Abstract
Chondrosarcoma is notable for its lack of response to conventional cytotoxic chemotherapy, propensity for developing lung metastases, and poor survival. Therefore, a better understanding of angiogenic and metastatic pathways is needed. Multiple pathways regulate angiogenesis and metastasis, including chemokines and their receptors. In this study, we investigated CHEMOKINE (C-X-C MOTIF) RECEPTOR 4 (CXCR4) signaling in chondrosarcoma and tested the hypotheses that CXCR4 inhibition suppresses tumor angiogenesis and metastasis. CXCR4 expression, analyzed by real-time PCR and Western blot, was increased in human chondrosarcoma cell line JJ compared to normal chondrocytes, and was further increased in JJ by hypoxia (2% O2), VASCULAR ENDOTHELIAL GROWTH FACTOR A (VEGFA) (10ng/ml), and in xenograft tumors in nude mice. The CXCR4 ligand CHEMOKINE (C-X-C MOTIF) LIGAND 12 (CXCL12) (10 ng/ml) doubled secreted VEGFA, measured with ELISA, under hypoxic conditions and this conditioned media increased HUVEC tube formation. These effects were inhibited by CXCR4 siRNA or AMD3100 (5 μg/mL). In a xenograft mouse model, four weeks of AMD3100 treatment (1.25 mg/kg, ip, bid) inhibited tumor angiogenesis, tumor growth, and metastasis. VEGFA content in tumor extracts was decreased (7.19 ± 0.52 ng/mL control vs. 3.96 ± 0.66 treatment) and bioimaging of angiogenesis was decreased by 56%. Tumor volumes averaged 4.44 ± 0.68 cm3 in control compared to 2.48 ± 0.61 cm3 in the treatment group. The number of lung metastatic nodules was 23 ± 9 in control compared to 10 ± 6 in the treatment group (N=8/group). Therefore, CXCR4 targeted therapy may be a treatment strategy for chondrosarcoma.
Keywords: bone cancer, cartilage tumor
Introduction
Chondrosarcoma is a devastating disease without effective systemic treatment. It is the second most common primary malignant bone tumor in large epidemiologic series(1). Five-year survival of patients with this cancer is 10% to 25% and there has been no progress over the last several decades (2;3). The consensus is that conventional cytotoxic chemotherapy does not improve survival(4). Patients with chondrosarcoma typically succumb to pulmonary metastases. Angiogenesis is necessary for both tumor growth and metastasis (5;6). Virtually all of the research on tumor vascularity and the factors that stimulate angiogenesis have shown correlations between vascularity, expression of proangiogenic factors, biologic aggressiveness, high pathologic grade, and poor survival(7). We found grade II and III chondrosarcomas have more microvascularity than benign or grade I tumors (8). Microvascularity correlates with clinical behavior, since it is primarily grade II and III chondrosarcomas that metastasize (9). Thus, chondrosarcoma development may be linked to angiogenesis.
The angiogenic switch typically involves hypoxia and oncogenes as activators of expression of the primary proangiogenic factor VASCULAR ENDOTHELIAL GROWTH FACTOR A (VEGFA) (6). We have shown grade II and III chondrosarcoma have higher expression of VEGFA and the major transcription factor related to hypoxia, HYPOXIA-INDUCING FACTOR 1 (HIF1) (10). In addition to VEGFA, MATRIX METALLOPEPTIDASES (MMPs) are factors related to angiogenesis and metastatic behavior and MMP1 expression in chondrosarcoma is inversely correlated with survival(11). Directly blocking expression of these factors in vivo is not yet feasible and our understanding of the regulation of these factors in chondrosarcoma is incomplete.
