film. Dot images were captured by an ImageQuant 350 CCD camera controlled by ImageQuant 350 capture software (GE Healthcare), at 3, 5 and 6 minutes exposure time. The antibody directed against pIGF-1R in this kit is selective and does not cross-react with pINSR (the phosphorylated form of the insulin receptor).
In vivo experiments (including ethical requirements)
In vivo experiments were carried out at the Institut Gustave Roussy (Animal Care license N° 94-076-11). Seven-week old female athymic Nude mice were purchased from Janvier (CERT, Le Genest St. Isle), maintained in laminar flow cabinets under pathogen-free conditions. Two different cell lines (H146 and H526) were selected for the in vivo experiments, according to recent guidelines(15). Mice were injected with 5×106 H526 cells into the flank. Animals were randomized to different treatment groups of 5–7 mice each when xenograft diameters attained 5mm. R1507 was administered via an intraperitoneal injection (IP) once weekly at a dose of 18 mg/kg and continued until the end of the experiment. Cisplatin was administered at a dose of 3 mg/kg IP at day 1, day 8 and day 15. IR was delivered in 4 fractions of 2 Gy on days 1 to 4 (A 200-kV X-ray irradiator, dose rate = 0.45 Gy min-1), using mouse jigs to exclusively expose the tumor beds. Bidimensional tumor measurement on each mouse was performed twice weekly to measure the tumor volume using the following formula: (length × width 2)/2. Animals were sacrified when the main tumor volume per group had attained 2200–2800 mm3.
Statistical analysis
The description of the results are based on classic statistical methods: median, range and 95% confidence intervals. For clonogenic assays and WST-1 proliferation assays, data are presented as the mean ± SEM from a minimum of three independent experiments and were compared using the Student’s t test. The synergy effect between different conditions was assessed using the two-way ANOVA interaction test. Tumor sizes were compared using Student’s t tests. Tumor growth delay (days) was analyzed using the Kaplan Meier method, considering any event as the moment when the tumor volume had attained 5-fold the baseline volume (RTV5). For all the tests performed, a p value of less than 0.05 was considered statistically significant, and all tests were two-tailed. The statistical analyses were conducted using the R statistical software and Bioconductor packages (affy, limma).
Results
IGF-1R and pAkt were frequently expressed in human SCLC tissue specimens
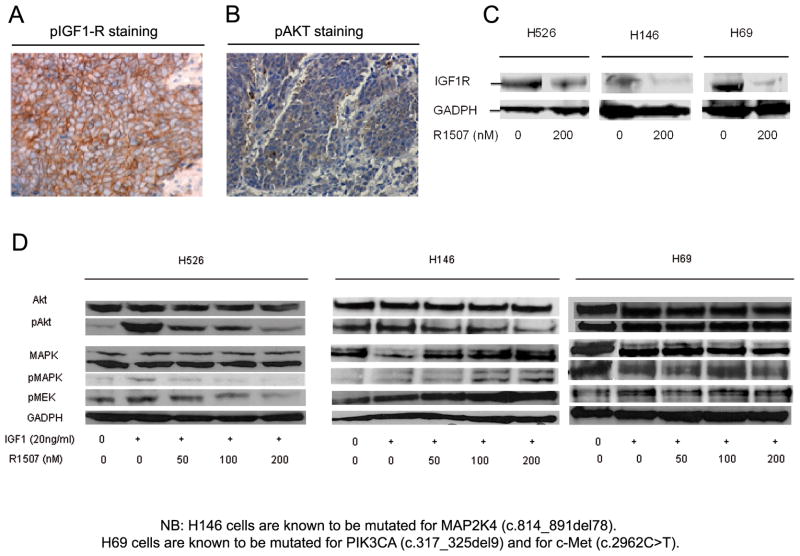
We assessed IGF-1R expression and Akt phosphorylation in one of the largest cohort of SCLC human specimens (83 patients). Positive IGF-1R immunohistochemical staining (Fig. 1A) was observed in 44 patients (53%), while pAkt (Fig. 1B) was detected in 31 patients (37%). A total of 17 (21%) and 22 (26%) of all tumors exhibited a minimum of 2+ staining intensity for IGF-1R and pAkt, respectively. Concomitant expression of both proteins was present in 24 samples (29%) suggesting that IGF pathway could be a relevant target in SCLC.
Figure 1.
