Abstract
Background
Type 1 diabetes mellitus (DM) patients surviving myocardial infarction (MI) manifest an increased incidence of subsequent heart failure (HF). We have previously shown that after MI, type 1 DM is associated with accentuated myocardial oxidative stress (OS) and concomitant worsening of left ventricular (LV) function. However, the precise mechanisms whereby type 1 DM-enhanced OS adversely affects HF after MI remain obscure. As carbonylation of proteins is an irreversible post-translational modification induced only by OS that often leads to the loss of function, we analyzed protein-bound carbonyls in the surviving LV myocardium of MI and DM + MI rats in relation to residual LV function.
Methods
Type 1 DM was induced in rats via administration of streptozotocin. Two weeks after induction of type 1 DM, MI was produced in DM and non-DM rats by coronary artery ligation. Residual LV function and remodeling was assessed at 4 weeks post-MI by echocardiography. Myocardial carbonylated proteins were detected through OxyBlot analysis, and identified by mass spectrometry.
Results
Compared with MI rats, DM + MI rats exhibited significantly poorer residual LV systolic function and elevated wet to dry weight ratios of the lungs. Protein carbonyl content in cardiac tissue and isolated heart mitochondria of DM + MI rats was 20% and 48% higher, respectively, versus MI rats. Anti-oxidative enzymes and fatty acid utilization proteins were among the carbonylated protein candidates identified.
Conclusions
These findings implicate myocardial protein carbonylation as part of the molecular pathophysiology of aggravated HF in the type 1 diabetic post-infarction heart.
Keywords: Carbonylation, Heart Failure, Myocardial Infarction, Oxidative Stress, Type 1 Diabetes Mellitus
Introduction
After myocardial infarction (MI), patients with diabetes mellitus (DM) have a higher incidence of morbidity and mortality compared to the non-DM patient population [1-4]. The reduced survival rate and poor prognosis of DM patients following MI has been reported to be attributable to the more frequent development of heart failure (HF) [2, 5-7]. Subgroup analyses from randomized clinical trials have suggested that modern treatment strategies (thrombolytic agents, beta blockers, antiplatelets, angiotensin converting enzyme [ACE] inhibitors) have been unable to reduce the differences in survival after MI between DM and non-DM patients [8-10]. Despite the higher risk for DM patients to develop HF after MI, our understanding of the pathobiology by which DM exacerbates the incidence of this syndrome remains obscure.
Although it has been documented that HF after MI in the presence of DM is characterized by greater myocardial oxidative stress (OS) burden [11], it remains unknown as to how DM-induced myocardial OS predisposes to HF after MI at the molecular level. Proteins are major targets for reactive oxygen species (ROS) because of their abundance in biological systems and because they are primarily responsible for most functional processes within cells [12]. While proteins can undergo oxidative modification in more than 35 ways, carbonylation is the most common and general type of protein oxidation [13]. Carbonylation is an irreversible post-translational modification induced only by OS that often leads to the loss of protein function and alteration of biological activity [13, 14]. Therefore, increased levels of carbonylated proteins are likely to have serious deleterious effects on cellular and organ function.
Little is known about changes in myocardial protein carbonylation during post-MI HF associated with DM. Accordingly, in the present study we: (a) analyzed the presence of carbonylated proteins via OxyBlot analysis in cardiac tissue and heart mitochondria isolated from the remote surviving left ventricular (LV) myocardium of MI and DM + MI rats in relation to residual LV function and remodeling at 4 weeks after MI, and (b) identified specific candidates of carbonylation in protein fractions that via OxyBlot analysis contained increased carbonylated proteins using mass spectrometry (MS) techniques.
Methods
Experimental Animals
Sprague-Dawley rats were obtained from Harlan laboratories. All experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center and conforms with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health.
Induction of Type 1 DM
Type 1 DM was induced in male Sprague-Dawley rats by administering a single intraperitoneal (i.p.) injection of streptozotocin (STZ) (65 mg/kg body wt) (Sigma) prepared daily in citrate buffer pH 4.5 for maximal stability. The control group was injected with the vehicle only. Development of DM was confirmed 48 hours later by the presence of glycosuria (>2000 mg/dl) along with polyuria as described previously [11]. Rats with urine glucose values of <2000 mg/dL 24-48 hours after STZ injection were not considered to be diabetic and were excluded from further study.
Induction of Myocardial Infarction
Diabetic and non-diabetic male Sprague-Dawley rats (200-225 g body weight) were anaesthetized intraperitoneally with Nembutal (30 mg/kg). Absence of a response to pinching the toe was used as an indicator of the appropriate level of anesthesia. Rats were then rapidly intubated and mechanically ventilated (tidal volume, 1 ml/100 g body weight; ventilation rate, 65 strokes/min) by a constant volume small animal ventilator (Model 683, Harvard Apparatus). A left thoracotomy was performed at the fourth intercostal space, and the left anterior descending (LAD) coronary artery was then ligated by irreversible tightening of a 6-0 suture loop. Myocardial infarction (MI) was confirmed by regional cyanosis of the myocardial surface distal to the suture, accompanied by S-T segment elevation on the electrocardiogram. Following successful induction of MI, the chest cavity was compressed to evacuate any air before being tightly sealed. Sham-operated animals underwent the same surgical procedure with the exception that the left coronary artery was not ligated. The rats were given buprenorphine (0.05 mg/kg) pre-operatively, then Q 8-12 hours post-operatively by subcutaneous injection for 48 h. Animals that survived through to the end of 4 weeks post-MI were used for different studies outlined below.
Measurement of Blood Glucose
Prior to MI surgery and at the time of sacrifice, blood samples were collected from the tail vein for the determination of blood glucose levels using Accutrend Plus test strips and meter (Roche).