Pathways related to angiogenesis and metastasis could involve chemokines and their receptors. There are four groups of chemokine receptors: C, CC, CXC, and CX3C. Under normal circumstances they are important in immune cell function and migration of stem cells to sites of injury; however, in cancer, they regulate invasion, angiogenesis, migration, and metastasis. CHEMOKINE (C-X-C MOTIF) RECEPTOR 4 (CXCR4) is the chemokine receptor most commonly expressed in tumors(12). The ligand for CXCR4 is CHEMOKINE (C-X-C MOTIF) LIGAND 12 (CXCL12) (13;14). CXCR4 and CXCL12 together promote metastasis in some tumors by mediating proliferation and migration of tumor cells and enhancing tumor-associated angiogenesis (15–17). CXCR4 is a transmembrane G-protein-coupled receptor, whose activation also leads to intracellular signaling cascades, downstream targets of which include MMP1 and VEGFA (17;18). The increased expression of chemokine receptors has been mostly investigated in carcinomas (19–22). We (23) and others (24) have found CXCR4 is overexpressed in chondrosarcoma cells and primary chondrosarcoma tissue, and we have also shown that one mechanism of increased CXCR4 expression is hypoxia, specifically HIF1(23). VEGFA has also been shown to upregulate CXCR4 expression in endothelial cells, and in some, but not all tumor cells (25;26). Development of agents that block CXCR4 has been propelled by the fact that it is a coreceptor for HIV. The drug AMD3100 is a bicyclam (1,1′-[1,4-Phenylenebis-(methylene)]-bis-(1,4,8,11-tetraazacyclotetradecane) octahydrochloride) (27) with high specificity for CXCR4 and is the prototypical CXCR4-blocking drug (28). It is already approved for human use as a stem cell mobilizer. In chondrosarcoma cells, CXCR4 blockade with AMD3100 inhibits expression of MMP1 and invasion in vitro (23). CXCR4, therefore, is an attractive target since it is the most common chemokine receptor expressed in cancer cells, upregulates factors related to invasiveness and angiogenesis in chondrosarcoma, and agents exist that can block this receptor. As an orphan disease, it is unlikely that novel agents will be developed for chondrosarcoma and repurposing the use of drugs approved for one use to the treatment of orphan diseases is an NIH priority (29). Thus, it is important to identify pathways and agents that may have applicability for chondrosarcoma treatment. In this study, we (1) investigated another potential mechanism of CXCR4 overexpression in chondrosarcoma involving the mutual regulation of VEGFA and CXCR4 by each other, (2) tested the hypothesis that CXCR4 inhibition decreases VEGFA expression and angiogenesis in vitro and in vivo, and (3) tested the hypothesis that CXCR4 inhibition decreases metastasis.
Material and Methods
Chondrosarcoma cell line
The human chondrosarcoma cell line JJ, derived from a human Grade II chondrosarcoma (a gift from Dr. Joel Block, Rush Medical School, Chicago, IL, in 1999), was cultured in complete medium (40% Dulbecco’s Modified Eagle’s Medium, 40% Minimal Essential Medium, 20% F12) with 10% fetal bovine serum (30), in a humidified incubator under 5% CO2 and ambient oxygen (20%) or hypoxia (2%) (31). CXCL12, VEGFA (R&D Systems, Minneapolis, MN), and/or AMD3100 (Sigma-Aldrich Co, St Louis, MO) were added to the medium as indicated. The cell line was authenticated using short tandem repeat (STR) profiling and was performed (ATCC, Manassas, VA) on the source cell line in 1999, 2007, and repeated in 2012. There is 94% similarity between the different time points, the cells are human, and there are no matches with any cell lines in the ATCC data base. Normal human chondrocytes were isolated from articular cartilage obtained from tumor resections.
siRNA
CXCR4 knockdown with siRNA was performed by transfecting JJ cells with CXCR4 or control siRNA (Qiagen, Valencia, CA, USA) using the HiPerFect® transfection reagent (Qiagen).
Quantitative real-time PCR analysis for mRNAs
For quantification of mRNA, total RNA was isolated from chondrocytes, JJ cells, and xenograft mouse tumors using the RNAqueous® Kit (Ambion, Austin, TX, USA). SYBR real-time PCR was carried out using two-step real-time qRT-PCR (Qiagen) with normalization to 18S rRNA (18S). The primers used for VEGFA, CXCR4, and 18S were previously reported (23;31). 18S was used as an internal control since it is reportedly the optimal reference gene (32). The comparative threshold cycle (Ct) method, i.e., the 2−ΔΔCt method, was used for the calculation of fold amplification (33). Each experiment was evaluated with three PCR reactions and each experiment was repeated three times, the sample size necessary to maintain power at 0.80 to detect a 50% decrease with an alpha of 0.025 (one-tailed t-test).
Western Blot
Cell lysates containing forty μg of protein were separated via SDS-PAGE (Bio-Rad, Hercules, CA). Western Blot analyses were performed as previously described with CXCR4 antibody (IMGNEX, San Diego, CA) and actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA)(34). Protein concentrations were determined using the Quick Start Bradford protein assay (Bio-Rad).
ELISA
Conditioned medium was obtained 72 hours after transfection or AMD3100 treatment. Soluble VEGF-165 was detected using VEGF Immunoassay kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions (34). VEGF-165 levels were measured two times for each condition and with normalization to the number of cells at the end of the culture period. Each experiment was repeated three times. Twenty mg of xenograft tumor tissue in RIPA buffer containing proteinase inhibitors (Roche) was homogenized on ice using TissueRuptor (Qiagen). Tissue lysates were centrifuge at 14000 rpm for 30 min, supernatant saved at −80°C for later use. VEGF-165 levels in xenograft tumor lysates were normalized to total protein.
