Abstract
Rationale
In animals, nicotine enhances reinforcement from stimuli unrelated to nicotine intake. Human research is suggestive but has not clearly shown a similar influence of nicotine.
Objectives
We assessed acute effects of nicotine via smoking on enhancement of positive (money, music) or negative (termination of noise) reinforcers, or no “reward” (control). These different rewards determined the generalizability of nicotine effects.
Materials and Methods
Dependent (n=25) and nondependent (n=27) smokers participated in three sessions, each after overnight abstinence. Using a within-subjects design, sessions involved no smoking or smoking denicotinized (0.05 mg) or nicotine (0.6 mg) QuestR brand cigarettes. For comparison, a fourth session involved no abstinence prior to smoking one’s own brand to gauge responses under typical nicotine satiation. Reinforcement was assessed by responses on a simple operant computer task for the rewards, each available singly on a progressive ratio schedule during separate trials.
Results
The reinforcing effect of music, but not other rewards, was greater due to the nicotine cigarette, compared to the denicotinized cigarette or no smoking. Reinforcement enhancing effects of nicotine did not differ between dependent and nondependent groups, indicating no influence of withdrawal relief. Responding due to acute nicotine after abstinence was very similar to responding one’s own brand after no abstinence.
Conclusions
Acute nicotine intake per se from smoking after abstinence enhances the reinforcing value of rewards unassociated with smoking, perhaps in a manner comparable to ad lib smoking after no abstinence. Nicotine’s reinforcement enhancing effects may be specific to certain rewards, perhaps those sensory in nature.
Keywords: nicotine, reinforcement enhancement, reward, smoking, dependence
Introduction
Nicotine is clearly a primary reinforcer, as self-administration behavior of humans and non-human animals is increased by contingent nicotine per se (e.g., Harvey et al. 2004; Kenny and Markou 2006; LeFoll and Goldberg 2006). Nicotine intake also can be influenced by secondary reinforcement, in that stimuli directly associated with nicotine can increase responding (i.e. “cues”; Caggiula et al. 2001; Perkins et al. 1994; Rose et al. 2010). Research with animal models (Caggiula et al. 2009; Chaudhri et al., 2006; Olausson et al. 2004; Palmatier et al. 2012; Raiff and Dallery 2008) indicates that nicotine has a third reinforcing function, that of enhancing reinforcement from rewards not directly associated with nicotine intake. This action of nicotine may reflect activation of the mesolimbic dopamine system and perhaps other brain reward pathways (Paterson 2009; Wise 1998). Yet, nicotine’s reinforcement enhancing effects may be specific to certain types of rewards and not to any available reinforcing stimulus. For example, some animal studies suggest nicotine may enhance rewards that are “sensory” in nature, such as visual stimuli, but perhaps not most primary rewards, such as food, although this selectivity may also stem from other differences (see Caggiula et al. 2009; Raiff and Dallery 2008). Other stimulant drugs may similarly enhance reinforcement from non-drug rewards (e.g. Sheppard et al. 2012), including sensory types (Lloyd et al. 2012).
In contrast to this animal work, very few studies have directly assessed reinforcement enhancing effects of nicotine in humans. This lack of study is curious since nicotine’s ability to enhance reinforcement from stimuli in the environment could help explain the persistence of nicotine intake in humans via tobacco smoking and perhaps the decline in responding for reward after tobacco abstinence (e.g. Powell et al. 2002). Even enhancement of selective rewards may be important; because sensory rewards are common in the natural environment (Fowler 1971; Raiff et al. 2012), including while smoking (e.g. van Gucht et al. 2010), nicotine enhancement of immediate sensory reinforcement could be a common experience in smokers. Consistent with the idea that nicotine enhances reinforcement from sensory (including visual) rewards, nicotine via smoking may increase subjective attractiveness ratings of viewing faces (Attwood et al. 2009) and self-reported pleasure in response to watching movie clips (Dawkins et al. 2007). On the other hand, very recent research found a modest, but significant, self-reported increase in perceived enjoyment of various “rewarding events” in the first weeks after quitting smoking, although only post-quit perceived change in perception was assessed (Snuggs and Hajek 2013). However, such self-report measures are only indirectly related to reinforcement per se, which generally requires access to a stimulus (i.e. reward) that is contingent on behavioral responding of some kind (e.g. Honig and Staddon 1977).
Other human research has assessed nicotine’s influence on responding positively reinforced by money, with somewhat mixed findings. No effect of nicotine via inhaler on gambling behavior for monetary reinforcement was recently reported (McGrath et al. 2012). Nicotine via lozenge increased card-sorting speed for money in heavier smokers (>15 cigs/day), but not in lighter smokers (Dawkins et al. 2006), raising the possibility that these nicotine effects on monetary reward were secondary to dependence, or that they reflected withdrawal relief and not necessarily enhancement of reinforced responding directly. Yet, transdermal nicotine increased response bias on a difficult signal detection task reinforced by money in non- or ex-smokers (Barr et al. 2008), ruling out withdrawal relief. However, because nicotine marginally improved cognitive processing task ability, this responding by nicotine may have been independent of enhancement of reinforcement per se (e.g. Evans and Drobes 2009).