(A) Representative sections of positive IGF1R (B) and pAkt staining in human SCLC specimens at × 200 magnification. Immunohistochemical analyses were conducted using the Discovery XT automated platform (Ventana Medical Systems, Tucson, AZ) (see (C) Effect of R1507 on IGF-1R protein expression in H526, H146 and H69 cell lines (Western Blots). Cells were exposed to R1507 or to vehicle during one hour following overnight serum starvation. R1507 (Robatumumab, a fully human neutralizing IgG1 monoclonal antibody directed against IGF-1R was provided by Roche Diagnostics and stored at −80°C. (D) Effect of R1507 on IGF-1R downstream pathways: Akt, pAkt, MAPK, pMAPK and pMEK in H526, H146 and H69 cell lines (Western Blots). Human established SCLC cell lines NCI-H146, NCI-H69 and NCI-H526 were obtained from the American Type Culture Collection (ATCC-LGC Standards) and were cultured in the following medium: RPMI 1640 (GIBCO®-Invitrogen) supplemented with 1% GlutaMAX, 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells growing in floating aggregates were maintained in plastic flasks and incubated at 37°C in 5% CO2 humidified atmosphere. Serum-starved cells were exposed to increasing doses of R1507, and incubated in the presence or absence of IGF-1. All the western blots displayed are representative of three independent experiments, and were performed as previously described [12]. The full list of antibodies with their dilutions is provided in supplementary information file. Signals were detected using the Super Signal® West Pico chemoluminescent system (Pierce Biotechnology).
R1507 downregulated IGF-1R protein expression and disrupted its downstream signaling in selected SCLC cell lines
Three SCLC cell lines were selected according to their high basal IGF1R protein expression and their specific mutation profiles: H146 cells are mutated for MAP2K4 (c.814_891del78), whereas H69 cells are mutated for both PIK3CA (c.317_325del9) and c-Met (c.2962C>T) (Cosmic database). WB analyses showed a down-regulation of IGF-1R protein expression in the presence of R1507 (200nM) in all the selected cell lines (Fig. 1C).
R1507 decreased IGF-1-induced downstream effector pAKT in H526 and H146 cells but not in H69 cells (Fig. 1D). R1507 also inhibited ligand-induced phosphorylation of MEK in H526 cells. However, the MAPK pathway (pMAPK, pMEK) appeared to be stimulated by R1507 in a dose-dependent manner in H146 cells.
R1507 inhibited colony formation of selected SCLC cell lines in vitro
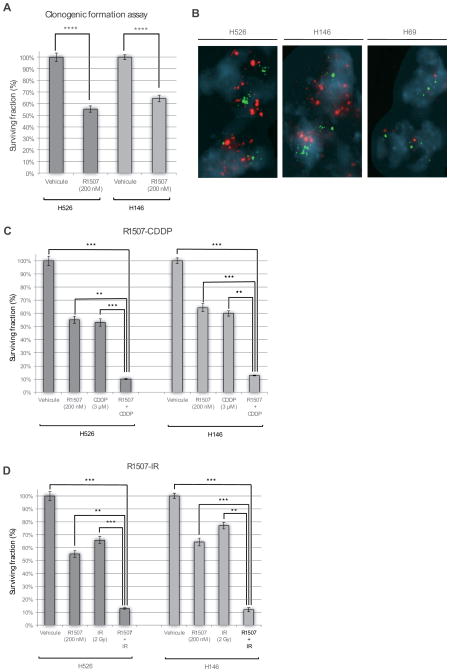
Exploring the sensitivity of selected SCLC cell lines to R1507, we observed a 35% and 45% relative reduction in colony formation of H526 cells (P < .0001) and H146 cells (P < .0001), respectively (Fig. 2A). No significant reduction in the colony-forming capacity was observed in H69 cells (data not shown). Although we did not study any SCLC cell lines without IGF-1R basal expression, such feature does not appear to be correlated with sensitivity to R1507. Indeed, R1507 treatment had no effect on H69 cells, while these cells present IGF1R basal expression (Fig. 1D). Interestingly, only the cell lines (H526 and H146) with IGF-1R gene amplification exhibited sensitivity to R1507 whereas those devoid of IGF1R gene amplification (H69) did not respond to R1507 (Fig. 2B).
Figure 2.