HUVEC Angiogenesis Assay
Human umbilical vein endothelial cell (HUVEC) (ATCC, Manassas, VA) tube formation in three-dimensional BD Matrigel™ matrix (BD Biosciences, San Jose, CA) was determined after culture with conditioned medium from JJ cells transfected with control or anti-CXCR4 siRNA or treated with AMD3100. Tube formation was quantified by seeding HUVECs on BD Matrigel™ cultures at 5 × 104 cells/well, 96 well plates and culturing for one day after addition of conditioned medium. The entire well of HUVECs were photographed, the tubular structures traced, and total length/well measured using Elements software (Nikon, Inc, Melville, NY).
Mouse model, bioimaging, tumor growth, metastasis analysis
JJ cells (100 μl of 1 × 106 cells) were mixed with 300 μL Matrigel™ (BD Biosciences, San Jose, CA) and injected subcutaneously in the back of eight nude mice per group (nu/nu 6–8 week old female, Charles River Laboratory, Wilmington, MA). Treatment with AMD3100 (1.25 mg/kg) or control (phosphate-buffered saline) twice daily intraperitoneally 5 days/week was started on Day 14. All animal studies were approved by the Institutional Animal Care and Use Committee at Rhode Island Hospital and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In vivo bioimaging of angiogenesis in xenograft tumors was performed with fluorescence molecular tomography (FMT) (PerkinElmer, Waltham, MA) using AngioSense® 750 (PerkinElmer), a probe with an excitation wavelength of 750 nm and an emission wavelength of 780 nm at the start and after 3 weeks of treatment. Fluorochrome concentration in the target was calculated from reconstructed images and expressed as femtomoles of fluorochrome per defined target volume (the primary tumor). Tumors were measured with calipers and volumes were calculated with the formula for a scalene ellipsoid: height × width × length × 0.52. Weight was determined after 4 weeks of treatment at the time of excision. Lungs were analyzed with microscopy after fixation in 10% formalin. Transverse sections were be made at 100μm intervals. H&E stained slides were scanned with an Aperio Scanscope (Aperio Technologies, Inc.). Metastatic burden was quantified as the number of nodules per lung (35).
Immunohistochemistry
Tissue sections were deparaffinized and blocked with 3% serum and then stained with anti-CD31 antibody (1:100 dilution) (SC-56; Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) using Vectastain® ABC kit (VECTOR Laboratories, Burlingame, CA, USA). Antibody staining was visualized with peroxidase-conjugated anti-mouse antibody and counterstained with hematoxylin. A negative control was performed on each tumor tissue stained with mouse IgG. Immunostained slides were photographed with a Spot RT™ camera (Diagnostic Imaging, Sterling Heights, MI, USA) and Nikon E800 microscope at 200×.
Statistical analysis
