Abstract
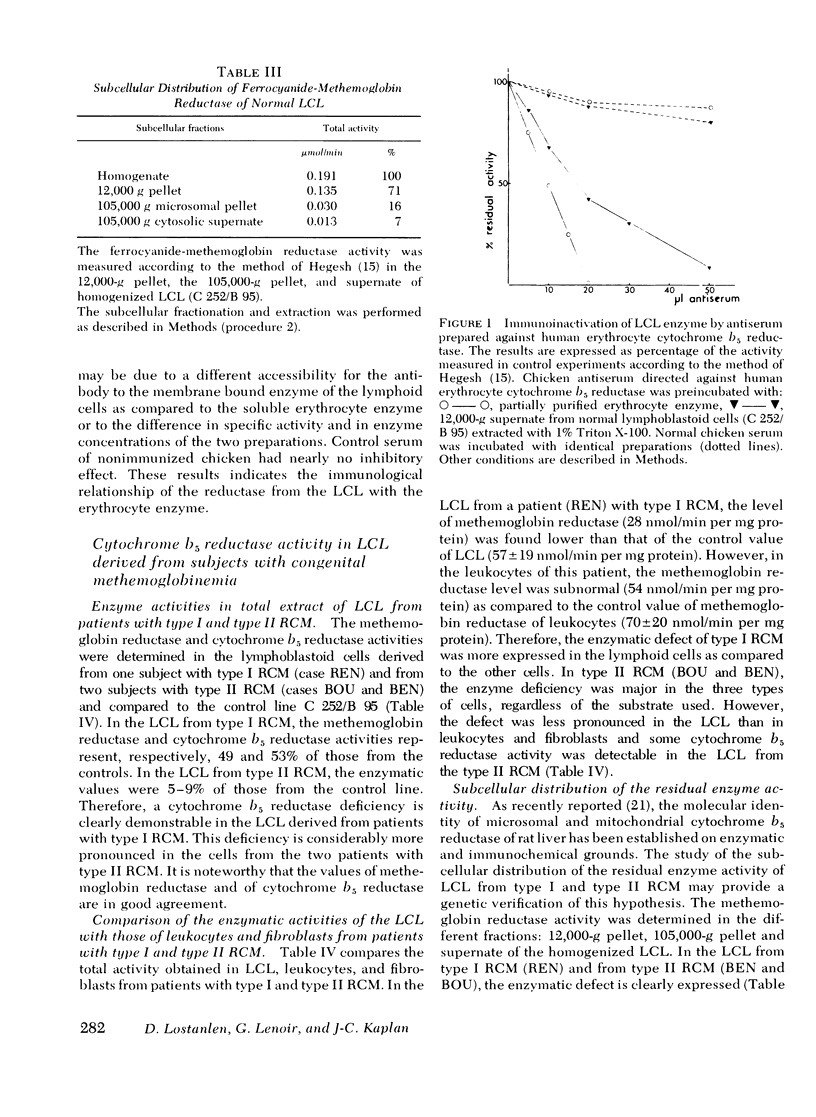
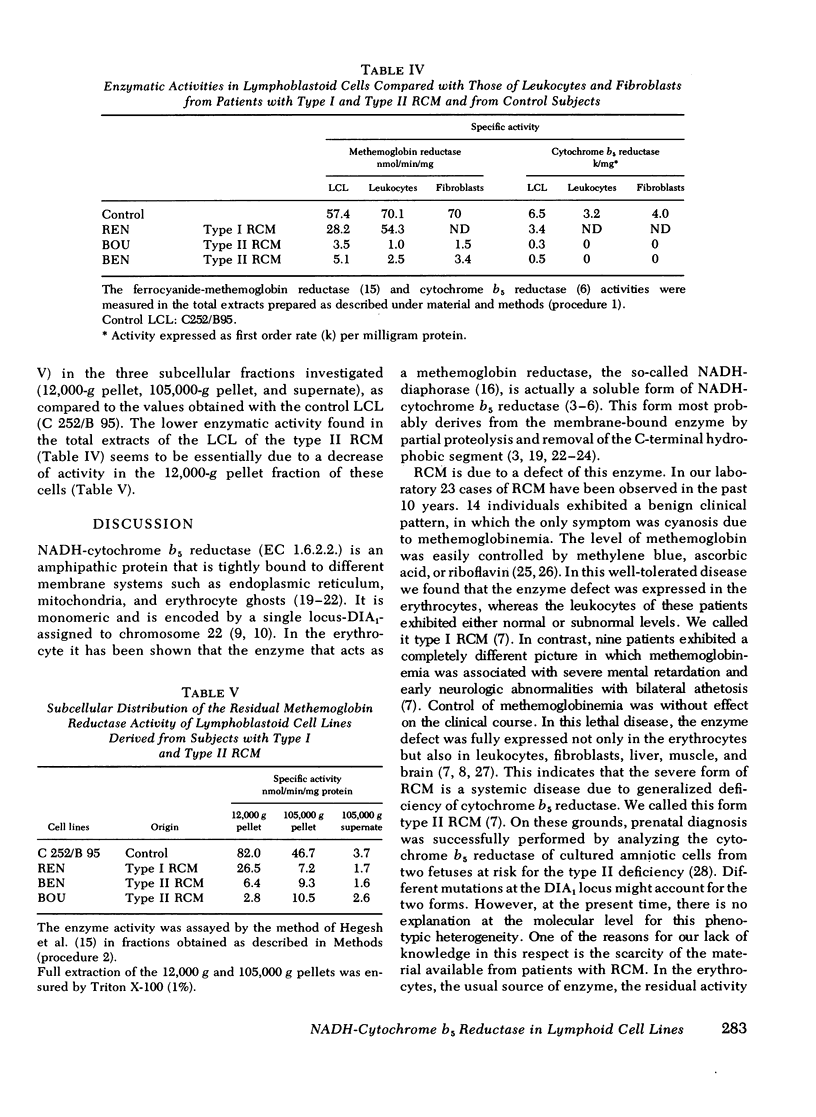
Recessive congenital methemoglobinemia (RCM) is due to the homozygous deficiency of NADH-cytochrome b5 reductase (EC 1.6.2.2.). In type I disease, in which the patients are only methemoglobinemic, the enzyme defect is fully expressed in the erythrocytes, whereas the leukocytes are much less affected. In type II disease, in which the patients are, in addition, mentally retarded, the defect is generalized to all the tissues including cultured fibroblasts. In the present study we have investigated Epstein-Barr virus (EBV) transformed lymphoid cell lines (LCL) derived from patients with both types of cytochrome b5 reductase deficiency and from nondeficient individuals. The total cytochrome b5 reductase activity of the control LCL was found to be similar whatever the LCL origin, except for one lymphoma line (Daudi). The enzyme from the control LCL (c 252/B 95) was found to be immunologically related to the human soluble erythrocyte cytochrome b5 reductase, indicating that it is the product of the same gene: the DIA1 (diaphorase) locus. The LCL derived from one patient with the type I disease and two patients with the type II disease were investigated.l In the former the defect was expressed to a lesser degree than in the cases with mental retardation in which the defect was much pronounced, and involved both the mitochondrial and the microsomal fraction. This indicated that all the subcellular forms of the cytochrome b5 reductase are under the same genetic control. Altogether, these data show that the LCL are a favorable material for studying both types of cytochrome b5 reductase deficiency and for investigating in depth the molecular aspects of this metabolic disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choury D., Kaplan J. C. Diaphorase P: a new fetal isozyme identified in human placenta. Biochim Biophys Acta. 1980;613(1):18–25. doi: 10.1016/0005-2744(80)90187-4. [DOI] [PubMed] [Google Scholar]