In Vivo Residual LV Function Evaluation by Echocardiography
Transthoracic echocardiographic images of hearts from all groups of rats were obtained using an ultra high-resolution ultrasound scanner (Vevo 2100; VisualSonics, Inc.) under light anesthesia (Nembutal, 30 mg/kg, i.p.). For M-mode recordings, the parasternal short-axis view was used to image the heart in two dimensions at the level of the papillary muscles to obtain LV fractional shortening (LVFS) and LV ejection fraction (LVEF). All measurements were averaged in five consecutive cardiac cycles and analyzed off-line by a single blinded observer using software resident on the ultrasonograph. All calculations were derived using standard formulas. LV internal dimension during diastole and systole (LVIDd and LVIDs) were measured from M-mode tracings obtained at the mid-papillary level and analyzed according to modified American Society for Echocardiography standards. After echocardiographic assessment, the rats were sacrificed, and the hearts were removed for further studies.
Tissue Harvest
Following echocardiographic assessment, hearts from all groups of rats were rapidly excised and perfused with ice-cold physiological saline and weighed. The atria and ventricles were dissected and the infarcted (scar) and non-infarcted regions of the LV was separated, weighed, and frozen in liquid nitrogen. The non-infarcted LV tissue was used for all molecular analyses.
Pieces of tissues from the lungs and liver were removed and weighed. For the determination of dry weight, these were placed in an oven at 65°C until a constant weight was reached. Ratios of wet to dry weight were calculated for both lungs and liver.
Cardiac Tissue and Heart Mitochondrial Protein Lysate Preparation for OxyBlot
For each animal in all of the groups, one piece of frozen non-infarcted LV myocardial tissue was used for measuring carbonylated proteins in cardiac tissue homogenate while another separate piece of immediately adjacent frozen non-infarcted LV myocardial tissue from the very same animal was used for isolating mitochondria in order to measure heart mitochondrial carbonylated proteins.
Frozen viable LV tissues were homogenized in ice-cold Rippa buffer (pH 7.4) containing a mixture of protease inhibitors followed by sonication. Following sonication, tissue lysates were centrifuged at 13,500 rpm for 15 min at 4°C. Supernatant was collected and the remaining pellet was saved for a second extraction. The procedure was repeated and this second supernatant was added to the previously collected extract. Protein concentration of tissue extracts was measured using the Modified Lowry Protein Assay Reagent Kit (Pierce Biotechnology).
Intact cardiac mitochondria were isolated from surviving LV tissues using a mitochondria isolation kit for tissue (MitoSciences, MS850). Briefly, 0.2-0.4 g of cardiac tissue was washed twice with 1.5 ml of wash buffer provided in the kit. Tissue was minced and placed in a pre-chilled Dounce homogenizer. Up to 2.0 ml of isolation buffer provided with the kit was then added to the tissue in the homogenizer. Cells were ruptured by homogenizing tissue with the dounce homogenizer. The resulting homogenate was centrifuged at 1,000 g for 10 minutes at 4°C. The supernatant was saved and transferred into two new tubes and each tube was filled to 2.0 ml with isolation buffer. The supernatant was centrifuged at 12,000 g for 15 minutes at 4°C. The pellet was collected and washed by resuspending in isolation buffer supplemented with protease inhibitor cocktail and then centrifuged at 12,000 g for 15 minutes at 4°C. Pellets were collected and centrifugation was repeated. Pellets were combined and resuspended in isolation buffer supplemented with protease inhibitor cocktail. Aliquots were then frozen at −80°C until use. Heart mitochondria isolated were assayed for protein concentration using the BCA protein assay kit (Pierce Biotechnology) as recommended by the mitochondria isolation kit for tissue.
Protein Carbonyl Immunodetection After 1D-OxyBlot
Immunodetection of carbonyl groups introduced into cardiac tissue and heart mitochondrial proteins by oxidative stress was performed using an OxyBlot kit according to the manufacturer’s instructions (Millipore). Using this OxyBlot kit, protein carbonyl groups may be derivatized with 2, 4-dinitrophenylhydrazine (DNPH), generating a stable dinitrophenylhydrazone (DNP) product that can be immunodetected by specific anti-DNP antibodies. For 1D-OxyBlot, 15 μg of protein extracts derived from the viable LV tissues and 5 μg of protein extracts derived from heart mitochondria were first derivatized with DNPH in the presence of sodium dodecyl sulfate (SDS). After 15 min incubation at room temperature, the reaction was stopped with the addition of the supplied neutralization solution. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was then performed with a Novex Midi Gel System using NuPAGE Novex 4%-12% Bis-Tris Midi gels (Invitrogen). After electrophoresis, the proteins were transferred to a PVDF membrane (Millipore) using a Novex Semi-Dry Blotter (Invitrogen). Membranes were then blocked in PBS Tween + 5% BSA overnight and incubated with a rabbit anti-DNP primary antibody (1:500) supplied with the Oxyblot kit for 1-2 hours. The primary antibody was detected using a horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody (1:300) supplied with the Oxyblot kit. Immunoreactivity was visualized by chemiluminescent detection. Molecular weight protein standards containing attached DNP residues were included with the OxyBlot kit and served as an internal control for the OxyBlot procedure. Treatment of samples with a control solution also supplied in the kit served as a negative control to the DNPH treatment. As an additional control, the anti-DNP antibody was omitted. The blots were scanned and densitometric analysis was performed using NIH Image J software. For all samples, two gels were performed in parallel, one for Coomassie staining of total protein (Colloidal Blue Staining Kit, LC6025, Invitrogen) and the other for transfer to PVDF membranes.