(A) Colony-forming assays were performed on H526, H146 and H69 cells treated or not with R1507. Clonogenic survival assays were performed in methylcellulose as previously described [12]. Colonies consisting of at least 50 viable cells were estimated 21 days post treatment. The surviving fraction was normalized to the corresponding untreated control. Data presented are means ± SEM from at least three independent experiments. Comparisons were computed using the Student’s t test. The symbols ****, *** and ** represent P< .0001, P< .001 and P<.01, respectively. (B) Fluorescent in situ hybridization (FISH) analysis shows amplification of IGF-1R (red probes) in H525 and H146 cells but not in H69 cells (C) Clonogenic formation assays were performed on H146 and H526 cells combining: R1507 (200nM) and Cisplatin (CDDP 3μM). Cisplatin was purchased from Mylan Pharmaceuticals and stored at room temperature, protected from light. (D) R1507 (200nM) and Ionizing Radiation (2 Gy). A total irradiation dose of 2 Gy was delivered using a linear accelerator (200-kV X-ray irradiator, dose rate = 0.96 Gy min-1) within the hour after seeding. Clonogenic survival assays were performed in methylcellulose as previously described [12]. Colonies consisting of at least 50 cells were counted at day 21. The surviving fraction was compared to the corresponding untreated control. Data presented are means ± SEM from three independent experiments. Comparisons were computed using the Student’s t test. The symbols ****, *** and ** represent P< .0001, P< .001 and P<.01, respectively.
R1507 sensitized H146 and H526 cells to cisplatin and IR effects
Since cisplatin (CDDP)-based chemotherapy and IR are the standard treatments for SCLC, we investigated whether R1507 could enhance their efficacy in SCLC cell lines. To formally assess the synergy relationship, two-way ANOVA were computed. We observed that the combination of R1507 and CDDP decreased their colony-forming capacity by 90% and 87% in H526 and H146 cells, respectively (P<.001) (Fig. 2C). We observed a synergistic inhibitory effect of the CDDP-R1507 combination on the colony-forming capacity of H146 cells (two-way ANOVA interaction test; P=.008) and an additive inhibitory effect in H526 cells (two-way ANOVA interaction test; P=.691). The R1507-IR combination decreased the colony-forming capacity by 87% and 88% in H526 and H146 cells, respectively (P<.001) (Fig. 2D). We also noted a synergistic effect of R1507-IR on the colony-forming capacity of H146 cells (two-way ANOVA interaction test; P=.0001), and an additive inhibitory effect in H526 cells (two-way ANOVA interaction test; P=0.101).
The triple combination of R1507-Cisplatin-IR potentiated the antitumor effect of Cisplatin-IR (current standard treatment) in H146 and H526 cells in vitro
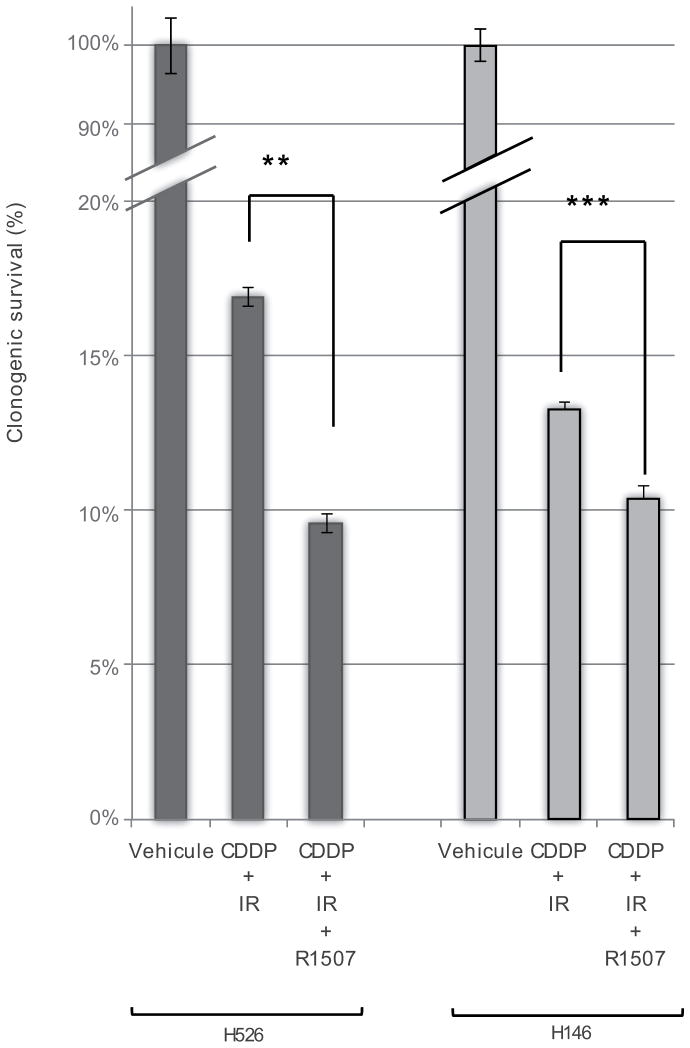
Cisplatin (CDDP) combined with IR remains the standard of care for the treatment of limited-stage SCLC. We herein observe that the addition of R1507 to CDDP-IR resulted in a 32% and 45% relative reduction in the colony-forming capacity compared to CDDP-IR in H146 and H526 cells, respectively (P<.001 and P<.0001) (Fig. 3).