The current study assessed effects of nicotine vs denicotinized cigarette smoking and vs no smoking, all after overnight abstinence, on reinforced responding during a simple operant computer task for various rewards in nondependent and dependent smokers. Comparing nicotine vs denicotinized cigarettes determined nicotine effects per se, and comparing denicotinized cigarettes vs no smoking determined nonspecific effects of any smoking behavior on reinforced responding. We hypothesized that nicotine per se would increase reinforced responding for one or more of the available rewards, compared to no reward (control for nonspecific responding), in both nondependent and dependent smokers. To gauge generalizability of nicotine effects across types of reward, we compared responding for each of two positive reinforcers, preferred music (e.g., a sensory reward) and money, as well as for the negative reinforcing effects of terminating an aversive loud noise, in a mostly within-subjects design.
We previously reported (Perkins et al. 2009) two studies examining the influence of very low doses of nicotine (via nasal spray or cigarettes) on responding for these same rewards, and no increase in reinforced responding for any rewards was observed with nicotine administration. However, we studied only nondependent, very occasional smokers (<5 cigarettes/week) to completely eliminate any potential effects of withdrawal relief (since nondependent do not experience withdrawal; Shiffman 1989). Consequently, the amount of nicotine intake was limited (e.g. 4 puffs/trial) in these intermittent smokers to avoid aversive responses, although this intake was sufficient to cause increased subjective responses (e.g. “liking”). Moreover, some procedures may have made the reinforcement task insensitive for assessing maximal responding for reward (breakpoint) within each 15-min trial, namely a 1-sec response delay and a very gradual progressive ratio (PR10%) schedule. Thus, in the current study, we included dependent, as well as nondependent, smokers, provided 6 puffs/trial, eliminated the response delay, and used a PR30% schedule of reinforcement. Also, for comparison, we added a fourth session involving ad lib smoking of one’s preferred brand after no overnight abstinence, to examine the degree to which acute smoking of a nicotine cigarette after abstinence would match reinforced responding under typical non-abstinent smoking conditions.
Method
Participants
Study participants (N=52) comprised 25 dependent (11 men, 14 women) and 27 nondependent (11 men, 16 women) smokers. Dependent smokers were those who smoked at least 10 cigarettes per day for at least one year and met DSM-IV criteria for nicotine dependence (APA 1994), according to a structured interview updated from Breslau et al. (1994). Nondependent smokers were those who reported never smoking more than 5 cigarettes per day and who did not ever meet DSM-IV nicotine dependence criteria. Dependent and nondependent smokers differed on some smoking characteristics, as expected, with respective means (± SD) of 14.3±3.8 vs 1.5±1.1 cigarettes/day and 4.6±1.9 vs 0.2±0.6 on the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al. 1991). The respective nicotine yield of their preferred brand was 1.0±0.2 vs 0.9±0.1 mg, 56% vs 74% were non-menthol smokers, and they were 29.2±11.4 vs 21.3±3.2 years of age. Men and women did not differ on these smoking characteristics or age. Participants were recruited from the surrounding community and most self-identified as Caucasian (82.7%), with 9.6% African-American, and 7.7% Asian. We excluded those currently taking medications to treat serious psychological problems (e.g. psychosis, major depression).
CO, Withdrawal
Expired-air carbon monoxide (CO) was assessed upon arrival via Breathco CO monitor (Lenexa, KS) to confirm overnight (>12 hr) smoking abstinence (CO< 10 ppm; SRNT Subcommittee 2002) in all subjects on three sessions and to confirm non-abstinence (i.e. satiation; CO>10 ppm) in dependent smokers on the comparison ad lib smoking session. CO was also assessed at the end of each session to gauge exposure from session smoking intake (although puff volume prior to each trial was the primary measure; see below). Nicotine withdrawal was assessed by the Minnesota Nicotine Withdrawal Scale (Hughes and Hatsukami, 2007) using the following 6 items: depressed mood/sad, irritable/angry/frustrated, anxious/nervous, difficulty concentrating, restless/impatient, and drowsiness. Items were each rated on a 0 (“not at all”) to 100 (“extremely”) visual analog scale (VAS) and averaged across symptoms to get a total withdrawal score.
Cigarettes
Subjects smoked on three sessions, involving smoking a moderate nicotine brand, a denicotinized brand, and, for comparison, their own preferred brand. The moderate “nicotine” brand was QuestR 1 (yield of 0.6 mg nicotine, 9 mg tar), and the denicotinized brand was QuestR 3 (yield < 0.05 mg nicotine, 9 mg tar), both formerly sold commercially by Vector Group, Ltd. (Miami, FL). Menthol smokers received menthol Quest; non-menthol smokers received non-menthol Quest. All Quest cigarettes had identifiable markings covered and subjects were kept blind to brand, but markings of their preferred brand were not covered in the non-abstinent comparison session and so were administered unblind. We included the own brand/satiation condition to compare the reinforcement enhancing effects of nicotine between the acute intake from Quest 1 after overnight abstinence and ad lib smoking intake after no abstinence (typical of most smoking in the natural environment).