1D-OxyBlot Data Acquisition and Analysis
Band detection and quantification were carried out using NIH Image J software. For each sample, total pixel intensity of carbonylated bands identified from the OxyBlot were divided by the total pixel intensity of the corresponding Colloidal Blue-stained bands to account for any differences in loading of protein samples. Resulting normalized densitometric intensity of protein carbonylation for each of the samples were used in calculating the mean densitometric intensity of protein carbonylation in each of the groups. From the Coomassie-stained gels of myocardial tissue samples, protein bands in corresponding gel regions to immunoreactive bands detected following the OxyBlot procedure were excised for in-gel digestion and subsequent mass spectrometric analysis.
Mass Spectrometry (MS) Protein Identification and Database Search
In-gel protein digestion using proteomics-grade trypsin and LC-MS/MS analysis of extracted peptides were performed as previously described (15) using a LTQ Orbitrap Velos mass spectrometer (Thermo Scientific). Using an Eksigent NanoLC Ultra and AS2 Autosampler, MudPIT analysis was performed using a biphasic C18/SCX capillary column and an 11-step salt pulse gradient, as detailed previously (15). Peptides were resolved on a C18 analytical column (18cm x 100 um i.d.) with a 90 min reverse gradient (2-40% acetonitrile, 0.1% formic) for the first 10 salt pulses, and a 100 min gradient (2-98% acetonitrile, 0.1% formic acid) for the last salt pulse. Gradient-eluted peptides were introduced into the mass spectrometer via nanoelectrospray ionization.
Data were collected using a 17-scan event, data-dependent method. Full scan (m/z 350-2000) spectra were acquired with the Orbitrap as the mass analyzer (resolution 60,000), and the sixteen most abundant ions in each MS scan were selected for fragmentation via collision-induced dissociation (CID) in the LTQ Velos ion trap. An isolation width of 2 m/z, an activation time of 10 ms, and 35% normalized collision energy, a maximum injection time of 100ms and an AGC target of 1×104 were used to generated MS/MS spectra. Dynamic exclusion was enabled, using a repeat count of 1, repeat duration of 10 sec, and an exclusion duration of 15 sec.
For identification of proteins, tandem mass spectra were extracted and searched with SEQUEST (Thermo Fisher Scientific) as the database-searching algorithm as previously described (15) and the protein database consisted of a concatenated forward and reversed (decoy) rat subset database created from the Uniprot KB protein database (www.uniprot.org). Searches were configured to use variable modification of +57.0214 on Cys (carbamidomethylation) and +15.9949 on Met (oxidation). Search results were assembled using Scaffold 3.5.1 (Proteome Software), where threshold filtering criteria applied consisted of 95% peptide probability and 99% protein probability.
Statistical Analysis
Data were expressed as the mean ± S.E.M. Comparisons between two groups were performed by Student’s t-test. Multiple groups were compared by one-way analysis of variance (ANOVA) with pair wise comparisons, and ANOVA followed by Bonferroni’s correction was used for multiplicity adjustment. Values of P< 0.05 were considered statistically significant.
Results
General Characteristics
Blood glucose levels were observed to be significantly elevated in the DM and DM + MI groups of rats compared with the non-diabetic animal groups (Table 1). Animals in the DM and DM + MI groups exhibited decreased body weight gain as compared to their non-diabetic counterparts (Table 1). Heart weight was found to be increased in the MI and DM + MI groups (Table 1). No significant differences in the weight of myocardial scar caused by the infarction were observed between MI and DM + MI groups of rats (Table 1). The wet to dry weight ratio of the lungs were observed to be elevated only in the DM + MI animals (Table 1), suggesting that severe HF was present only in this group. Mortality within the 4 weeks post-MI period was higher among the DM + MI group compared to the MI group (47% vs. 33%).
Table 1.
General Characteristics of Experimental Rats at 4 Weeks After MI
| Parameter | CV | DM | Sham MI | MI | DM + MI |
|---|---|---|---|---|---|
| Body Weight, g | 365±10.6 | 225±11.6* | 356±7.0 | 376±9.4# | 227±7.7*†‡ |
| Blood Glucose, mg/dl | 152±6.1 | 547±20.2* | 142±7.3 | 163±5.2# | 549±13.9*†‡ |
| Total Heart Weight, g | 1.2±0.03 | 0.9±0.08* | 1.3±0.03 | 1.5±0.05†# | 1.3±0.06#‡ |
| Scar Weight, g | ND | ND | ND | 0.20±0.03 | 0.19±0.02 |
| Lung Wet/Dry | 4.8±0.03 | 4.8±0.08 | 5.1±0.11 | 5.0±0.09 | 5.3±0.08*#‡ |
| Liver Wet/Dry | 3.3±0.12 | 3.0±0.11 | 3.2±0.05 | 3.1±0.12 | 3.3±0.04 |
Values are mean ± standard error of the mean of four to six animals. ND: not detectable. CV, control vehicle; DM, diabetes mellitus; Sham MI, sham myocardial infarction, MI, myocardial infarction; DM + MI, diabetes mellitus + myocardial infarction.
P<0.05 vs. CV;
P<0.05 vs. Sham MI;
P<0.05 vs. DM;
P<0.05 vs. MI.