Figure 3.
Clonogenic formation assays were performed on H146 and H526 cells combining: R1507 (200nM), Cisplatin (CDDP 3μM) and Ionizing Radiation (2 Gy) Colony-forming assays were performed in methylcellulose as previously described [12]. Colonies consisting of at least 50 cells were counted at day 21. The surviving fraction was compared to the corresponding untreated control. Data presented are means ± SEM from three independent experiments. Comparisons were computed using the Student’s t test. The symbols *** and ** represent P< .001 and P< .01, respectively.
The addition of R1507 to CDDP or IR induced non-significant delays in tumor growth in vivo
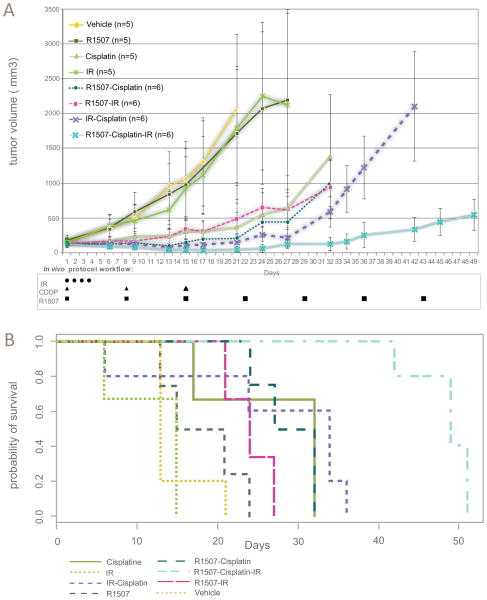
To evaluate the delay in tumor growth according to the various treatment conditions in vivo, we assessed the RTV5 in H526 and H146 xenografts (Fig. 4A, B and Supplementary Fig. 1&2). Single agent R1507 induced a non-significant delay in tumor growth in H526 and in H146 cells (Supplementary table. 1). Similarly, the R1507-CDDP and R1507-IR combination produced a non-significant delay in tumor growth in both H146 cells and H526 cells (Fig. 4B).
Figure 4.
(A) Effect of R1507 (18mg/kg IP qw), Cisplatin (3mg/kg IP Day 1,8,15), IR (4 fractions of 2 Gy; days 1–4) and their combinations on H526 xenografts in Nude mice. Mice were injected with 5×106 H526 cells into the flank. Animals were randomized to different treatment groups of 5–7 mice each when xenograft diameters attained 5mm. R1507 was administered via an intraperitoneal injection (IP) once weekly at a dose of 18 mg/kg and continued until the end of the experiment. Cisplatin was administered at a dose of 3 mg/kg IP at day 1, day 8 and day 15. IR was delivered in 4 fractions of 2 Gy on days 1 to 4 (A 200-kV X-ray irradiator, dose rate = 0.45 Gy min-1), using mouse jigs to exclusively expose the tumor beds. Bidimensional tumor measurement on each mouse was performed twice weekly to measure the tumor volume using the following formula: (length × width2)/2. Animals were sacrified when the main tumor volume per group had attained 2200–2800 mm3. Data presented are means ± SEM of xenograft tumor volumes. (B) Kaplan-Meier plots showing the tumor progression of nude mice with H526 xenografts. Median RTV5: R1507-Cisplatin-IR=50 days vs. Cisplatin-IR=34 days, p=0.006. The endpoint, RTV5, is defined as the moment when tumor volume reached five times the baseline volume. Differences in median RTV5 were computed with log rank test.