Reinforcement Task
Reinforced responding was assessed with a modified version of a simple computer task (“Applepicker”; Norman and Jongerius 1985) used in many prior studies by us and others (e.g., Epstein et al.1991; Perkins et al. 1994; 1997; 2009). The task involves using arrow keys to move a cursor on a monitor around a “field” and pressing a button (a “pick”) when the cursor lands on one of the “trees” to look for “apples”. (The graphical representations are kept rudimentary to minimize any visual entertainment value from responding on the task.) When an “apple” is found, a symbol briefly lights up as feedback, signaling that a unit of the reward designated for that trial (i.e. one reinforcer) has been earned. The number of responses, or “picks,” required to find an “apple” constitute the reinforcement schedule, which in this study was a progressive ratio, incrementing by 30% after each completed ratio (i.e. PR30%), starting with an FR10.
As in prior research (Perkins et al. 2009), each of the four trials per session differed in the reward that was available as a reinforcer over the 15-min of task responding: 1) money (counter incrementing by $.10); 2) preferred music (playing of 30 sec of preferred music from participant’s compact disc through headphones; see Procedures below), 3) termination for 30 sec of aversive intermittent noise (4 bursts @ 30 secs of loud [106 dB] acoustic stimuli through headphones; see Perkins et al. 2009), and 4) no reward (control). The reward available for the current trial was displayed on the monitor once the trial began, and these reward trials were presented in counter-balanced order between subjects. The total money earned was displayed on the counter and added to the total amount for participating. The experimenter was not in the room with participants during task trials; the playing of the music reward was initiated by the computer and began immediately after a reinforcer was earned. Similarly, the intermittent noise was discontinued for 30 sec by the computer immediately after a reinforcer was earned, resulting in no audio through the headphones. Upon earning a reinforcer, subjects could continue responding on the task without interruption, to earn additional units of the reinforcer (i.e. further increment money counter, extend the time of music or termination of aversive noise). They were free to stop responding at any point and simply wait quietly, or read available magazines provided (intentionally routine in nature), until the end of the 15-min task period. (To eliminate any other alternative reinforcers, they were not allowed to bring their own material to sessions.) Virtually all did so on each trial, as mean (SE) duration of responding per trial was 8.7±0.7 min (11.0±0.6 min for money, 10.0±0.5 min for music, 8.7±0.6 min to terminate noise, and 5.2±0.7 min for no reward), indicating that maximal responding for reward was reached (i.e. breakpoint).
Procedure
Participants were first screened by phone on smoking and health history and scheduled for an introductory session in the laboratory to obtain written informed consent and verify eligibility. They also brought with them a compact disc of their own preferred music for use as the music reward in experimental sessions. Participants were instructed to select their 5 most highly preferred “tracks”, and 4 tracks rated at least 75 on a 0–100 VAS of “liking” were selected as the music reinforcer, totaling more than 10 mins of music. They were then introduced to the Applepicker task without reinforcement to become familiar with it during subsequent experimental sessions.
All engaged in four 2-hr experimental sessions, with three following overnight abstinence and differing only in the acute smoking condition in effect: “nicotine” (Quest 1) or denicotinized (Quest 3, “denic”) cigarettes presented under blind conditions, or no smoking. A fourth session involved no overnight abstinence and smoking of one’s preferred brand (unblinded), provided by the experimenter (to equate the “free” access to all available cigarettes). The order of these smoking conditions across the four sessions was counter-balanced between subjects and separated by at least one day. Upon arrival to each session, participants completed the withdrawal and CO measures. Then, prior to each of the 4 task trials on the 3 smoking sessions, participants self-administered six puffs over 3 mins, one puff every 30 sec, on the cigarette assigned for that session. All smoking was done via the portable Clinical Research Support System (“CReSS Pocket”; Borgwaldt KC, Inc., Richmond VA; www.plowshare.com), which assesses puff volume (in ml; Lee et al. 2003; Perkins et al. 2012). Exact puff timing and duration were guided by computer-presented puffing instructions to standardize intake at about 60 ml per puff (consistent with ad lib puffing; e.g. Perkins et al., 2012), as in our prior studies of controlled smoke exposure, including with the CReSS (Perkins et al. 2010). Also, CO was again obtained after the last trial of each session to gauge exposure during this intermittent smoking from the nicotine and denicotinized cigarettes.
During each trial, participants were instructed that they were free to work on the task for as long as they wanted the designated reward (money, preferred music, termination of aversive noise, no reward). The 24 different orders of rewards across trials were counter-balanced between subjects but remained constant across sessions within subjects. Trials lasted 25 mins each, to provide time for controlled intake of 6 puffs, completion of the 15-min task period, and 5-min brief rest until the next trial. This study protocol was approved by the University of Pittsburgh Institutional Review Board.
