Abstract
Background
Cutaneous metastases of malignant melanoma (CMMM) can be confused with other skin lesions. Dermoscopy could be helpful in the differential diagnosis.
Objective
To describe distinctive dermoscopic patterns that are reproducible and accurate in the identification of CMMM
Methods
A retrospective study of 146 dermoscopic images of CMMM from 42 patients attending a Melanoma Unit between 2002 and 2009 was performed. Firstly, two investigators established six dermoscopic patterns for CMMM. The correlation of 73 dermoscopic images with their distinctive patterns was assessed by four independent dermatologists to evaluate the reproducibility in the identification of the patterns. Finally, 163 dermoscopic images, including CMMM and non-metastatic lesions, were evaluated by the same four dermatologists to calculate the accuracy of the patterns in the recognition of CMMM.
Results
Five CMMM dermoscopic patterns had a good inter-observer agreement (blue nevus-like, nevus-like, angioma like, vascular and unspecific). When CMMM were classified according to these patterns, correlation between the investigators and the four dermatologists ranged from κ = 0.56 to 0.7.
71 CMMM, 16 angiomas, 22 blue nevus, 15 malignant melanoma, 11 seborrheic keratosis, 15 melanocytic nevus with globular pattern and 13 pink lesions with vascular pattern were evaluated according to the previously described CMMM dermoscopy patterns, showing an overall sensitivity of 68% (between 54.9–76%) and a specificity of 81% (between 68.6–93.5) for the diagnosis of CMMM.
Conclusion
Five dermoscopic patterns of CMMM with good inter-observer agreement obtained a high sensitivity and specificity in the diagnosis of metastasis, the accuracy varying according to the experience of the observer.
Keywords: dermoscopy, dermatoscopy, cutaneous metastases, differential diagnosis, polarized light, melanoma
Introduction
Melanoma has the potential to metastasize to any organ and the route and pattern of metastatic disease is unpredictable.1 The most common sites for the clinical presentation of metastases are the skin, subcutaneous tissue and lymph nodes followed by visceral organs.1 Skin metastases (cutaneous and/or subcutaneous) represent a relatively frequent event in the natural history of melanoma.2 The reported incidence varies widely, ranging from 2 to 20%.2 It can represent an early as well as a late phase of disease progression.3 Up to 70% of first recurrences of primary melanomas are locoregional, representing the first site of recurrence after surgical excision of the primary melanoma (satellites or in transit metastases), but cutaneous metastases of malignant melanoma (CMMM) may appear as the only manifestation and onset of disseminated disease.1,3
The risk of developing metastasis depends on the anatomical location, clinical presentation and microscopic characteristics commonly associated with more aggressive primary tumours.4,5 Skin metastases are classified as ‘satellitosis’ or ‘in-transit disease’ and ‘distant cutaneous metastases”.2,6
The clinical presentation can be heterogeneous, being solitary or multiple, pigmented or erythematous3,4,7, dermal or subcutaneous mimicking other benign and malignant neoplasms.8
The differential diagnosis of CMMM with other cutaneous tumours can be challenging and especially important in the early detection and correct management of progressive disease. Misdiagnosing a cutaneous metastatic melanoma as a benign lesion can result in significant under-staging of a patient, as cutaneous metastases/in-transit melanoma under the current American Joint Committee on Cancer guidelines is considered N2C or stage IIIB disease and equivalent to lymph node involvement. Alternatively, misdiagnosing a benign lesion as a metastatic melanoma falsely labels a patient as having stage III disease and may lead to significant morbidity from unnecessary toxic adjuvant therapy as well as emotional strain and potential loss of health insurance.9
Dermoscopy demonstrated to be useful in the differential diagnosis of skin tumours.10 The widespread use of dermoscopy in recent years has provided new morphologic features for the differentiation of malignant melanoma from other melanocytic and non-melanocytic skin lesions. Systematic reviews of the diagnostic accuracy of dermoscopy in detecting malignant melanoma, reported an improvement of 49% with sensitivity and specificity values of approximately 86% and 89%, respectively (compared with inspection by the unaided eye).11 Some studies have been published on dermoscopy in the diagnosis of CMMM and several features and patterns have been described but no precise dermoscopic patterns for the definition of CMMM has been provided.4,12,13
In the present study, a specific nomenclature for classifying CMMM patterns based on dermoscopic features is established. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPP), and reproducibility of the dermoscopic patterns associated with CMMM were investigated.