The triple combination CDDP-IR-R1507 caused a dramatic delay in tumor growth in H526 xenografts as compared with the CDDP-IR combination
The triple combination R1507-CDDP-IR produced a significant delay in tumor growth compared to the standard CDDP-IR regimen in H526 cells (median RTV5: R1507-Cisplatin-IR=50 days vs. Cisplatin-IR=34 days, P=0.006) (Fig. 4A, B). Notably, the experimental regimen yielded an 84% (P=.01) reduction of the mean tumor volume compared to the CDDP-IR regimen (tumor assessment at day 42) (Supplementary table. 1).
Integrated genomic and functional analysis revealed adaptive mechanisms under R1507
Since R1507 as a single agent demonstrated clear antitumor activity in vitro but not in vivo, we hypothesized that: (i) R1507 as a single agent would not be sufficient to inhibit IGF-1R in vivo, and/or (ii) molecular adaptive mechanisms would occur under anti-IGF-1R treatment in vivo.
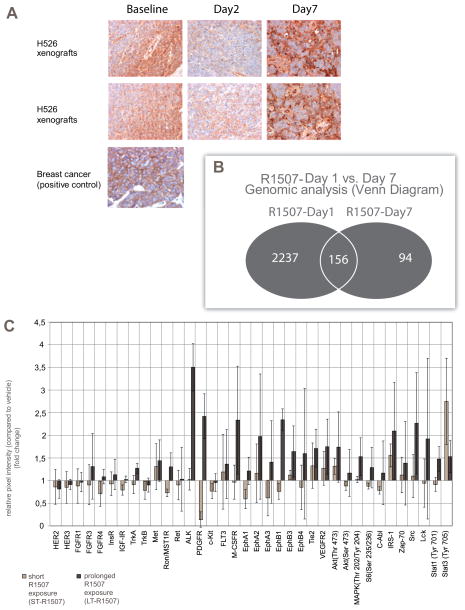
To evaluate whether R1507 could efficiently inhibit IGF-1R in tumor tissue, we examined IGF-1R expression in H526-xenografted mice at different treatment times. We observed an expected decrease in IGF-1R expression on day 2, but a secondary increase on day 7 (Fig. 5A). This did not appear to be attributable to a lack of R1507 availability on day 7 since the product half-life in mice is estimated at 10 days(11).
Figure 5.
(A) Representative sections of IGF-1R staining in H526 xenografts according to various treatment times: baseline (no treatment), day 2 and day 7 after R1507. Nude mice bearing H526 xenografts were treated with R1507 (18 mg/kg, IP) at day 1 only.. Immunohistochemical analyses were conducted using the Discovery XT automated platform (Ventana Medical Systems, Tucson, AZ) (B) Gene expression profiles (Human Genome U133 Plus 2.0 Array, Affymetrix) derived from H526 xenografts treated according to different time-related conditions: vehicle, 24 hours after R1507 treatment (R1507 day1) and seven days after R1507 treatment (R1507 day 7). Microarray probe sets down- or upregulated (2 fold change, P < 0.05, Benjamini Hochberg adjusted) in R1507 Day 1 and R1507 Day 7 groups were compared using Venn diagrams. Numbers of probe sets are indicated. (C) Low-scale protein antibody array. H526 cells were exposed to R1507 treatment (200 nM) for 3 hours (ST-R1507) or for 4 weeks (LT-R1507). The phosphorylation of 28 Receptor Tyrosine Kinases and 11 Signaling nodes are detected using the PathScan RTK Signaling Antibody Array Kit. Data represented are the means ± SEM of the fold-changes in relative pixel intensities (quantification of the protein pixel intensities relative to the vehicle treatment group) of three independent experiments.
We thus hypothesized that some adaptive mechanisms occurred upon R1507 exposure and resulted in the secondary increase in IGF1R expression on day 7. The overall regulation of gene expression induced by R1507 in H526 xenografts at the following time points: baseline (vehicle), R1507 day 1 and R1507 day 7. A total of 2396 probe sets were differentially expressed at R1507 day 1, whereas only 254 probe sets were differentially expressed at R1507 day 7. Interestingly, only 6.7% (n= 156) of the initially deregulated probe sets (R1507 day 1) remained differentially expressed at R1507 day 7 (Fig. 5B, Supplementary Fig. 3). To investigate the biological basis of the gene expression alterations, we computed the overlap between the differentially expressed probes and gene set databases. The following pathways were enriched in the R1507-Day 1 condition: Cell-to-Cell Adhesion Signaling, Map Kinase Pathway, Beta-Arrestin and Vitamin D Receptor (Supplementary table. 2). Interestingly, the downregulation of the MAPK pathway for R1507 day 1 was consistent with our previous results in vitro (Fig. 1D). However, some pathways like MAPK signaling appeared to be inversely deregulated between R1507-day 1 and R1507–day 7 (Supplementary table. 2), suggesting a role in the escape to R1507 alone in vivo.