Data Analyses
All analyses were conducted using IBM SPSS 20.0. The primary dependent measure was number of task responses on the PR schedule for the various available rewards (money, music, termination of aversive noise, no reward). Preliminary analyses showed no effects of cigarette condition order across sessions, and so data for the three sessions involving overnight abstinence were collapsed across orders in subsequent analyses. In the analyses of variance (ANOVAs) of these responses, the between-subjects factor was dependence level (dependent vs nondependent), and the within-subjects factors were smoking condition (no smoke, denicotinized cigarette, nicotine cigarette) and type of reward (4). Because no differences in reinforced responding due to dependence were significant (as noted below), most analyses were completely within-subjects, which greatly increased statistical power (e.g. Cohen 1988). Significant effects of smoking condition in all analyses were followed up with paired comparisons of responses (Huitema 1980). Similarly, we also assessed differences in duration of reinforced responding during the 15-min trial, due to reward and cigarette condition. Effect sizes for reinforced responding of particular interest are presented by partial eta-squared values (
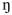 p2), which indicate the percent of variance explained.
p2), which indicate the percent of variance explained.
We also conducted similar analyses of responses between the nicotine cigarette session (Quest 1) versus the ad lib/own brand session (i.e. no abstinence), to explore whether acute nicotine intake after overnight abstinence differed from the non-abstinent, satiated condition. Finally, we assessed differences between the ad lib smoking and no smoking conditions to determine effects of complete smoking abstinence on reinforced responding.
Results
Withdrawal and dependence level
Mean (SE) withdrawal upon arrival was significantly increased by overnight abstinence, versus the no abstinence comparison session, for the dependent smokers, 28.6±3.3 vs 16.9±2.6, respectively, but not for the nondependent smokers, 14.9±3.2 vs 15.9±2.5. Thus, the interaction of abstinence x dependence level on withdrawal severity was significant, F(1,50)=9.30, p<.005, and nondependent smokers showed no withdrawal due to this brief abstinence, as expected.
Control over smoke intake
Mean (SE) puff volume from the 6 puffs on each trial was greater on the denicotinized vs nicotine cigarette smoking sessions, 339±13 vs 265±12 ml, respectively, F(1,50)=40.07, p<.001. Yet, there was no main effect of reward (range of 298 ml for money to 308 ml for no reward) and no interaction of nicotine/denicotinized cigarette x reward on puff volume, both F(3,150)<1, ns. There were also no significant effects of dependence, F(1,49)=2.65, p=.11, cigarette x dependence, F(1,49)=2.18, p=.15, or of the interactions of reward x dependence or of reward x cigarette x dependence, both F(3,147)<1, ns. Moreover, there was no effect of cigarette x dependence level on CO increase (assessed only at baseline and after the last trial; +14.9±0.8 ppm), F(1,50)<1, ns. In sum, although smoke intake was greater for the denicotinized cigarette, consumption did not differ by reward type or dependence level.
Reinforcing value of rewards
In the 3 sessions following overnight abstinence, overall responding for rewards was significantly influenced by the main effects of smoking condition, F(2,100)=10.03, p<.001,
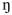 p2 = .167, reward, F(3,150)=48.93, p<.001,
p2 = .167, reward, F(3,150)=48.93, p<.001,
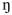 p2 = .495, and the smoking x reward interaction, F(6,300)=2.13, p=.05,
p2 = .495, and the smoking x reward interaction, F(6,300)=2.13, p=.05,
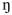 p2 = .041. For the main effects of smoking condition, pairwise comparisons (i.e. within-subjects) showed greater mean (SE) reinforced responding per trial due to the nicotine cigarette, 788±42, which was 116±26 more than the denicotinized cigarette, F(1,51)=19.90, p<.001,
p2 = .041. For the main effects of smoking condition, pairwise comparisons (i.e. within-subjects) showed greater mean (SE) reinforced responding per trial due to the nicotine cigarette, 788±42, which was 116±26 more than the denicotinized cigarette, F(1,51)=19.90, p<.001,
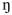 p2 = .281, and 111±32 more than no smoking, F(1,51)=11.71, p<.001,
p2 = .281, and 111±32 more than no smoking, F(1,51)=11.71, p<.001,
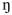 p2 = .187. In other comparisons, none of these effects varied by dependence/nondependence, as the main effect of dependence level, F(1,50) < 1, and interactions of dependence x smoking condition, F (2,100) < 1, dependence x reward, F(3,150) = 1.17, p=.32, and dependence x smoking x reward, F(6,300)=1.11, p=.35, were not significant. Therefore, these effects of smoking on responding for rewards did not differ between the dependent and nondependent subjects, despite their clear difference in withdrawal due to overnight abstinence (above).
p2 = .187. In other comparisons, none of these effects varied by dependence/nondependence, as the main effect of dependence level, F(1,50) < 1, and interactions of dependence x smoking condition, F (2,100) < 1, dependence x reward, F(3,150) = 1.17, p=.32, and dependence x smoking x reward, F(6,300)=1.11, p=.35, were not significant. Therefore, these effects of smoking on responding for rewards did not differ between the dependent and nondependent subjects, despite their clear difference in withdrawal due to overnight abstinence (above).