Materials and methods
A retrospective evaluation of 146 dermoscopic images of CMMM from 42 consecutive patients with well documented images from the database of the Melanoma Unit of the Hospital Clinic of Barcelona, between 2002 and 2009, was performed. Dermoscopic images of each lesion were obtained using a digital microscopy system (DermLite Foto - 3Gen LLC, Dana Point, CA, U.S.A.; Canon PowerShot G7). Clinical data were obtained for each patient, including age at time of primary melanoma diagnosis, sex, skin type, anatomical location of primary cutaneous malignant melanoma, Clark’s level of invasion, Breslow tumour thickness, presence of ulceration and nodal metastases at the time of melanoma diagnosis. At least one metastasis from each patient was confirmed by histopathology. The clinical evolution confirmed other metastases as rapid growth together with other histopathological confirmed metastases and response or progression with melanoma specific treatments (e.g. intra-lesional interleukin 2).
In the first part of the study, two investigators (one with experience in evaluating dermoscopic images, J.M. and one without, J.C.,) established six dermoscopic patterns of CMMM (Fig. 1) based on the dermoscopic features described in previous studies4,12,14 and formulated a tutorial with written descriptions of the patterns and two representative images demonstrating each of the patterns as seen in Table 1. The images used in the tutorial were not included in the evaluation. Half of the dermoscopic images from the 146 CMMM included in the study (75 images) were evaluated for the presence of these patterns by four dermoscopists (V.B., C.C., S.P. and G.S.) and the inter-observer reproducibility of these patterns was assessed. Data analysis was performed using statistical program Statistical Package for the Social Sciences (SPSS) version 12.0, which calculated coefficient kappa (k) statistics and average percentage. With regard to the interpretation of k statistics, a value of 1.00 indicates perfect agreement, values greater than 0.80 are considered excellent, values between 0.61 and 0.80 are good, values between 0.40 and 0.60 are fair, and values less than 0.40 are poor. As regards the interpretation of average percentage, a value of 100% was considered perfect agreement, values greater than 80% were considered excellent, values between 60 and 80% were good, values between 40 and 60% were fair, and values less than 40% were poor.
Figure 1.
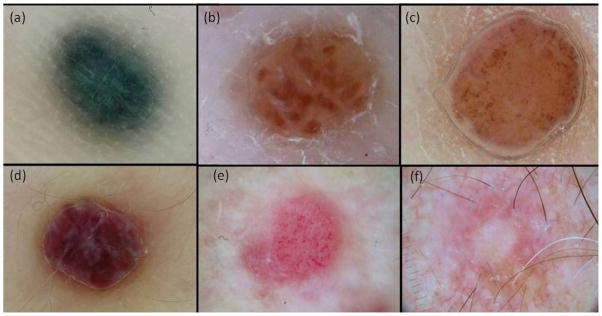
CMMM patterns: a. blue nevus-like pattern; b. nevus-like globular pattern; c. nevus-like nonglobular pattern; d. angioma like pattern; e. vascular pattern; and unspecific pattern.
Table 1.
Dermoscopic Patterns of CMMM.
| Pattern | Description |
|---|---|
| Blue nevus-like | Homogeneous diffuse grey-blue to grey-black or grey- brownish pigmentation indistinguishable from blue nevus. |
| Globular Nevus-like | Aggregation of brown to black or bluish globules. Some reddish globules may be present. If all the globules are reddish, an angioma-like pattern is considered. Atypical vessels can be present. |
| Non globular Nevus-like | Homogenous brown pigmentation that can be associated with multiple blue-grey dots or atypical vessels. |
| Angioma-like | Homogeneous reddish or purplish coloration and/or lacunae with a red, red-blue, purplish or nearly black hue separated from each other by whitish septa. If vessels are present, it is classified as vascular pattern. Pink homogeneous areas and pyogenic granuloma-like are included. |
| Vascular | Three or more atypical vessels (dotted, linear irregular, corkscrew, irregular-polymorphous). The vascular component is predominant and light brown pigmentation may be present, but not aggregates of brown globules. |
| Unspecific | Presence of fewer (< 3) nonspecific vessels and neither another pattern nor homogeneous brown pattern. |
In the second part of the study, dermoscopic images of 71 CMMM (8 angioma-like, 22 blue nevus-like, 15 nevus-like globular, 11 nevus-like non globular, 2 unspecific and 13 vascular pattern), 16 haemangiomas, 22 blue nevus, 15 primary malignant melanoma, 11 seborrheic keratosis, 15 melanocytic nevus with globular pattern and 13 pink lesions with vascular pattern (pyogenic granuloma, psoriasis, basal cell carcinoma, squamous cell carcinoma, molluscum contagiosum, dermal nevus and seborrheic keratosis) were evaluated by the same four dermoscopists (V.B., C.C., S.P. and G.S.) in order to classify the images in these diagnoses. The primary melanomas included did not exhibit reticular or multicomponent pattern but globular, homogeneous or unspecific similar to the patterns present in CMMM. Nevi included also exhibited globular pattern. Dermoscopic images of each lesion were obtained using DermLite Foto (3Gen LLC, Dana Point, CA, U.S.A.; Canon PowerShot G7). All CMMM and non-CMMM lesions were evaluated for the presence of the same dermoscopic structures and patterns commonly seen in CMMM, as shown in Table 2, and the average percentage of agreement was calculated between the four authors with the aforementioned statistical program.