To confirm the micro-array results at the protein level, we performed low-scale protein arrays (Fig. 5C, Supplementary Fig. 4). H526 cells were previously cultured with continuous four-week R1507 exposure (200nM) as follows: (i) vehicle, (ii) short-term R1507 exposure (ST-R1507: R1507 200nM – three-hour exposure), (iii) long-term R1507 exposure (LT-R1507: R1507 200nM – four-week exposure). We observed several striking results: (i) a transient inhibitory effect on the IGF-1R axis after ST-R1507 exposure, with reduced expression of the phosphorylated forms of IGF-1R, (Insulin Receptor) (INSR) and Akt (Ser 473), respectively. After LT-R1507 exposure, we observed a return to the baseline expression of the activated forms of IGF-1R and INSR. This is consistent with our previous results in vivo (Fig. 5A), (ii) After LT-R1507 exposure, we observed a major increase in the expression of receptors and signaling proteins involved in angiogenesis (PDGFR, VEGFR2, EphA2, EphA3, EphB3, EphB4), in proliferation (Akt, MAPK, S6 Ribosomal protein, STAT3, ALK,), and in survival (Akt, ALK). In this respect, the low-scale TK array results are also consistent with our previous genomic analyses (Supplementary table. 2).
Integrated genomic and functional analysis revealed the involvement of NER and mitochondrial apoptotic pathways in the response to CDDP- and IR-R1507 combinations
To identify the molecular mechanisms involved in the response to CDDP- and IR-R1507 combinations, we performed global gene expression profiling on mice bearing H526 xenografts treated with the following treatment conditions: vehicle, R1507 CDDP, IR, CDDP-R1507 and IR-R1507 (Fig. 6A, B, Supplementary Fig. 5&6, Supplementary table. 2). To investigate the biological basis of the gene expression variation, we computed the overlap between the differentially expressed probes and gene set databases.
Figure 6.
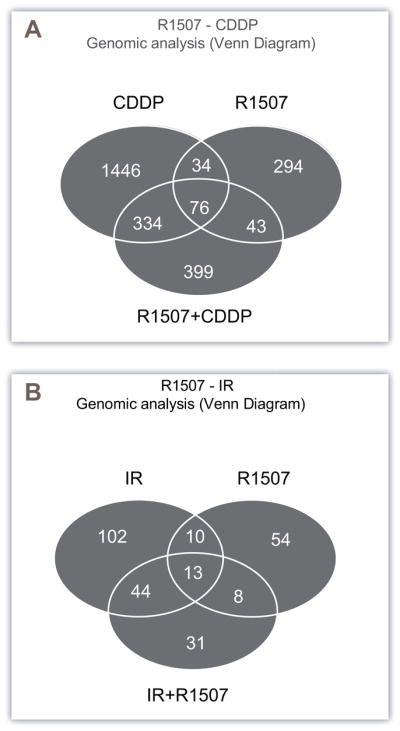
Venn diagrams derived from gene expression profiles (Human Genome U133 Plus 2.0 Array, Affymetrix) of H526 xenografts treated according to the following conditions: vehicle, R1507 alone (18mg/kg, day 1, IP), Cisplatin alone (2mg/kg, day 1, IP), ionizing radiation (IR) alone (2 Gy, day 1) and the combination of both. All tumors were removed 7 days after treatment. (A) Microarray probe sets down- or upregulated (2 fold change, P < 0.05, Benjamini Hochberg adjusted) in the R1507, Cisplatin and R1507+Cisplatin conditions were compared using Venn diagrams. The numbers of probe sets are indicated. (B) Microarray probe sets down-or upregulated (2 fold change, P < 0.05, Benjamini Hochberg adjusted) in the R1507, IR and R1507+IR conditions were compared using Venn diagrams. The numbers of probe sets are indicated.
The probes differentially expressed exclusively in the CDDP-R1507 combination were associated with downregulation of the NER pathway (Nucleotide Excision Repair) (P=0.02) (through decreases in ERCC1, XAB2 and RAD23A gene expression) and upregulation of the apoptotic mitochondrial response to DNA damage pathway (through gene expression modifications in BCL2L1 and IFI6) (P=0.04) (Fig. 6A, Supplementary table. 2, Supplementary Fig. 5).