Gene expression in multiple groups in vitro were compared using one-way ANOVA, followed by Bonferroni’s multiple comparison test. We determined differences in gene and protein expression in vivo, bioimaging, and tumor weight and volume between control and AMD3100 or CXCR4siRNA groups using Student’s t-test. Lung metastatic burden was compared using Mann-Whitney U test. (GraphPadPrism, v 5.03, GraphPad Software, Inc., San Diego, CA).
Results
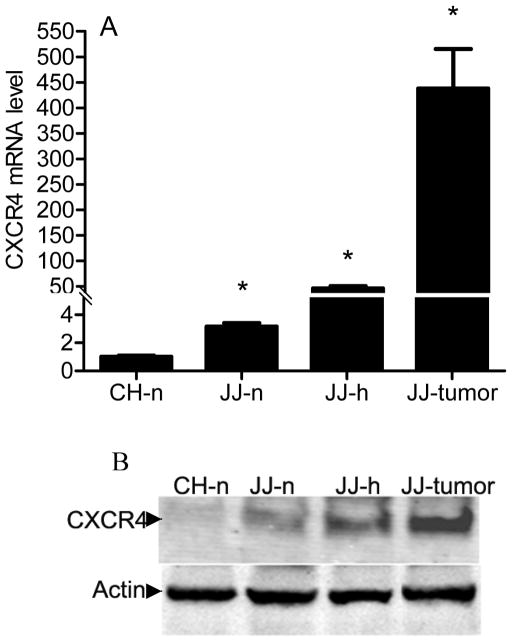
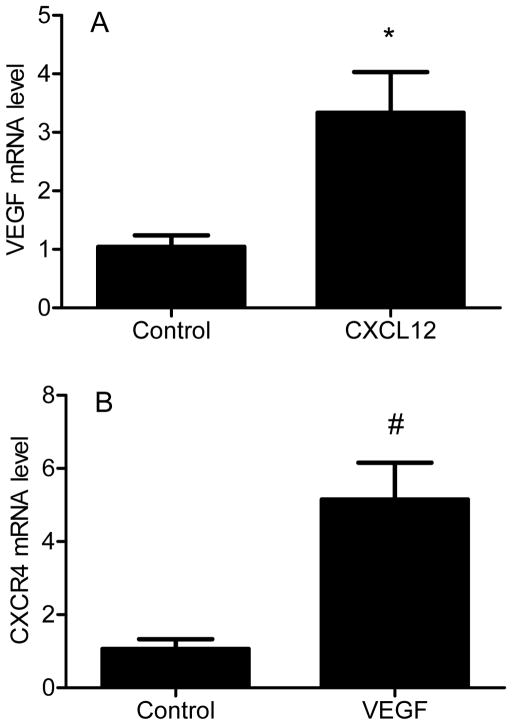
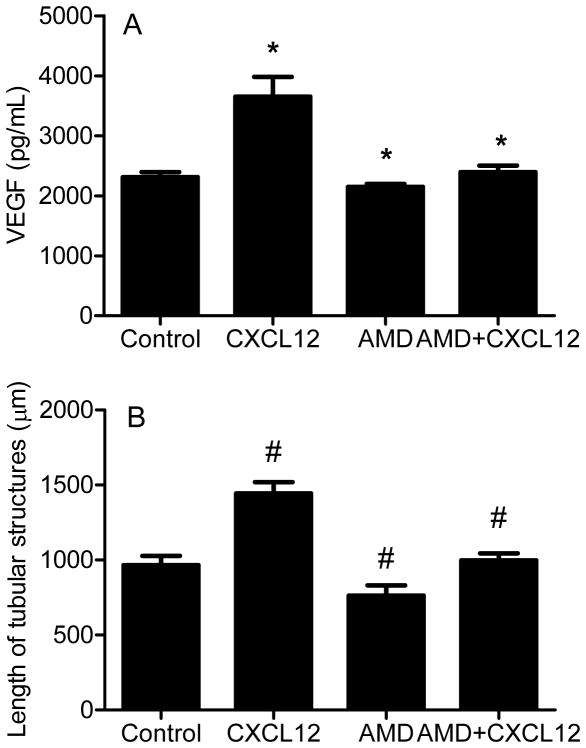
While it is known that CXCR4 is upregulated in primary human chondrosarcoma, we evaluated CXCR4 expression in xenograft tumors to confirm the model could be used to study CXCR4 inhibition. CXCR4 was expressed at higher levels in JJ cells compared to normal chondrocytes, and expression was further increased (p < 0.001) by hypoxia 46-fold and in xenograft tumors 438-fold, both relative to chondrocytes. Western blot confirmed the results (Fig. 1), suggesting CXCR4 may be a relevant treatment target. JJ cells were treated with CXCL12 or VEGFA to evaluate if CXCR4 signaling regulates expression of VEGFA, if VEGFA regulates CXCR4, and if CXCR4 blockade would inhibit angiogenesis in vitro. CXCL12 during normoxia increased VEGFA mRNA threefold (p < 0.03) (Fig. 2A). Similarly, CXCR4 mRNA increased fivefold (p < 0.02) after cells were treated with VEGFA (Fig. 2B). CXCL12 treatment during hypoxia increased VEGFA protein concentration in conditioned media (Figure 3A), HUVEC tube formation (Figure 3b), and the effects were attenuated by AMD3100.
Figure 1. CXCR4 expression is increased in xenograft chondrosarcoma tumors.
CXCR4 expression was evaluated with real-time PCR with normalization to 18S (A) and Western blot (B) as described in Materials and Methods. CH: chondrocytes, JJ: chondrosarcoma cell line, n: normoxia; h: hypoxia (2% O2); JJ-tumor: xenograft tumor. Levels of mRNA are shown as mean ± SD for 3 replicate determinations, * p < 0.001 compared to CH-n.
Figure 2. Effects of CXCL12 on VEGFA expression and VEGFA on CXCR4 expression.
VEGFA (A) and CXCR4 (B) mRNA were quantified with real-time PCR in JJ cells cultured in normoxia after exposure to CXCL12 (10 ng/mL) or VEGFA (10 ng/mL) respectively. Levels of mRNA are shown as mean ± SD for 3 replicate determinations, *, p<0.03; #, p<0.02.
Figure 3. CXCR4 blockade decreases VEGFA expression and in vitro angiogenesis.
JJ cells were cultured in hypoxia with CXCL12 (10 ng/mL), AMD3100 (5 μg/mL), or both. (A) VEGFA in media was measured with ELISA. Data are shown as mean ± SD for three replicates. (B) HUVEC were cultured with conditioned media from JJ cells and total length of tubular structures measured after twenty-four hrs. Data are shown as mean ± SD. *, # p<0.001, CXCL12 compared to control, AMD3100 or AMD3100 plus CXCL12 compared to CXCL12 alone.