Echocardiography
Post-Infarction HF is Worsened by the Presence of Type 1 DM
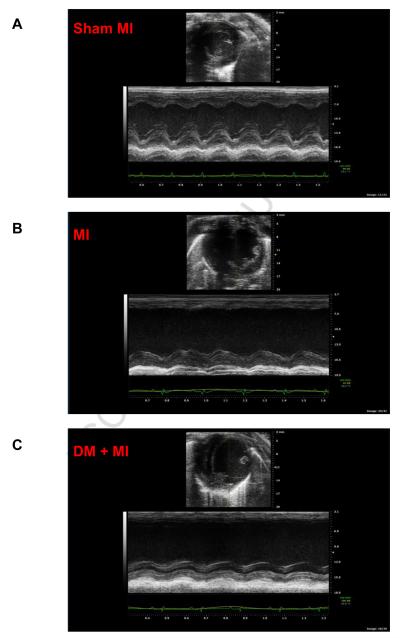
Echocardiographic studies revealed differences in residual LV function between MI and DM + MI groups of rats. Representative echocardiograms obtained from a sham MI, MI and DM + MI rat are shown in Fig. 1. Echocardiographic data are summarized in Table 2. LVFS and LVEF were significantly lower in the DM + MI group compared to the MI group, indicating an increased functional severity of HF in the diabetic post-MI group. Although a statistically significant reduction in LVEF and LVFS was also observed in the DM group without MI, the magnitudes of such decreases were significantly less as compared to the DM + MI group. Heart rate was significantly lower in the DM and DM + MI groups (Table 2).
Figure 1.
Representative echocardiographic images consisting of 2-dimensional echocardiography at parasternal short-axis plane (top) and M-mode short axis of LV (bottom) of type 1 diabetic and non-diabetic rat hearts at 4 weeks post-MI. (A): Sham MI; (B): MI; (C): DM + MI.
Table 2.
LV Echocardiographic Parameters in Experimental Rats at 4 Weeks After MI
| Parameter | CV | DM | Sham MI | MI | DM + MI |
|---|---|---|---|---|---|
| LVFS, % | 43.4±1.9 | 34.3±1.1* | 40.4±1.0 | 25.2±2.2†# | 17.9±0.7*†#‡ |
| LVEF, % | 73.3±2.3 | 61.5±1.5* | 69.5±1.2 | 47.5±3.5†# | 35.5±1.4*†#‡ |
| LVIDd, mm | 6.3±0.22 | 7.7±0.20* | 6.6±0.24 | 9.3±0.55†# | 9.2±0.67*† |
| LVIDs, mm | 3.6±0.23 | 5.1±0.17* | 4.2±0.19 | 7.0±0.55†# | 7.8±0.37*†# |
| Heart Rate, bpm | 350±23.5 | 241±9.0* | 376±16.5 | 361±11.1# | 299±9.3†#‡ |
LVFS, left ventricular fractional shortening; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal dimension, diastolic; LVIDs, left ventricular internal imension, systolic. Values are mean ± standard error of the mean of four to six animals.
P<0.05 vs. CV;
P<0.05 vs. Sham MI;
P<0.05 vs. DM;
P<0.05 vs. MI.
Type 1 DM Does Not Exacerbate LV Remodeling After MI
As shown in Table 2, MI and DM + MI groups of rats had a significantly larger LV chamber size as compared to the sham MI group as indicated by significant increases in LVIDd and LVIDs. However, no differences in the extent of the increases in LVIDd and LVIDs were observed between the MI and DM + MI groups. Although an increase in LVIDd and LVIDs was observed in DM rats in the absence of MI, the extent of such increases were significantly less pronounced compared with the DM + MI group of rats.
Cardiac Tissue Formation of Oxidatively-Induced Carbonylated Proteins is Augmented During Post-MI HF Associated With Type 1 DM
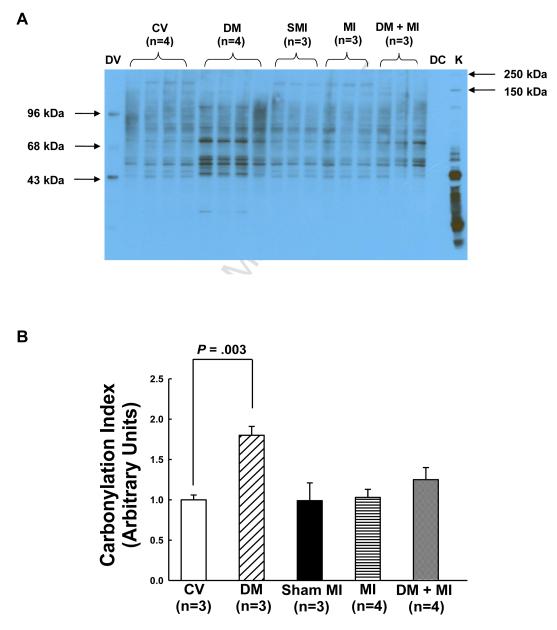
Although not statistically different from the non-diabetic MI group, LV myocardial tissue levels of carbonylated proteins were increased by 20% in the DM + MI group compared to the non-diabetic MI group (Fig. 2). Interestingly, in the DM group without MI, a nearly 2-fold increase (P<0.05) in myocardial carbonylated protein levels was observed relative to the control vehicle group (Fig. 2). No changes in myocardial protein carbonylation was observed in the MI group compared to the sham MI group (Fig. 2).
Figure 2.
Levels of carbonylated proteins in the surviving LV myocardial tissue of MI and DM + MI groups of rats at 4 weeks post-MI. (A): Representative OxyBlot. DV, derivatized molecular weight markers; DC, derivatization-control (negative control); K, Kaleidoscope protein standard markers. (B): Densitometric analysis of DNP-immunoreactivity for CV, DM, sham MI, MI and DM + MI groups of rats. In all samples, the densitometric measurements of DNP-immunoreactivity were normalized to total protein. OxyBlots were performed in duplicate.