The probes that were exclusively differentially expressed in the R1507-IR combination were associated with a downregulation of the TGF-Beta pathway (Fig. 6B, Supplementary table. 2, Supplementary Fig. 6), suggesting that the IR-R1507 combination induced the downregulation of this pathway.
We then showed that the R1507-CDDP combination decreased ERCC1 but not XPA protein expression in H526 cells (Supplementary Fig. 7A), and also decreased cleaved caspase 9 expression (Supplementary Fig. 7C). When we examined the effect of R1507-IR on the TGF-beta pathway, we did not observe any impact on SMAD4 protein expression (Supplementary Fig. 7D). We next investigated whether the R1507-IR combination could modify ERCC1 (and XPA at the protein expression level. Interestingly, the R1507-IR combination also decreased ERCC1 but not XPA protein expression in H526 cells (Supplementary Fig. 7C).
Discussion
The importance of the IGF1-IGF-1R autocrine axis in SCLC growth and its role in DNA damage repair processes suggests a benefit of combining anti-IGF-1R with cisplatin and ionizing radiation(3–5). To our knowledge, this is the first time that such a dramatic delay in tumor growth is observed after adding an IGF-1R inhibitor to the CDDP-IR standard combination against SCLC.
We have demonstrated that R1507 efficiently inhibited cell growth in vitro in H146 and H526 SCLC cell lines. Conversely, H69 cells failed to respond to IGF-1R inhibition, despite effective R1507-induced IGF-1R receptor downregulation. Interestingly, sensitive cell lines (H526 and H146) exhibited both higher IGF-1R gene copy number and lower level of basal phosphorylated Akt expression as compared to resistant cells (H69). Interestingly, these findings are consistent with those of Gong et al. (16) and of Yeh et al.(9), respectively; but warrant further studies to be validated.
We did not observe a significant effect of R107 monotherapy in H526 xenografts. Interestingly, long lasting responses have been observed with anti-IGF1R agents delivered in monotherapy, in patients diagnosed with Ewings sarcoma or with adrenocortical carcinoma(17,18). However, such responses have not yet been described in the SCLC setting. In this study, we observed that - compared to the short-term setting, the chronic administration of R1507 as a single agent led to both sustained IGF-1R pathway activity and to the concomitant relief of multiple alternative signaling pathways including the HER family receptors, angiogenesis, STATs, etc. These late effects are likely to promote tumor escape under R1507 exposure. Similar results have already been observed for antibodies targeting tyrosine kinase receptors (19,20). Though, we did not observe clear compensatory effect mediated by the insulin receptor in our model(21). These results require to be validated in human specimens to be confirmed. Since most antineoplastics are delivered over the long term and in a recurrent manner, we believe that greater emphasis should be given to long-term preclinical analyses when evaluating a new drug.
The latter findings also support strategies combining anti-IGF-1R drugs with other antineoplastics. In this regard, the long-lasting R1507-induced sensitizing effect with CDDP and IR is noteworthy. This can be partially explained by the role of IGF-1R in DNA damage repair processes. A large body of literature already exists regarding the influence of IGF-1R on DNA damage repair through ATM(7,8). Here, we describe that the R1507-CDDP and R1507-IR combinations were associated with a downregulation of the NER pathway in vitro and in vivo. Given the importance of CDDP and IR as antineoplastic agents across cancer types, the causal implication of NER in the response to combinations of anti-IGF1R antibody with CDDP and IR should be further evaluated.
The triple combination R1507-Cisplatin-IR demonstrated major and durable antitumor effects in vitro and in vivo in a SCLC model. Given these encouraging results and the very poor prognosis of SCLC, it is thus time for clinical testing of this triple combination.
Supplementary Material
Acknowledgments
None to be reported.
Grant support: This work was supported by a grant U54CA149237 from the Integrative Cancer Biology Program of the National Cancer Institute (C.Ferté) and by a fellowship from the Ministère de l’Enseignement Superieur et de la Recherche (France) (C.Ferté). A significant financial grant was also provided by Roche Diagnostics (Penzberg, Germany) to support this work (INSERM U1030).
Footnotes
Disclosure of Potential Conflicts of Interest:
The authors declare no conflict of interest.