Because dependence level had no significant impact on these effects, subsequent analyses examined the source of the smoking x reward interaction on amount of responding in all subjects combined (again using within-subjects comparisons). These follow-up ANOVAs showed significant effects of smoking condition for music reward only, F(2,102)=10.49, p<.001,
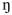 p2 = .171. Smoking condition only marginally affected responding for money, F(2,102)=2.72, p=.07,
p2 = .171. Smoking condition only marginally affected responding for money, F(2,102)=2.72, p=.07,
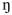 p2 = .051, and for termination of aversive noise F(2,102)=2.37, p=.10,
p2 = .051, and for termination of aversive noise F(2,102)=2.37, p=.10,
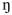 p2 = .044, and had no effect on responding for no reward (control), F(2,102)=1.58, p=.21. “Earnings” for each respective reward trial averaged 5.9 mins of music, $1.20, and 5.2 mins of terminating/avoiding intermittent aversive noise.
p2 = .044, and had no effect on responding for no reward (control), F(2,102)=1.58, p=.21. “Earnings” for each respective reward trial averaged 5.9 mins of music, $1.20, and 5.2 mins of terminating/avoiding intermittent aversive noise.
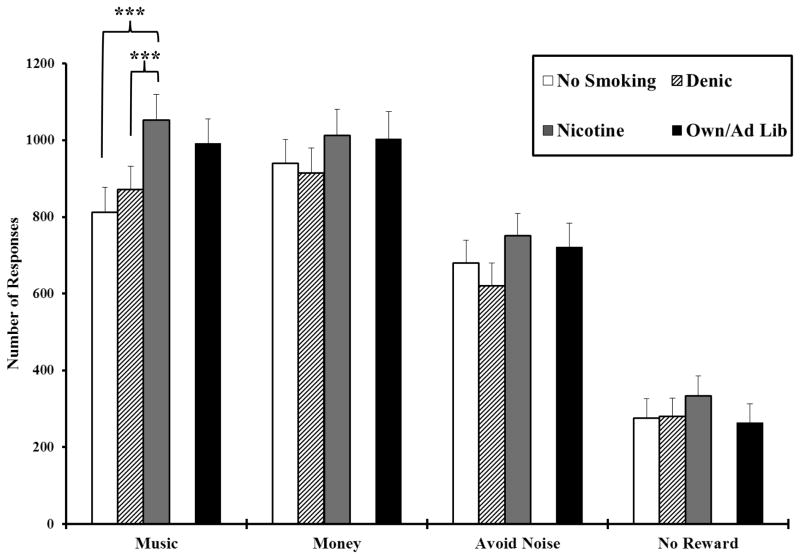
Mean (SE) responding for each reward is shown in Figure 1 for all subjects by the 3 smoking conditions after overnight abstinence. Responding was greater for each of the three rewards compared to no reward, confirming they were reinforcers (Honig and Staddon 1977). As in the figure, pairwise comparisons found that reinforced responding for music during the nicotine cigarette increased 181±38 vs the denicotinized cigarette, t(51)=4.76, p<.001, and 241±62 vs no smoking, t(51)=3.88, p<.001. The denicotinized and no smoking conditions did not differ from each other (t=0.98, p=.33), indicating that nicotine intake per se, and not simply smoking behavior in general, influenced reinforced responding for music reward. Responding for music reward was increased by the nicotine vs denicotinized cigarette condition in 40 of the 52 subjects (77%), and music reward responding was correlated 0.80, p<.001, between the nicotine vs denicotinized cigarette, showing high consistency.
Fig. 1.
Mean±SE total number of responses per trial for reinforcers, by reward type and smoking condition (N=52). Only music reward was significantly influenced by nicotine intake. *** p<.001 for within-subjects paired comparisons of the difference in responding from smoking the nicotine cigarette after overnight abstinence.
Although the order of rewards across the 4 trials per session was counter-balanced, we also assessed reinforced responding for music reward by session trial number (i.e. 1–4, in which reward order remained the same for each individual across sessions). We found no effects of trial number, F(3,48)=1.25, p=.30, or the interaction of smoking condition x trial number, F(6, 96) < 1, ns, suggesting that nicotine’s enhancement of music reinforcement was similar regardless of whether it was available during earlier or later trials within the session.