Oxidatively-Induced Carbonylated Protein Formation is Increased in Isolated Heart Mitochondria From Failing Type 1 Diabetic Post-Infarction Hearts
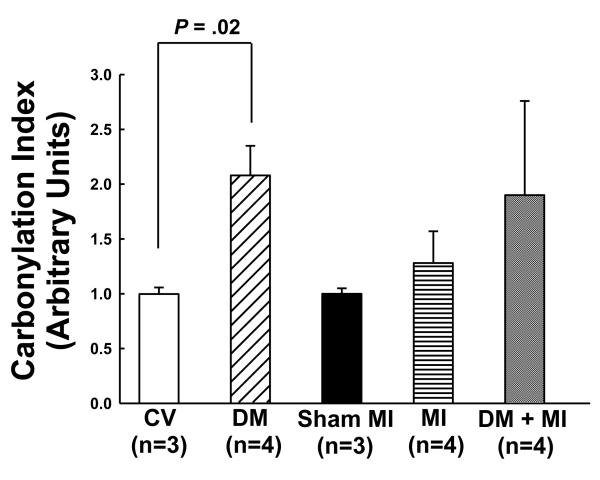
Protein carbonyl content in isolated heart mitochondria from the DM + MI group was 48% higher versus the non-diabetic MI group (Fig. 3). Although this increase did not reach statistical significance, the increase in protein carbonylation in heart mitochondria from the DM + MI group was larger in magnitude compared to that observed in the cardiac tissue of this same group. A statistically significant increase in heart mitochondrial carbonylated protein levels was observed in the DM group without MI (Fig. 3).
Figure 3.
Levels of carbonylated proteins in mitochondria isolated from the surviving LV myocardial tissue of MI and DM + MI groups of rats at 4 weeks post-MI. Densitometric analysis of DNP-immunoreactivity is shown for CV, DM, sham MI, MI and DM + MI groups of rats. In all samples, the densitometric measurements of DNP-immunoreactivity were normalized to total protein. OxyBlots were performed in duplicate.
Identification of Myocardial Carbonylated Protein Candidates in Diabetic Post-MI Hearts With Advanced HF
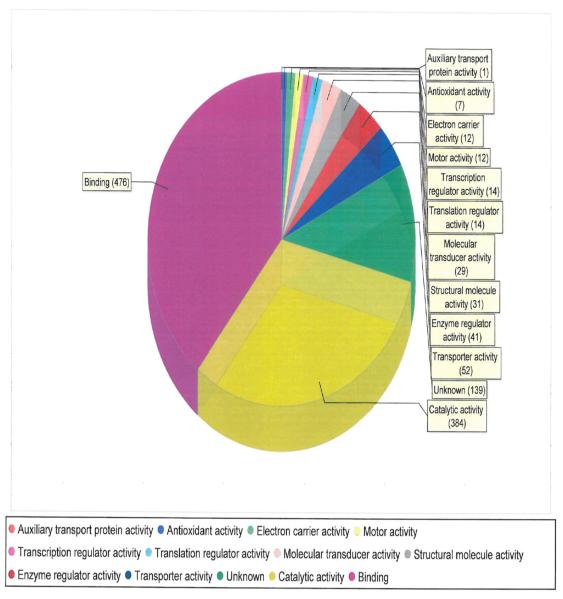
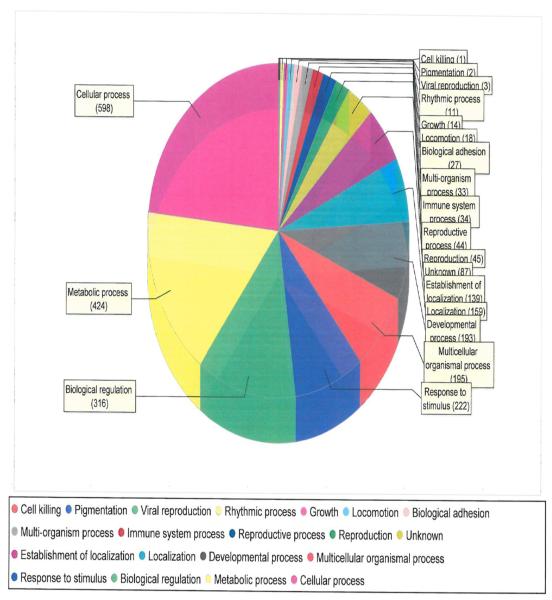
We used MS to characterize myocardial tissue protein fractions from diabetic post-MI hearts with advanced HF that via OxyBlot contained increased carbonylated proteins. The myocardial proteins identified were classified according to known molecular function (Fig. 4) as well as by the biological processes that they mediate (Fig. 5). Particularly relevant to our biology was the fact that there were 7 anti-oxidants that were identified as being among the increased carbonylated proteins that were readily detected via OxyBlot analysis (Fig. 4 and Table 3). The most prominent involved the peroxiredoxin system, in particular peroxiredoxins 1, 2, 3, and 6 (Table 3). Proteins linked to metabolic processes, molecular transport, and binding were also identified as being among the increased carbonylated proteins that were readily detected via OxyBlot analysis (Fig. 4 and Fig. 5). A large majority of the metabolic proteins identified were involved in fatty acid metabolism, including acyl coenzyme A thioester hydrolase, acetyl-CoA acetyl transferase, acyl CoA dehydrogenase, 3-ketoacyl-CoA thiolase, enoyl-CoA hydratase, and heart-type fatty acid-binding protein (H-FABP).
Figure 4.
Molecular functions of the proteins found to be more highly carbonylated in the failing type 1 diabetic post-MI rat myocardium.
Figure 5.
Biological processes that are carried out by the proteins found to be more highly carbonylated in the failing type 1 diabetic post-MI rat myocardium.
Table 3.