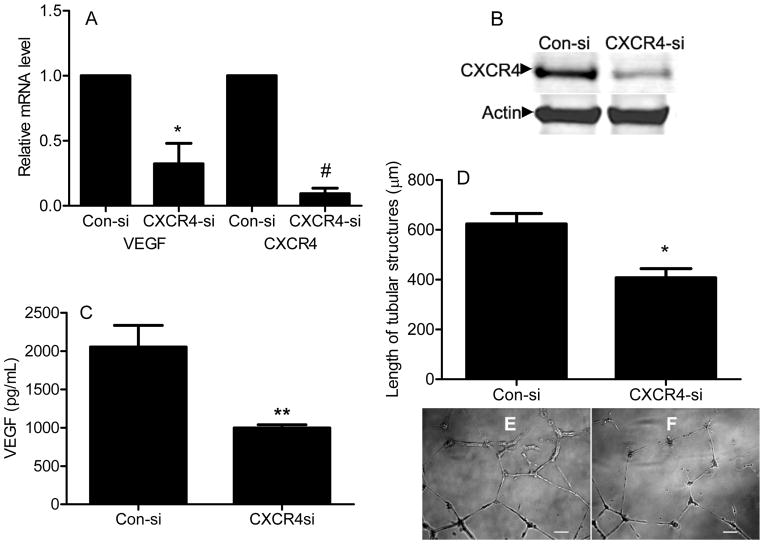
The effect of CXCR4 siRNA on VEGFA mRNA and protein expression and HUVEC tube formation were similar to treatment with AMD3100, suggesting the effects of AMD3100 result from inhibition of CXCR4 signaling. CXCR4 siRNA efficiently knocked down CXCR4 expression (90% knockdown of mRNA) (Fig 4A, B), decreased VEGFA mRNA by 68% (Fig. 4A, p < 0.01), and VEGFA protein in conditioned medium by 51% (Fig. 4C, p < 0.02). Conditioned medium from JJ cells cultured in hypoxia after CXCR4 knockdown had 35% less effect on formation of HUVEC tube length (Fig. 4D, p < 0.01).
Figure 4. CXCR4 knockdown reduces VEGFA expression and in vitro angiogenesis.
JJ cells were cultured in hypoxia after transfection with CXCR4 siRNA or control siRNA. (A)VEGFA and CXCR4 mRNA were quantified relative to 18S with real-time PCR as described in Material and Methods. (B) CXCR4 was evaluated with Western blot and VEGFA in conditioned media with ELISA (C). (D) HUVEC were cultured with conditioned media from JJ cells and length of tubular structures measured after twenty-four hrs. (E, F) Representative bright field images are shown. Data are shown as mean ± SD for three replicates (A,C) and four replicates (D). * < 0.01, # p < 0.0001; ** p < 0.02. Scale bar, 100 μm. Con-si: control siRNA, CXCR4-si: CXCR4 siRNA.
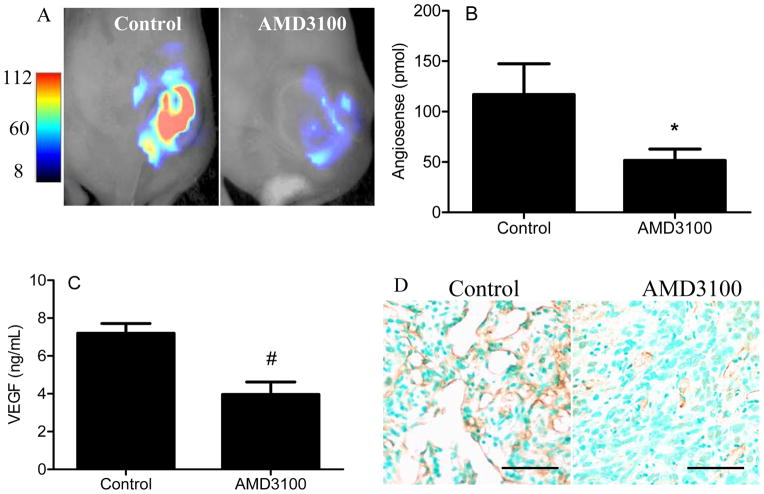
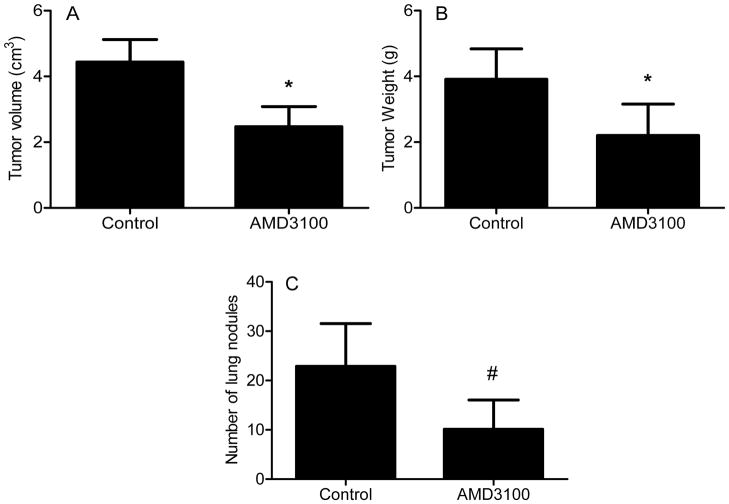
In order to determine if AMD3100 could be used as an antiangiogenic and antimetastatic agent in vivo, mice bearing xenograft tumors were treated with AMD3100. Bioimaging of xenograft tumors demonstrated AMD3100 decreased angiogenesis as measured by tumor content of AngioSense® probe. Probe content in treated tumors was decreased 56% compared to control (p < 0.03) (Fig. 5A–B). VEGFA content in treated tumors was decreased 45% of control (p < 0.002) (Fig. 5C). Representative immunohistochemical staining shows less CD31-positive microvessels within treated tumors (Fig. 5D). Final tumor weight and volume were decreased by 44% and 45% respectively (Fig. 6A,B) (p< 0.05). Evaluation of lungs demonstrated that AMD3100 treatment decreased the number of lung nodules by 56% (Fig. 6C) (p < 0.04).