Table 2.
Frequencies of the predominant dermoscopic structures in CMMM and other cutaneous lesions included in the study (n: 163).
| Homogeneous blue | Lacunae red and/or purplish |
Cork screw vessels | Horney plugs and/or millium cysts |
Arborizing vessels | Globules | Irregular vessels polymorphous and/or dotted and/or linear |
Crown vessels | Coiled vessels | Hairpin vessels | Unspecific | Homogenous brown pigmentation |
Pink homogenous | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMMM | 22 | 8 | 5 | 0 | 1 | 15 | 7 | 0 | 0 | 0 | 2 | 11 | 0 |
| Angioma | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blue nevus | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MM | 2 | 0 | 1 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 6 | 0 | 0 |
| SK | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Melanocytic nevus with globular pattern | 0 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SCC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| BCC | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Others (P, PG, MC, DN) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (DN) | 1 (MC) | 1 (P) | 0 | 0 | 0 | 1 (PG) |
Abbreviations: CMMM, cutaneous malignant melanoma metastases; MM, malignant melanoma; SK, seborrheic keratoses; P, psoriasis; PG, pyogenic granuloma; MC, molluscum contagiosum; DN, dermal nevus; BCC, basal cell carcinomas; SCC, squamous cell carcinomas.
On the basis of these evaluations, we determined the diagnostic significance of CMMM patterns. The criteria for calculating diagnostic variables in CMMM were defined as follows: true-positive (TP) metastatic lesions considered as such by the observer, true-negative (TN) non-metastatic lesions considered as such by the author, false-negative (FN) metastatic lesions not detected by the author, and false-positive (FP) non-metastatic lesions considered as metastatic by the author. Sensitivity was the fraction of CMMM lesions detected among all CMMM and was calculated as TP/(TP+FN). Specificity was the fraction of non-CMMM lesions detected among all non-CMMM and was calculated as TN/(TN+FP). The PPV was the fraction of CMMM lesions detected as such by the author among all lesions considered as CMMM by the same and was calculated as TP/(TP+FP). The NPV was the fraction of non-CMMM lesions detected as such by the author among all lesions considered as non-CMMM by the same and was calculated as TN/(TN+FN). All these parameters were calculated for each of the four authors and globally. Data analysis was performed with the same statistical program cited above, giving 2 × 2 contingency tables.
Results
Description of CMMM lesions and patients included
The lesions were obtained from 42 patients, 20 men (47.6%) and 22 women (52.4%) ranging in age from 13 to 79 years (median age, 54.8 years) at diagnosis of primary melanoma. From these, 146 dermoscopic images of CMMM were obtained.
Twenty-one patients (50%) were phototype II, 14 (33.3%) were phototype III, 3 (7.1%) were phototype I and 1 patient (2.4%) each for phototypes IV and V, data was unavailable in two patients (4.8%).
Regarding the type of primary melanoma, 16 patients (38%) presented superficial spreading melanoma, 11 (26.2%) presented acral lentiginous melanoma, six (14.3%) presented nodular melanoma, two (4.7%) presented melanoma on nevus and one (2.4%) desmoplastic melanoma. Data was unavailable for six (14.3%) patients.
As regards the site of the primary melanoma, it was more frequent in lower limbs (15 patients 35.7%) followed by sole (10 patients, 23.8%), trunk (8 patients, 19%), upper limbs (3 patients, 7.1%) and less frequently face, hand and scalp with one patient (2.4%) for each of these sites.
The mean Breslow of primary melanoma was 4.5 mm (SD ± 4.51) ranging in depth from 0.8 to 22 mm. Half of the primary melanomas were not ulcerated. Just over half of the patients (54.8%) had nodal metastases at the time of melanoma diagnosis.
On average, the time delay between melanoma diagnosis and CMMM onset was two years and nine months (range: two months to 14 years).
Part 1: Dermoscopy patterns of CMMM
The six dermoscopic patterns of CMMM described were blue nevus-like (22 images), nevus-like globular (15 images), nevus-like non globular (12 images), angioma-like (9 images), vascular (14 images) and unspecific (3 images).