Carbonylated Anti-Oxidant Proteins in the Failing Diabetic Post-MI Heart
| Identified Protein Name | Accession No. | MW (kDa) | Sequence Coverage (%) |
No. of Spectra |
|---|---|---|---|---|
| Glutathione Peroxidase-1 | P04041 | 22 | 36 | 15 |
| Glutathione-S-Transferase | P08010 | 26 | 39 | 26 |
| Superoxide Dismutase-2 | P07895 | 25 | 23 | 15 |
| Peroxiredoxin-1 | Q63716 | 22 | 48 | 20 |
| Peroxiredoxin-2 | P35704 | 22 | 19 | 11 |
| Peroxiredoxin-3 | Q9Z0V6 | 28 | 25 | 14 |
| Peroxiredoxin-6 | O35244 | 25 | 36 | 19 |
Proteins were identified by LC-MS/MS as described in the Methods.
Discussion
The findings of this study reveal that the levels of carbonyl proteins are higher in both the cardiac tissue and heart mitochondria of type 1 diabetic post-MI hearts compared to non-diabetic post-MI hearts concomitant with an increased severity of HF. Additionally, our parallel MS analysis identified several cardiac proteins as candidates for increased carbonylation in the failing type 1 diabetic infarcted heart, most notably anti-oxidant enzymes. These results highlight the potential role of myocardial protein carbonylation in the increased incidence of HF after MI associated with type 1 diabetes.
While it has been reported that after MI, type 1 DM is associated with enhanced OS [11], studies aimed at investigating the underlying mechanisms by which type 1 diabetes-induced OS adversely affects HF following MI remain elusive. The demonstration in the present study that the level of total protein carbonylation is higher in both the cardiac tissue and heart mitochondria of failing type 1 diabetic post-MI rat hearts identifies a potential molecular mechanism by which enhanced OS in the type 1 diabetic post-infarction heart potentiates subsequent HF. Since carbonylation is an irreversible post-translational modification that damages proteins [12-14], higher carbonyl content of cardiac proteins is likely to result in a reduction in the healthy myocardial protein pool, thereby negatively impacting myocyte survival and function. Carbonyls, however, are not an index of all oxidative modifications of proteins [12, 13]. Thus, we cannot exclude the possibility that other types of oxidative modifications may occur in the setting of diabetic post-MI HF.
To our surprise, the levels of carbonylated proteins in both the cardiac tissue and heart mitochondria of DM + MI hearts were not observed to be higher than in DM hearts without MI. This was not expected as myocardial levels of OS have been shown to be increased to a greater extent in diabetic hearts with concurrent MI [11]. Prior reports in the literature have documented an increase in proteolysis of oxidized and glycated proteins with increasing OS which may reflect severe tissue damage [16]. Thus, the absence of a further increase in myocardial carbonylated proteins observed in the DM + MI group compared to the DM group alone may be a result of enhanced degradation of carbonylated proteins owing to the more severe OS in this group.
Among the cardiac proteins identified in diabetic post-MI myocardial tissue lysates containing increased protein carbonylation were proteins involved in the anti-oxidant response. The peroxiredoxin system, in particular peroxiredoxins 1, 2, 3, and 6, accounted for the vast majority of anti-oxidant proteins identified as being affected by carbonylation. Peroxiredoxins are a family of thiol-specific antioxidant enzymes that catalyze the reduction of peroxides. Oxidation of peroxiredoxins has been demonstrated to result in an accumulation of inactive forms of peroxiredoxin [17]. Their potential inactivation through carbonylation could reduce the ability of surviving myocytes in diabetic infarcted hearts to cope with OS, resulting in amplified myocardial protein carbonylation and an attendant acceleration of the post-infarction progression of HF as observed in this study.
Although the present study was not designed to specifically study post-MI remodeling processes, our echocardiographic results showed that DM is not associated with greater LV enlargement after MI. This observation is consistent with the findings obtained in patients enrolled in the Survival and Ventricular Enlargement (SAVE) echocardiographic substudy which demonstrated that after MI, diabetes is not associated with greater ventricular enlargement despite an increased incidence of HF [5]. However, our results are contrary to previously published findings in animals which have reported that DM exacerbates LV enlargement and remodeling after MI [18-20]. The divergent results between the present study and the aforementioned studies [18-20] may be secondary to differences in the species examined (mice vs. rats), the dynamicity of post-MI periods evaluated, and the severity and duration of diabetes. While beyond the scope of this study, further research is needed to clarify the propensity of the diabetic post-MI heart for LV remodeling and its contribution to the greater degree of HF in conjunction with MI in the setting of DM [6].
The significantly lower HR observed in the STZ-diabetic groups of rats is consistent with our previous observations [21, 22] as well as prior reports in the literature [23-27] and may be due to cardiac autonomic neuropathy [23, 24]. Although reductions in heart rate are routinely associated with cardiac depression, studies undertaking functional comparison at comparable heart rates via pacing in STZ-diabetic rats have reported similar alterations in myocardial contractility despite correcting for heart rate [28, 29]. Therefore, the LV systolic dysfunction observed in the DM and DM + MI groups cannot merely be the result of the much reduced heart rate and instead points to greater impairment of myocardial contractile function.
The tendency of clinical trials to focus on cardiovascular disease in type 2 DM (as opposed to type 1 DM) in spite of the fact that the age-adjusted relative risk for cardiovascular disease in type 1 DM far exceeds that of type 2 DM has resulted in an under-appreciated link between type 1 DM and cardiac disease [30]. As such, in the present study, we aimed to bridge this gap by employing un-treated STZ-induced diabetes, a well-established model of type 1 DM that closely recapitulates uncontrolled hyperglycemia due to absolute insulin deficiency. While possible confounding effects of STZ administration have been suggested to occur acutely, the half-life of the drug is ~15 minutes, and it is cleared from the organs within 4 hours [31]. Given that the data reported in the present study was obtained at 6 weeks after STZ injection, the results reported herein are likely related to the development of diabetes. Furthermore, the reversal of accompanying mechanical changes in cardiac function induced by STZ with insulin [32, 33] renders the possibility that the abnormalities observed among the diabetic animals in the present study being due to drug toxicity, rather than the diabetic condition itself, extremely unlikely.