Figure 5. AMD3100 decreases angiogenesis in xenograft tumors.
(A) Representative Fluorescence-based Quantitative Tomography images performed with AngioSense probe are shown. (B) AngioSense content in xenograft tumors was measured after three weeks of treatment with AMD3100, N = 8/group. * p< 0.03. (C) VEGFA expression in xenograft tumor lysates was quantified with ELISA after four weeks of treatment, N = 8/group. *, p < 0.002. (D) Representative immunohistochemical analysis for CD31 positive microvessels in tumors are shown. 200×, bar = 100μm.
Figure 6. AMD3100 decreases tumor weight, volume, and metastasis.
Tumor volume (A), tumor weight (B), and number of lung nodules are shown after four weeks of treatment. N=8/group, *, p<0.05. #, p<0.04, Mann-Whitney U test, one-tail.
Discussion
Chondrosarcoma is notable for its lack of response to systemic treatment and poor survival. Chemokines and their receptors regulate invasion, angiogenesis, migration, and metastasis. CXCR4 is the most common chemokine receptor expressed in cancer cells and upregulates factors related to invasiveness and angiogenesis. We therefore determined whether CXCR4 signaling contributes to VEGFA expression in chondrosarcoma and whether blocking CXCR4 would inhibit angiogenesis and metastasis in this tumor.
Regulation of VEGFA expression in chondrosarcoma, as in other tumors, involves a physiologic response to hypoxia and genetic aberrations found in tumors, with some overlap. VEGFA is directly upregulated by the transcription factor HIF1, which is increased during hypoxia as a result of decreased degradation (31). Here we show VEGFA is also upregulated by CXCR4 signaling in a positive feedback loop. The mechanisms of CXCR4 signaling include the MAP kinases ERK, JNK, and p38(36), which are known to upregulate VEGFA promoter activity (37). CXCR4 has been previously reported to be upregulated by VEGFA in normal endothelial cells and in breast cancer (26), however this is the first report of such upregulation in chondrosarcoma. CXCR4 is also upregulated by hypoxia via HIF1 (23). Therefore, there are multiple levels of regulation and amplification of VEGFA and CXCR4 expression. VEGFA expression is directly regulated by HIF1, which binds to the VEGFA promoter, and indirectly by CXCR4 signaling, which itself is increased by HIF1, VEGFA, and other factors such as PEA3, PAUF, PAX3-FKHR, nuclear respiratory factor 1, and estrogen (23;38;39). VEGFA receptors also signal through the MAP kinase and phosphatidylinositol 3-kinase pathways, which in turn upregulate CXCR4 (40). Our results are consistent with investigations of transcriptional regulation of CXCR4 and VEGFA in other systems and emphasize the notion that any one factor can be regulated by multiple pathways. From a therapeutic standpoint, our data show inhibiting CXCR4 signaling can decrease VEGFA expression, albeit partially. This is important since directly inhibiting the effects of hypoxia on VEGFA expression is clinically not yet possible. Clinical translation will require a multipronged approach.