- Choury D., Leroux A., Kaplan J. C. Membrane-bound cytochrome b5 reductase (methemoglobin reductase) in human erythrocytes. Study in normal and methemoglobinemic subjects. J Clin Invest. 1981 Jan;67(1):149–155. doi: 10.1172/JCI110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Thé G., Ho H. C., Kwan H. C., Desgranges C., Favre M. C. Nasopharyngeal carcinoma (NPC). I. Types of cultures derived from tumour biopsies and non-tumorous tissues of Chinese patients with special reference to lymphoblastoid transformation. Int J Cancer. 1970 Sep 15;6(2):189–206. doi: 10.1002/ijc.2910060206. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Edwards Y. H., Putt W., Potter J., Hopkinson D. A. An interpretation of human diaphorase isozymes in terms of three gene loci DIA1, DIA2 and DIA3. Ann Hum Genet. 1977 Oct;41(2):139–149. doi: 10.1111/j.1469-1809.1977.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Povey S., Bobrow M., Solomon E., Boyd Y., Carritt B. Assignment of the DIA1 locus to chromosome 22. Ann Hum Genet. 1977 Oct;41(2):151–155. doi: 10.1111/j.1469-1809.1977.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Glade P. R., Beratis N. G. Long-term lymphoid cell lines in the study of human genetics. Prog Med Genet. 1976;1:1–48. [PubMed] [Google Scholar]
- Goto-Tamura R., Takesue Y., Takesue S. Immunological similarity between NADH-cytochrome b5 reductase of erythrocytes and liver microsomes. Biochim Biophys Acta. 1976 Feb 16;423(2):293–302. doi: 10.1016/0005-2728(76)90186-9. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Calmanovici N., Avron M. New method for determining ferrihemoglobin reductase (NADH-methemoglobin reductase) in erythrocytes. J Lab Clin Med. 1968 Aug;72(2):339–344. [PubMed] [Google Scholar]
- Hultquist D. E., Passon P. G. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat New Biol. 1971 Feb 24;229(8):252–254. doi: 10.1038/newbio229252a0. [DOI] [PubMed] [Google Scholar]
- Junien C., Leroux A., Lostanlen D., Reghis A., Boue J., Nicolas H., Boue A., Kaplan J. C. Prenatal diagnosis of congenital enzymopenic methaemoglobinaemia with mental retardation due to generalized cytochrome b5 reductase deficiency: first report of two cases. Prenat Diagn. 1981 Jan;1(1):17–24. doi: 10.1002/pd.1970010106. [DOI] [PubMed] [Google Scholar]