Consistent with the amounts of responding, as expected, results were similar for the duration of task responding per trial, with a significant main effect of reward, F(3,150)=33.77, p<.001,
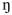 p2 = .678, and the smoking x reward interaction, F(6,300)=2.09, p=.05,
p2 = .678, and the smoking x reward interaction, F(6,300)=2.09, p=.05,
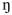 p2 = .252, but no main effect of smoking condition, F(2,100)<1, ns. Overall duration of responding per trial was marginally longer in dependent vs non-dependent smokers, 9.5±0.6 vs 8.0±0.6 mins, F(1,50)=2.99, p=.09, but duration did not vary by the interactions of reward x dependence or smoking x dependence, both F’s<1, ns, or of smoking x reward x dependence, F(6,300)=1.49, p=.18.
p2 = .252, but no main effect of smoking condition, F(2,100)<1, ns. Overall duration of responding per trial was marginally longer in dependent vs non-dependent smokers, 9.5±0.6 vs 8.0±0.6 mins, F(1,50)=2.99, p=.09, but duration did not vary by the interactions of reward x dependence or smoking x dependence, both F’s<1, ns, or of smoking x reward x dependence, F(6,300)=1.49, p=.18.
Comparisons with Ad Lib Smoking (No Abstinence)
Also shown in Figure 1 is the reinforced responding for each reward during the ad lib smoking of own brand (no abstinence) comparison condition. Responding for reward was very similar between the ad lib condition and the Quest 1 nicotine cigarette condition after overnight abstinence, F(1,51)=2.18, p=.15,
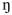 p2 = .041, and there was no significant interaction of ad lib/Quest 1 x reward on reinforced responding, F(3,153)<1, ns. So, acute nicotine intake after abstinence enhanced reinforcement as much as ad lib nicotine intake after no abstinence.
p2 = .041, and there was no significant interaction of ad lib/Quest 1 x reward on reinforced responding, F(3,153)<1, ns. So, acute nicotine intake after abstinence enhanced reinforcement as much as ad lib nicotine intake after no abstinence.
However, overall reinforced responding was significantly different for the ad lib smoking vs. no smoking conditions, F(1,51)=5.69, p=.02,
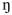 p2 = .100, and for the interaction of ad lib smoking/no smoking x reward, F(3,153)=3.31, p=.02,
p2 = .100, and for the interaction of ad lib smoking/no smoking x reward, F(3,153)=3.31, p=.02,
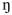 p2 = .061. Again, only reinforcement of music reward was enhanced by ad lib smoking, F(1,51)=10.18, p=.002,
p2 = .061. Again, only reinforcement of music reward was enhanced by ad lib smoking, F(1,51)=10.18, p=.002,
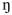 p2 = .166, while responding for money, F(1,51)=1.56, p=.22, or for termination of aversive noise or for no reward, both F(1,51)<1, were not different between ad lib smoking vs no smoking, further suggesting that nicotine’s reinforcement enhancing effects may be specific to certain rewards.
p2 = .166, while responding for money, F(1,51)=1.56, p=.22, or for termination of aversive noise or for no reward, both F(1,51)<1, were not different between ad lib smoking vs no smoking, further suggesting that nicotine’s reinforcement enhancing effects may be specific to certain rewards.
Discussion
To our knowledge, this may be the first clear demonstration in humans that nicotine via smoking has reinforcement enhancing effects, i.e. that it increases reinforcement from stimuli unassociated with nicotine. Because reinforced responses were significantly greater following smoking of nicotine cigarettes, compared to smoking denicotinized cigarettes or no smoking, this reinforcement enhancing effect is due to acute nicotine intake per se and not a general response to smoking behavior. Nicotine did not affect responding for no reward (control trial), ruling out nonspecific effects of nicotine. These reinforcement enhancing effects of nicotine via smoking did not differ between dependent and nondependent smokers, despite their expected differences in withdrawal response to overnight abstinence, indicating these effects are not a consequence of dependence or a result of withdrawal relief. Consequently, animal research results demonstrating nicotine’s reinforcement enhancing effects may be very relevant to understanding nicotine effects in humans (Caggiula et al. 2009), suggesting inter-species consistency (e.g., O’Dell and Khroyan, 2009).
However, nicotine may enhance reinforcement from only specific types of rewards. With the three types available to smokers in our study, nicotine acted in rather narrow fashion, enhancing the “sensory” reward of music but not the non-sensory reward of money or the negatively reinforcing sensory reward of terminating aversive noise. We found similar overall amounts of responding for the money vs music rewards, ruling out differential magnitude of reinforcing efficacy as an explanation for this specificity of effects of nicotine on reward type (see Palmatier et al. 2012). Therefore, it is conceivable these nicotine effects may be very specific to certain types of rewards and not broadly enhancing of reinforcement from all types of rewards unassociated with nicotine, such as positive reinforcers not sensory in nature (e.g. money) or negative reinforcers, even if also immediately sensory in nature (terminating aversive noise). Yet, these rewards varied in ways other than their sensory characteristics, such as in immediacy vs delayed (money) delivery, and in terms of their secondary (money?) versus primary reinforcing actions. Clearly, further research in humans is needed with wider availability of other types of sensory and nonsensory rewards to replicate the specificity of our reinforcement enhancing effects of nicotine and assess whether nicotine may enhance reinforcement from other types of rewards. If our results are confirmed, they may appear consistent with findings from some animal research that nicotine enhances reinforcement from certain rewarding stimuli but not others (e.g. Caggiula et al. 2009; Raiff and Dallery, 2008).