Inhibition of CXCR4 signaling also decreased the angiogenic phenotype in vitro and in vivo. In vitro, HUVEC tube formation and in vivo, bioimaging with AngioSense and microvessel count were all decreased by CXCR4 inhibition, consistent with the decreased expression and content of VEGFA in JJ cells and xenograft tumors. Bioimaging in other tumor systems, primarily carcinomas, using other antiangiogenic strategies have shown similar results. CXCR4 blockade with AMD3100, peptides, antibodies, or knockdown with siRNA has resulted in decreased tumor volume, angiogenesis, and lung metastases in xenograft mouse models of breast, prostate, and thyroid carcinoma and osteosarcoma (41–45). In prior work, we found AMD3100 inhibited hypoxia-induced invasion and expression of MMP1 in vitro, an important negative prognosticator in chondrosarcoma(11;23). Our current data suggest systemic administration of AMD3100 to tumor-bearing mice also inhibited tumor angiogenesis and VEGFA content in xenograft tumors, so that blocking CXCR4 has multiple beneficial effects (23).
Antiangiogenic treatment as a stand-alone strategy has recently been called into question. Although knockdown of VEGFA with RNA interference downregulated CXCR4 and tumor growth and angiogenesis in a xenograft model of non-small cell lung carcinoma (46), in patients with rectal carcinoma, treatment with bevacizumab, an anti-VEGFA antibody, upregulated CXCL12 and CXCR4 in tumors and plasma CXCL12 levels, which correlated with development of lung metastases. This suggests anti-VEGF monotherapy can be accompanied by compensatory mechanisms and blocking CXCR4 in addition to VEGFA will be important in combination therapy (47). There is also the notion that normalization of tumor vasculature with anti-VEGF treatment will increase the efficacy of conventional cytotoxic chemotherapy (48). AMD3100 is a drug approved for human use as a stem cell mobilizer whose mechanism of action is specific inhibition of CXCR4. The extent to which CXCR4 inhibition with AMD3100 or other molecules alone or as part of combination therapy as treatment for chondrosarcoma in humans needs to be evaluated.
In summary, our results show that VEGFA expression increases CXCR4 expression and that CXCR4 blockade inhibits VEGFA production, angiogenesis, and metastasis in vitro and in a mouse chondrosarcoma xenograft model.
Acknowledgments
Financial Support: One or more of the authors have received funding from the National Institutes of Health and from the National Center for Research Resources, a component of NIH: R.M. Terek, Q. Chen (Grant 1P20RR024484-01 and 9P20GM104937-06), L. Wei (Grant R01AR059142), W. Yang (Grant R21AR57156).
We thank Joel Block, MD for the human chondrosarcoma cell line JJ. The authors thank Virginia Hovanesian for assistance with image analysis of lungs.
Abbreviations list
- CXCR4
CHEMOKINE (C-X-C MOTIF) RECEPTOR 4
- VEGFA
VASCULAR ENDOTHELIAL GROWTH FACTOR A
- HUVEC
human umbilical vein endothelial cell
- HIF1
HYPOXIA-INDUCING FACTOR 1
- MMPs
MATRIX METALLOPEPTIDASES
- CXCL12
CHEMOKINE (C-X-C MOTIF) LIGAND 12
Footnotes
The authors have no conflicts of interest to report.
Reference List
- 1.Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 2.Soderstrom M, Ekfors TO, Bohling TO, Teppo LH, Vuorio EI, Aro HT. No improvement in the overall survival of 194 patients with chondrosarcoma in Finland in 1971–1990. Acta Orthop Scand. 2003;74:344–350. doi: 10.1080/00016470310014292. [DOI] [PubMed] [Google Scholar]
- 3.Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–1072. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 4.D’Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin Oncol. 2011;38 (Suppl 3):S19–S29. doi: 10.1053/j.seminoncol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 8.McGough RL, Aswad BI, Terek RM. Pathologic neovascularization in cartilage tumors. Clin Orthop Relat Res. 2002:76–82. doi: 10.1097/00003086-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE, et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326–338. doi: 10.2106/00004623-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 10.McGough RL, Lin C, Meitner P, Aswad BI, Terek RM. Angiogenic cytokines in cartilage tumors. Clin Orthop Relat Res. 2002:62–69. doi: 10.1097/00003086-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Berend KR, Toth AP, Harrelson JM, Layfield LJ, Hey LA, Scully SP. Association between ratio of matrix metalloproteinase-1 to tissue inhibitor of metalloproteinase-1 and local recurrence, metastasis, and survival in human chondrosarcoma. J Bone Joint Surg Am. 1998;80:11–17. [PubMed] [Google Scholar]
- 12.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 13.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 14.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 15.Bartolome RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res. 2006;66:248–258. doi: 10.1158/0008-5472.CAN-05-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutkowski P, Kaminska J, Kowalska M, Ruka W, Steffen J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol. 2003;84:151–159. doi: 10.1002/jso.10305. [DOI] [PubMed] [Google Scholar]