- Junien C., Vibert M., Weil D., Van-Cong N., Kaplan J. C. Assignment of NADH-cytochrome b5 reductase (DIA1 locus) to human chromosome 22. Hum Genet. 1978 Jun 27;42(3):233–239. doi: 10.1007/BF00291301. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Chirouze M. Therapy of recessive congenital methaemoglobinaemia by oral riboflavine. Lancet. 1978 Nov 11;2(8098):1043–1044. doi: 10.1016/s0140-6736(78)92357-7. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Leroux A., Bakouri S., Grangaud J. P., Benabadji M. La lésion enzymatique dans la méthémoglobinémie congénitale récessive avec encéphalopathie. Description d'une nouvelle variante déficitaire de NADH-diaphorase (variante Beni-Messous) Nouv Rev Fr Hematol. 1974 Nov-Dec;14(6):755–770. [PubMed] [Google Scholar]
- Kaplan J. C., Leroux A., Beauvais P. Formes cliniques et biologiques du déficit en cytochrome b5 réductase. C R Seances Soc Biol Fil. 1979;173(2):368–379. [PubMed] [Google Scholar]
- Kuma F., Prough R. A., Masters B. S. Studies on methemoglobin reductase. Immunochemical similarity of soluble methemoglobin reductase and cytochrome b5 of human erythrocytes with NADH-cytochrome b5 reductase and cytochrome b5 of rat liver microsomes. Arch Biochem Biophys. 1976 Feb;172(2):600–607. doi: 10.1016/0003-9861(76)90113-2. [DOI] [PubMed] [Google Scholar]
- Kuwahara S., Okada Y., Omura T. Evidence for molecular identity of microsomal and mitochondrial NADH-cytochrome b5 reductases of rat liver. J Biochem. 1978 Apr;83(4):1049–1059. doi: 10.1093/oxfordjournals.jbchem.a131993. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leroux A., Junien C., Kaplan J., Bamberger J. Generalised deficiency of cytochrome b5 reductase in congenital methaemoglobinaemia with mental retardation. Nature. 1975 Dec 18;258(5536):619–620. doi: 10.1038/258619a0. [DOI] [PubMed] [Google Scholar]
- Leroux A., Kaplan J. C. Presence of red cell type NADH-methemoglobin reductase (NADH-diaphorase) in human non erythroid cells. Biochem Biophys Res Commun. 1972 Nov 15;49(4):945–950. doi: 10.1016/0006-291x(72)90303-8. [DOI] [PubMed] [Google Scholar]
- Leroux A., Torlinski L., Kaplan J. C. Soluble and microsomal forms of NADH-cytochrome beta 5 reductase from human placenta. Similarity with NADH-methemoglobin reductase from human erythrocytes. Biochim Biophys Acta. 1977 Mar 15;481(1):50–62. doi: 10.1016/0005-2744(77)90136-x. [DOI] [PubMed] [Google Scholar]
- Lostanlen D., Vieira de Barros A., Leroux A., Kaplan J. C. Soluble NADH-cytochrome b5 reductase from rabbit liver cytosol: partial purification and characterization. Biochim Biophys Acta. 1978 Sep 11;526(1):42–51. doi: 10.1016/0005-2744(78)90288-7. [DOI] [PubMed] [Google Scholar]