References
- 1.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006 Oct 1;24(28):4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Rossi A, Martelli O, Di Maio M. Cancer treatment reviews. Elsevier Ltd; 2012. Oct 17, Treatment of patients with small-cell lung cancer: From meta-analyses to clinical practice. [DOI] [PubMed] [Google Scholar]
- 3.Macaulay VM, Everard MJ, Teale JD, Trott PA, Van Wyk JJ, Smith IE, et al. Autocrine Function for Insulin-like Growth Factor I in Human Small Cell Lung Cancer Cell Lines and Fresh Tumor Cells Autocrine Function for Insulin-like Growth Factor I in Human Small Cell Lung Cancer Cell Lines and Fresh Tumor Cells. 1990. pp. 2511–7. [PubMed] [Google Scholar]
- 4.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature reviews cancer. Nature Publishing Group. 2012 Mar;12(3):159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 5.Gualberto a, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. Nature Publishing Group. 2009 Aug 27;28(34):3009–21. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein N, Servan K, Hildebrandt B, Pölitz A, Von Jonquières G, Wolf-Kümmeth S, et al. Hyperactivation of the insulin-like growth factor receptor I signaling pathway is an essential event for cisplatin resistance of ovarian cancer cells. Cancer research. 2009 Apr 1;69(7):2996–3003. doi: 10.1158/0008-5472.CAN-08-3153. [DOI] [PubMed] [Google Scholar]
- 7.Peretz S, Jensen R, Baserga R, Glazer PM. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proceedings of the National Academy of Sciences of the United States of America. 2001 Feb 13;98(4):1676–81. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macaulay VM, Salisbury aJ, Bohula Ea, Playford MP, Smorodinsky NI, Shiloh Y. Downregulation of the type 1 insulin-like growth factor receptor in mouse melanoma cells is associated with enhanced radiosensitivity and impaired activation of Atm kinase. Oncogene. 2001 Jul 5;20(30):4029–40. doi: 10.1038/sj.onc.1204565. [DOI] [PubMed] [Google Scholar]
- 9.Yeh J, Litz J, Hauck P, Ludwig DL, Krystal GW. Selective inhibition of SCLC growth by the A12 anti-IGF-1R monoclonal antibody correlates with inhibition of Akt. Lung cancer. 2008 May;60(2):166–74. doi: 10.1016/j.lungcan.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Warshamana-Greene GS, Litz J, Buchdunger E, García-Echeverría C, Hofmann F, Krystal GW. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005 Feb 15;11(4):1563–71. doi: 10.1158/1078-0432.CCR-04-1544. [DOI] [PubMed] [Google Scholar]
- 11.Croasdale R, Wartha K, Schanzer JM, Kuenkele K-P, Ries C, Mayer K, et al. Development of tetravalent IgG1 dual targeting IGF-1R-EGFR antibodies with potent tumor inhibition. Archives of biochemistry and biophysics. Elsevier Inc. 2012 Oct 15;526(2):206–18. doi: 10.1016/j.abb.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010 Apr 15;16(8):2458–65. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 13.Loriot Y, Mordant P, Brown BD, Bourhis J, Soria J-C, Deutsch E. Inhibition of BCL-2 in small cell lung cancer cell lines with oblimersen, an antisense BCL-2 oligodeoxynucleotide (ODN): in vitro and in vivo enhancement of radiation response. Anticancer research. 2010 Oct;30(10):3869–78. [PubMed] [Google Scholar]
- 14.Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jun 17;105(24):8387–92. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington KJ, Billingham LJ, Brunner TB, Burnet NG, Chan CS, Hoskin P, et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. British journal of cancer Nature Publishing Group. 2011 Aug 23;105(5):628–39. doi: 10.1038/bjc.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Y, Yao E, Shen R, Goel A, Arcila M, Teruya-Feldstein J, et al. High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507) PloS one. 2009 Jan;4(10):e7273. doi: 10.1371/journal.pone.0007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele K-P, Chawla SP, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011 Dec 1;29(34):4541–7. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer chemotherapy and pharmacology. 2010 Mar;65(4):765–73. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayan M, Wilken Ja, Harris LN, Baron AT, Kimbler KD, Maihle NJ. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer research. 2009 Mar 15;69(6):2191–4. doi: 10.1158/0008-5472.CAN-08-1056. [DOI] [PubMed] [Google Scholar]
- 20.Quesnelle KM, Grandis JR. Dual kinase inhibition of EGFR and HER2 overcomes resistance to cetuximab in a novel in vivo model of acquired cetuximab resistance. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011 Sep 15;17(18):5935–44. doi: 10.1158/1078-0432.CCR-11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proceedings of the National Academy of Sciences of the United States of America. 2010 Jun 15;107(24):10791–8. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.