Although speculative, our results on the reinforcement enhancing effects of nicotine may have clinical implications, even if the effects are specific to certain types of rewards. For example, even if limited to sensory rewards, nicotine via smoking could increase the degree of enjoyment from many rewarding activities involving “sensory” stimuli, such as watching television, listening to music (as in our task), viewing movies or certain internet websites, or other comparable activities (e.g., Hatsukami et al., 1990; Raiff et al. 2012; van Gucht et al. 2010). Moreover, loss of this reinforcement could help account for why quitting smoking may produce greater subjective deprivation than expected based solely on loss of direct reinforcing effects of nicotine per se (Baker et al. 2004). Thus, quitting smoking would not only remove primary and secondary reinforcing effects of nicotine (typically the focus of cessation treatments), but also these reinforcement enhancing effects of nicotine, which could decrease overall reinforcement from many daily rewards in a smoker’s environment. This notion is consistent with our results, as reinforced responding for music reward was significantly less during the no smoking condition after overnight abstinence, compared to responding during ad lib smoking of own brand after no abstinence, although our participants were not interested in quitting. It is also similar to our recent study in a small sample of smokers high in quit interest, in which responding for music reward was greater during an initial ad lib smoking session than during a subsequent 24-hr abstinence session on oral placebo (Perkins et al. in press). Consequently, a smoking lapse could abruptly increase reinforcement from rewarding activities. This was perhaps illustrated in our study by the fact that acute smoking of the Quest 1 following overnight abstinence was modest in nicotine intake but produced responding for reward similar to that during ad lib smoking of own brand after no abstinence. This finding potentially helps explain why smoking lapses may be particularly reinforcing after a period of cessation and how a single lapse can prompt full blown relapse (Shadel et al. 2011).
As noted, future research should examine the breadth of reinforcement enhancing effects of nicotine across the many types of rewards commonly available to humans when smoking, as well as via different methods of nicotine administration. Such studies may be able to ascertain whether greater reinforced responding stems from an increase in the hedonic value of a reward or from a decrease in the perceived cost of responding (e.g. Salamone 2006). Nicotine’s reinforcement enhancing effects also may depend on the nature of alternatives to responding for some rewards, or the specific conditions (e.g. feeling subjective distress may further enhance reinforced responding for certain rewards but attenuate responding for other rewards). Subsequent research also could examine individual differences in the magnitude of reinforced responding due to nicotine and/or the loss of such responding after quitting (e.g., Van Voorhees et al. 2012). Research should continue determining the changes in brain activation due to acute nicotine and relating these to its reinforcement enhancing effects, especially in smokers (Addicott et al. 2012; Guy & Fletcher 2013; Klinkenberg et al. 2013; Paterson 2009; Wise 1998). Finally, effects of other drugs on enhancement of reinforcement in humans warrant study (e.g. Lloyd et al. 2012; Perkins et al. in press; Sheppard et al. 2012).
Acknowledgments
This research was supported by NIH Grant DA31218 (KAP).
Footnotes
Neither author has any potential conflicts of interest to report.
References
- Addicott MA, Baranger DAA, Kozink RV, Smoski MJ, Dichter GS, McClernon FJ. Smoking withdrawal is associated with increases in brain activation during decision making and reward anticipation: a preliminary study. Psychopharmacology. 2012;219:563–573. doi: 10.1007/s00213-011-2404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual-IV. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Attwood AS, Penton-Voak IS, Munafo MR. Effects of acute nicotine administration on ratings of attractiveness of facial cues. Nic Tobacco Res. 2009;11:44–48. doi: 10.1093/ntr/ntn006. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;1:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatr. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. DSM-IIIR nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. doi: 10.1111/j.1360-0443.1994.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier M, Liu X, Chaudhri N, Sved A. The role of nicotine in smoking: A dual-reinforcement model. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use, Nebraska Symposium on Motivation. Vol. 55. New York: Springer-Verlag; 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the social sciences. 2. Hillsdale NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addict Behav. 2007;32:425–431. doi: 10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I—effects on incentive motivation. Psychopharmacology. 2006;189:355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Bulik CM, Perkins KA, Caggiula AC, Rodefer J. Behavioral economic analysis of smoking: money and food as alternatives. Pharmacol Biochem Behav. 1991;38:715–721. doi: 10.1016/0091-3057(91)90232-q. [DOI] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Fowler H. Implications of sensory reinforcement. In: Glaser R, editor. The nature of reinforcement. New York: Academic Press; 1971. pp. 151–195. [Google Scholar]
- Guy EG, Fletcher PJ. Nicotine-induced enhancement of responding for conditioned reinforcement in rats: role of prior nicotine exposure and α4β2 nicotinic receptors. Psychopharmacology. 2013;225:429–440. doi: 10.1007/s00213-012-2832-8. [DOI] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology. 2004;175:134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Morgan SR, Pickens RW, Champagne SE. Situational factors in cigarette smoking. Addict Behav. 1990;15:1–12. doi: 10.1016/0306-4603(90)90002-f. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Honig WK, Staddon JER. Handbook of operant behavior. Englewood Cliffs NJ: Prentice-Hall; 1977. [Google Scholar]
- Hughes JR, Hatsukami DK. Instructions for use of the Minnesota Withdrawal Scale-Revised. 2007 Retrieved from www/uvm.edu/~hbpl.