- 17.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Wang J, Sun Y, Song W, Nor JE, Wang CY, et al. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 2005;17:1578–1592. doi: 10.1016/j.cellsig.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Gelmini S, Mangoni M, Castiglione F, Beltrami C, Pieralli A, Andersson KL, et al. The CXCR4/CXCL12 axis in endometrial cancer. Clin Exp Metastasis. 2009;26:261–268. doi: 10.1007/s10585-009-9240-4. [DOI] [PubMed] [Google Scholar]
- 20.Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 21.Pan J, Mestas J, Burdick MD, Phillips RJ, Thomas GV, Reckamp K, et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer. 2006;5:56. doi: 10.1186/1476-4598-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, et al. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Wei L, Chen Q, Terek RM. CXCR4/SDF1 mediate hypoxia induced chondrosarcoma cell invasion through ERK signaling and increased MMP1 expression. Mol Cancer. 2010;9:17. doi: 10.1186/1476-4598-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai TH, Fong YC, Fu WM, Yang RS, Tang CH. Stromal cell-derived factor-1 increase alphavbeta3 integrin expression and invasion in human chondrosarcoma cells. J Cell Physiol. 2009;218:334–342. doi: 10.1002/jcp.21601. [DOI] [PubMed] [Google Scholar]
- 25.Zagzag D, Esencay M, Mendez O, Yee H, Smirnova I, Huang Y, et al. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer’s structures. Am J Pathol. 2008;173:545–560. doi: 10.2353/ajpath.2008.071197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203–7206. [PubMed] [Google Scholar]
- 27.Bridger GJ, Skerlj RT, Thornton D, Padmanabhan S, Martellucci SA, Henson GW, et al. Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J Med Chem. 1995;38:366–378. doi: 10.1021/jm00002a019. [DOI] [PubMed] [Google Scholar]
- 28.De Clercq E. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil) Biochem Pharmacol. 2009;77:1655–1664. doi: 10.1016/j.bcp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Committee on Accelerating Rare Diseases Research and Orphan Product Development, Institute of Medicine. Rare Diseases and Orphan Products: Accelerating Research and Development. The National Academies Press; 2011. [Google Scholar]
- 30.Block JA, Inerot SE, Gitelis S, Kimura JH. Synthesis of chondrocytic keratan sulphate-containing proteoglycans by human chondrosarcoma cells in long-term cell culture. J Bone Joint Surg Am. 1991;73:647–658. [PubMed] [Google Scholar]
- 31.Lin C, McGough R, Aswad B, Block JA, Terek R. Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes. J Orthop Res. 2004;22:1175–1181. doi: 10.1016/j.orthres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Asp J, Brantsing C, Lovstedt K, Benassi MS, Inerot S, Gamberi G, et al. Evaluation of p16 and Id1 status and endogenous reference genes in human chondrosarcoma by real-time PCR. Int J Oncol. 2005;27:1577–1582. [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Wei L, Chen Q, Terek RM. HDAC4 Represses Vascular Endothelial Growth Factor Expression in Chondrosarcoma by Modulating RUNX2 Activity. J Biol Chem. 2009;284:21881–21890. doi: 10.1074/jbc.M109.019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aherne WA, Dunhill MS. Morphometry. London: Edward Arnold; 1982. [Google Scholar]
- 36.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pages G, Pouyssegur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene--a concert of activating factors. Cardiovasc Res. 2005;65:564–573. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 38.Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 39.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iyer S, Acharya KR. Tying the knot: the cystine signature and molecular-recognition processes of the vascular endothelial growth factor family of angiogenic cytokines. FEBS J. 2011;278:4304–4322. doi: 10.1111/j.1742-4658.2011.08350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 42.Perissinotto E, Cavalloni G, Leone F, Fonsato V, Mitola S, Grignani G, et al. Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin Cancer Res. 2005;11:490–497. [PubMed] [Google Scholar]
- 43.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Diao X, Sun J, Chen Z. Regulation of VEGF, MMP-9 and metastasis by CXCR4 in a prostate cancer cell line. Cell Biol Int. 2011;35:897–904. doi: 10.1042/CBI20100744. [DOI] [PubMed] [Google Scholar]
- 45.De FV, Guarino V, Avilla E, Castellone MD, Salerno P, Salvatore G, et al. Biological role and potential therapeutic targeting of the chemokine receptor CXCR4 in undifferentiated thyroid cancer. Cancer Res. 2007;67:11821–11829. doi: 10.1158/0008-5472.CAN-07-0899. [DOI] [PubMed] [Google Scholar]
- 46.Feng Y, Hu J, Ma J, Feng K, Zhang X, Yang S, et al. RNAi-mediated silencing of VEGF-C inhibits non-small cell lung cancer progression by simultaneously down-regulating the CXCR4, CCR7, VEGFR-2 and VEGFR-3-dependent axes-induced ERK, p38 and AKT signalling pathways. Eur J Cancer. 2011;47:2353–2363. doi: 10.1016/j.ejca.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Xu L, Duda DG, di TE, Ancukiewicz M, Chung DC, Lauwers GY, et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69:7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]