Study Limitations-Although our echocardiographic results showed that DM is not associated with greater LV enlargement after MI, we cannot exclude the possibility that ventricular enlargement could have occurred as a pre-terminal event in the diabetic post-MI rats that died before the sacrifice, especially since we did not measure left ventricular chamber size in diabetic post-MI rats prior to the time of sacrifice. Moreover, it is very likely that the animals that lasted up to the sacrifice were not in a very end-stage HF. In addition, while we did not find any difference in terms of overall carbonylated protein levels between diabetic animals with or without MI, our measurement of carbonylated protein levels exclusively in the remote non-infarcted myocardium of diabetic post-MI rats precluded us from detecting any regional differences in carbonylated protein levels that may have existed in diabetic hearts with MI, such as between the regions bordering on and distant from the myocardial scar. Further studies are needed to answer these crucial questions.
Conclusions
The results from this study indicate that carbonylation of cardiac tissue and heart mitochondrial proteins is increased to a greater extent in the type 1 diabetic post-MI heart compared to the non-diabetic post-MI heart which may serve as a mechanistic link between amplified OS and exacerbation of post-infarction HF in the setting of diabetes.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute grant R01 HL089385 (to M.F. Hill).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mukamal KJ, Nesto RW, Cohen MC, Muller JE, Maclure M, Sherwood JB, Mittleman MA. Impact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarction. Diabetes Care. 2001;24:1422–1427. doi: 10.2337/diacare.24.8.1422. [DOI] [PubMed] [Google Scholar]
- 2.Woodfield SL, Lundergan CF, Reiner JS, Greenhouse SW, Thompson MA, Rohrbeck SC, Deychak Y, Simoons ML, Califf RM, Topol EJ, Ross AM. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1996;28:1661–1669. doi: 10.1016/s0735-1097(96)00397-x. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Califf RM, Young S, Candela R, Samaha J, Worley S, Kereiakes DJ, Topol EJ. Outcome of patients with diabetes mellitus and acute myocardial infarction treated with thrombolytic agents. The Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. J Am Coll Cardiol. 1993;21:920–925. doi: 10.1016/0735-1097(93)90348-5. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, St John Sutton M, Lamas GA, Plappert T, Rouleau JL, Skali H, Moye L, Braunwald E, Pfeffer MA. Survival And Ventricular Enlargement (SAVE) Investigators. Ventricular remodeling does not accompany the development of heart failure in diabetic patients after myocardial infarction. Circulation. 2002;106:1251–1255. doi: 10.1161/01.cir.0000032313.82552.e3. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar D, Solomon SD, Køber L, Rouleau JL, Skali H, McMurray JJ, Francis GS, Henis M, O’Connor CM, Diaz R, Belenkov YN, Varshavsky S, Leimberger JD, Velazquez EJ, Califf RM, Pfeffer MA. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation. 2004;110:1572–1578. doi: 10.1161/01.CIR.0000142047.28024.F2. [DOI] [PubMed] [Google Scholar]
- 7.Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, Turi ZG, Strauss HW, Willerson JT, Robertson T, et al. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol. 1989;14:49–57. doi: 10.1016/0735-1097(89)90053-3. [DOI] [PubMed] [Google Scholar]
- 8.Barbash GI, White HD, Modan M, Van de Werf F. Significance of diabetes mellitus in patients with acute myocardial infarction receiving thrombolytic therapy. Investigators of the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. J Am Coll Cardiol. 1993;22:707–713. doi: 10.1016/0735-1097(93)90180-9. [DOI] [PubMed] [Google Scholar]
- 9.Becker RC, Terrin M, Ross R, Knatterud GL, Desvigne-Nickens P, Gore JM, Braunwald E. Comparison of clinical outcomes for women and men after acute myocardial infarction. The Thrombolysis in Myocardial infarction Investigators. Ann Intern Med. 1994;120:638–645. doi: 10.7326/0003-4819-120-8-199404150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Mak KH, Moliterno DJ, Granger CB, Miller DP, White HD, Wilcox RG, Califf RM, Topol EJ. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. GUSTO-IInvestigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:171–179. doi: 10.1016/s0735-1097(97)00118-6. [DOI] [PubMed] [Google Scholar]