- Mihara K., Sato R., Sakakibara R., Wada H. Reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase: location of the hydrophobic, membrane-binding region at the carboxyl-terminal end and the masked amino terminus. Biochemistry. 1978 Jul 11;17(14):2839–2834. doi: 10.1021/bi00607a020. [DOI] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Povey S., Gardiner S. E., Watson B., Mowbray S., Harris H., Arthur E., Steel C. M., Blenkinsop C., Evans H. J. Genetic studies on human lymphoblastoid lines: isozyme analysis on cell lines from forty-one different individuals and on mutants produced following exposure to a chemical mutagen. Ann Hum Genet. 1973 Jan;36(3):247–266. doi: 10.1111/j.1469-1809.1973.tb00588.x. [DOI] [PubMed] [Google Scholar]
- SCOTT E. M., GRIFFITH I. V. The enzymic defect of hereditary methemoglobinemia: diaphorase. Biochim Biophys Acta. 1959 Aug;34:584–586. doi: 10.1016/0006-3002(59)90324-5. [DOI] [PubMed] [Google Scholar]
- SCOTT E. M., McGRAW J. C. Purification and properties of diphosphopyridine nucleotide diaphorase of human erythrocytes. J Biol Chem. 1962 Jan;237:249–252. [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. The purification and properties of microsomal cytochrome reductase. J Biol Chem. 1957 Oct;228(2):785–799. [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem. 1973 Feb 10;248(3):793–799. [PubMed] [Google Scholar]
- Swallow D. M., Gardiner S. E., Harris H., Arthur E., Steel C. M., Evans H. J. Lysosomal enzymes in human lymphoblastoid lines: unusual characteristics of RAJI and DAUDI. Ann Hum Genet. 1977 Jul;41(1):9–16. doi: 10.1111/j.1469-1809.1977.tb01957.x. [DOI] [PubMed] [Google Scholar]
- Yata J., Desgranges C., Nakagawa T., Favre M. C., De-The G. Lymphoblastoid transformation and kinetics of appearance of viral nuclear antigen (EBNA) in cord-blood lymphocytes infected by Epstein-Barr Virus (EBV). Int J Cancer. 1975 Mar 15;15(3):377–384. doi: 10.1002/ijc.2910150303. [DOI] [PubMed] [Google Scholar]
- Zamudio I., Canessa M. Nicotinamide-adenine dinucleotide dehydrogenase activity of human erythrocyte membranes. Biochim Biophys Acta. 1966 May 12;120(1):165–169. doi: 10.1016/0926-6585(66)90290-1. [DOI] [PubMed] [Google Scholar]