- Huitema B. Analysis of covariance and alternatives. New York: John Wiley & Sons; 1980. [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacol. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A, Riedel AJ, Sambeth A. Cholinergic modulation of auditory processing, sensory gating and novelty detection in human participants. Psychopharmacology. 2013;225:903–921. doi: 10.1007/s00213-012-2872-0. [DOI] [PubMed] [Google Scholar]
- Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nic Tobacco Res. 2003;5:673–679. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- LeFoll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Kausch MA, Gancarz AM, Beyley LJ, Richards JB. Effects of novelty and methamphetamine on conditioned and sensory reinforcement. Behav Brain Res. 2012;234:312–322. doi: 10.1016/j.bbr.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath DS, Barrett SP, Stewart SH, Schmid EA. The effects of acute doses of nicotine on video lottery terminal gambling in daily smokers. Psychopharmacology. 2012;220:155–161. doi: 10.1007/s00213-011-2465-3. [DOI] [PubMed] [Google Scholar]
- Norman WD, Jongerius JL. Apple Picker: Computer software for studying human responding on concurrent and multiple schedules. Behav Res Meth Instr Comput. 1985;17:222–225. [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, O’Brien LC, Hall MJ. The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology. 2012;219:1119–1131. doi: 10.1007/s00213-011-2439-5. [DOI] [PubMed] [Google Scholar]
- Paterson NE. The neuropharmacological substrates of nicotine reward: reinforcing versus reinforcement-enhancing effects of nicotine. Behav Pharmacol. 2009;20:211–225. doi: 10.1097/FBP.0b013e32832c7083. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe JE, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav. 1994;47:107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Fonte C. The influence of acute smoking exposure on the subsequent reinforcing value of smoking. Exper Clin Psychopharmacol. 1997;5:277–285. doi: 10.1037//1064-1297.5.3.277. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grottenthaler A, Wilson AS. Lack of reinforcement-enhancing effects of nicotine in non-dependent smokers. Psychopharmacology. 2009;205:635–645. doi: 10.1007/s00213-009-1574-8. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect measure and situation, but not on nicotine. Biol Psychiatry. 2010;67:707–714. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nic Tobacco Res. 2012;14:490–494. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC, Stratton E. Possible reinforcement enhancing effects of bupropion during initial smoking abstinence. Nic Tobacco Res. doi: 10.1093/ntr/nts224. (in press) in press. Online access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51:151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology. 2008;201:305–314. doi: 10.1007/s00213-008-1282-9. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Jarvis BP, Rapoza D. Prevalence of video game use, cigarette smoking, and acceptability of a video-game based smoking cessation intervention among online adults. Nic Tobacco Res. 2012;14:63–77. doi: 10.1093/ntr/nts079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Salley A, Behm FM, Bates JE, Westman EC. Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology. 2010;210:1–12. doi: 10.1007/s00213-010-1810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. Will the last person who uses the term ‘reward’ please turn out the lights? Comments on processes related to reinforcement, learning, motivation, and effort. Addiction Biol. 2006;11:43–44. doi: 10.1111/j.1369-1600.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Martino SC, Setodji C, Cervone D, Witkiewitz K, Beckjord EG, Scharf D, Shih R. Lapse-induced surges in craving influence relapse in adult smokers: an experimental investigation. Health Psychol. 2011;30:588–596. doi: 10.1037/a0023445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AB, Gross SC, Pavelka SA, Hall MJ, Palmatier MI. Caffeine increases the motivation to obtain non-drug reinforcers in rats. Drug Alc Depend. 2012;124:216–222. doi: 10.1016/j.drugalcdep.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Tobacco “chippers”—individual differences in tobacco dependence. Psychopharmacology. 1989;97:539–547. doi: 10.1007/BF00439561. [DOI] [PubMed] [Google Scholar]
- Snuggs S, Hajek P. Responsiveness to reward following cessation of smoking. Psychopharmacology. 2013;225:869–873. doi: 10.1007/s00213-012-2874-y. [DOI] [PubMed] [Google Scholar]
- SRNT subcommittee . Biochemical verification of tobacco use and cessation. Nic Tobacco Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Van den Bergh O, Beckers T, Vansteenwagen D. Smoking behavior in context: where and when do people smoke? J Behav Ther Exp Psychiatry. 2010;41:172–177. doi: 10.1016/j.jbtep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Van Voorhees EE, Mitchell JT, McClernon FJ, Beckham JC, Kollins SH. Sex, ADHD, and smoking outcomes: an integrative model. Med Hypoth. 2012;78:585–593. doi: 10.1016/j.mehy.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]