- 11.Smith HM, Hamblin M, Hill MF. Greater propensity of diabetic myocardium for oxidative stress after myocardial infarction is associated with the development of heart failure. J Mol Cell Cardiol. 2005;39:657–665. doi: 10.1016/j.yjmcc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Banfi C, Brioschi M, Barcella S, Veglia F, Biglioli P, Tremoli E, Agostoni P. Oxidized proteins in plasma of patients with heart failure: role in endothelial damage. Eur J Heart Fail. 2008;10:244–251. doi: 10.1016/j.ejheart.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Madian AG, Regnier FE. Proteomic identification of carbonylated proteins and their oxidation sites. J Proteome Res. 2010;9:3766–3780. doi: 10.1021/pr1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baraibar MA, Hyzewicz J, Rogowska-Wrzesinska A, Ladouce R, Roepstorff P, Mouly V, Friguet B. Oxidative stress-induced proteome alterations target different cellular pathways in human myoblasts. Free Radic Biol Med. 2011;51:1522–1532. doi: 10.1016/j.freeradbiomed.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Hill S, Luther JM, Hachey DL, Schey KL. Proteomic analysis of urine exosomes by multidimensional protein identification technology (MudPIT) Proteomics. 2012;12:329–338. doi: 10.1002/pmic.201100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed N, Babaei-Jadidi R, Howell SK, Beisswenger PJ, Thornalley PJ. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia. 2005;48:1590–1603. doi: 10.1007/s00125-005-1810-7. [DOI] [PubMed] [Google Scholar]
- 17.Rabilloud T, Heller M, Gasnier F, Luche S, Rey C, Aebersold R, Benahmed M, Louisot P, Lunardi J. Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J Biol Chem. 2002;277:19396–19401. doi: 10.1074/jbc.M106585200. [DOI] [PubMed] [Google Scholar]
- 18.Bäcklund T, Palojoki E, Saraste A, Eriksson A, Finckenberg P, Kytö V, Lakkisto P, Mervaala E, Voipio-Pulkki LM, Laine M, Tikkanen I. Sustained cardiomyocyte apoptosis and left ventricular remodelling after myocardial infarction in experimental diabetes. Diabetologia. 2004;47:325–330. doi: 10.1007/s00125-003-1311-5. [DOI] [PubMed] [Google Scholar]
- 19.Shiomi T, Tsutsui H, Ikeuchi M, Matsusaka H, Hayashidani S, Suematsu N, Wen J, Kubota T, Takeshita A. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J Am Coll Cardiol. 2003;42:165–172. doi: 10.1016/s0735-1097(03)00509-6. [DOI] [PubMed] [Google Scholar]
- 20.Song GY, Wu YJ, Yang YJ, Li JJ, Zhang HL, Pei HJ, Zhao ZY, Zeng ZH, Hui RT. The accelerated post-infarction progression of cardiac remodelling is associated with genetic changes in an untreated streptozotocin-induced diabetic rat model. Eur J Heart Fail. 2009;1:911–921. doi: 10.1093/eurjhf/hfp117. [DOI] [PubMed] [Google Scholar]
- 21.Hamblin M, Friedman DB, Hill S, Caprioli RM, Smith HM, Hill MF. Alterations in the diabetic myocardial proteome coupled with increased myocardial oxidative stress underlies diabetic cardiomyopathy. J Mol Cell Cardiol. 2007;42:884–895. doi: 10.1016/j.yjmcc.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamblin M, Smith HM, Hill MF. Dietary supplementation with vitamin E ameliorates cardiac failure in type I diabetic cardiomyopathy by suppressing myocardial generation of 8-iso-prostaglandin F2alpha and oxidized glutathione. J Card Fail. 2007;13:884–892. doi: 10.1016/j.cardfail.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda CY, Fernandes TG, Timm HB, Irigoyen MC. Autonomic dysfunction in short-term experimental diabetes. Hypertension. 1995;26:1100–1104. doi: 10.1161/01.hyp.26.6.1100. [DOI] [PubMed] [Google Scholar]
- 24.Hicks KK, Seifen E, Stimers JR, Kennedy RH. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. J Auton Nerv Syst. 1998;69:21–30. doi: 10.1016/s0165-1838(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto J, Barr RL, Kavanagh KM, Lopaschuk GD. Contribution of malonyl-CoA decarboxylase to the high fatty acid oxidation rates seen in the diabetic heart. Am J Physiol Heart Circ Physiol. 2000;278:H1196–H1204. doi: 10.1152/ajpheart.2000.278.4.H1196. [DOI] [PubMed] [Google Scholar]
- 26.Nagareddy PR, Xia Z, MacLeod KM, McNeill JH. N-acetylcysteine prevents nitrosative stress-associated depression of blood pressure and heart rate in streptozotocin diabetic rats. J Cardiovasc Pharmacol. 2006;47:513–520. doi: 10.1097/01.fjc.0000211744.93701.25. [DOI] [PubMed] [Google Scholar]
- 27.Borges GR, de Oliveira M, Salgado HC, Fazan R., Jr Myocardial performance in conscious streptozotocin diabetic rats. Cardiovasc Diabetol. 2006;5:26. doi: 10.1186/1475-2840-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banyasz T, Kalapos I, Kelemen SZ, Kovacs T. Changes in cardiac contractility in IDDM and NIDDM diabetic rats. Gen Physiol Biophys. 1996;15:357–369. [PubMed] [Google Scholar]
- 29.Banyasz T, Kovacs T. Altered [3H]ouabain binding to cardiac muscle in insulin dependent and non-insulin-dependent diabetic rats. Exp Physiol. 1998;83:65–76. doi: 10.1113/expphysiol.1998.sp004092. [DOI] [PubMed] [Google Scholar]
- 30.Retnakaran R, Zinman B. Type 1 diabetes, hyperglycaemia, and the heart. Lancet. 2008;371:1790–1799. doi: 10.1016/S0140-6736(08)60767-9. [DOI] [PubMed] [Google Scholar]
- 31.Adolphe AB, Glasofer ED, Troetel WM, Weiss AJ, Manthei RW. Preliminary pharmacokinetics of streptozotocin, an antineoplastic antibiotic. J Clin Pharmacol. 1977;17:379–388. doi: 10.1002/j.1552-4604.1977.tb04620.x. [DOI] [PubMed] [Google Scholar]
- 32.Fein FS, Strobeck JE, Malhotra A, Scheuer J, Sonnenblick EH. Reversibility of diabetic cardiomyopathy with insulin in rats. Circ Res. 1981;49:1251–1261. doi: 10.1161/01.res.49.6.1251. [DOI] [PubMed] [Google Scholar]
- 33.Pierce GN, Kutryk MJ, Dhalla NS. Alterations in Ca2+ binding by and composition of the cardiac sarcolemmal membrane in chronic diabetes. Proc Natl Acad Sci U S A. 1983;80:5412–5416. doi: 10.1073/pnas.80.17.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]