The inter-observer agreement for all patterns (considering as gold standard the classification of the investigators) measured by k, was the following: for dermoscopist A, k = 0.7, for dermoscopist B k = 0.562, for dermoscopist C k = 0.625, and for dermoscopist D k = 0.670.
The average percentage of agreement between the authors and investigators for each pattern individually were: greater than 80% for the haemangioma-like and blue nevus-like patterns and between 60 to 80% for the vascular and unspecific pattern. As regards the nevus-like globular and non globular patterns, the average percentage of agreement evaluated individually were around 50% and 60% respectively, but when only taking nevus-like (both globular and non globular) as a parameter, the concordance increases to 83% (Fig. 2).
Figure 2.
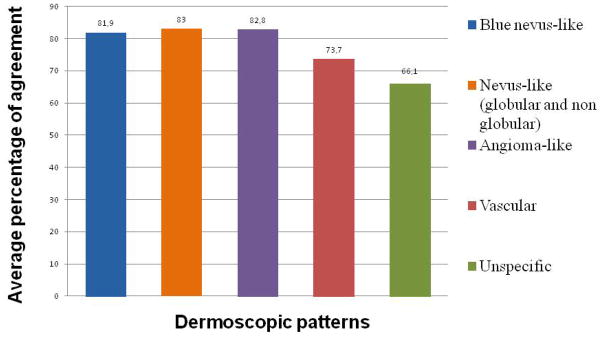
Patterns and inter-observer agreement.
Part 2: Accuracy of CMMM dermoscopy patterns
In the second study, we compared CMMM and other cutaneous lesions. The more common dermoscopy structures in the CMMMs included were: blue homogeneous (31%), globules (21%), homogeneous brown pigmentation (15.5%), lacunae red and/or purplish (11%) and to a lesser degree, irregular vessels (polymorphous, dotted, linear, cork screw and arborizing) and unspecific pattern. The findings are presented in Table 2.
Table 3 summarizes the results of the statistical analysis. These include the sensitivity, specificity, PPV and NPP for CMMM evaluated for four dermatologists. We obtained an overall sensitivity of 68% (between 54.9 – 76%) and specificity of 79.8% (between 68.6–93.5%) for the diagnosis of CMMM.
Table 3.
Diagnostic significance and reproducibility of patterns in CMMM.
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |
|---|---|---|---|---|
| Author A | 76 | 77.1 | 72 | 80.6 |
| Author B | 54.9 | 68.5 | 57.4 | 66.3 |
| Author C | 74.6 | 93.5 | 89.8 | 82.7 |
| Author D | 66.2 | 80.4 | 72.3 | 75.5 |
| Overall | 68 | 79.8 | 72.3 | 76.3 |
In this specific set of samples, the sensitivity of the dermoscopists in the diagnosis of benign non-CMMM was 80% for seborrheic keratoses, between 60 and 80% for angioma, blue nevus, nevus, psoriasis, between 40 and 60% for BCC, SCC, malignant melanoma and pyogenic granuloma and less than 40% for molluscum contagiosum (Fig. 3).
Figure 3.
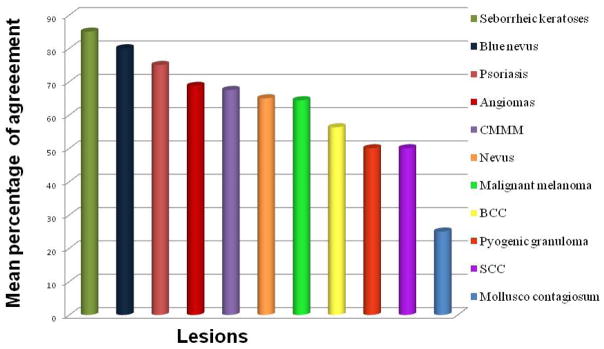
Mean sensitivity of the dermoscopist in detecting CMMM and other non-CMMM lesions.
Discussion
Malignant melanoma is a neoplasm with a capacity to metastasize to any organ, including the skin and here it can be confused with other benign or malignant skin lesions.
In contrast to previous studies, such as Bono et al4 who reported that the most common site of primary cutaneous malignant melanoma was the trunk (41%), in our study the most common sites were lower limbs (35%) followed by the sole (24%). Bono et al also noted the importance of the acral location of primary melanomas in five out of the 32 cases. In our study, the acral location (foot and hand) were seen in about one third (11 out of 42 patients). In the same study the median thickness calculated by Breslow was 2.9 mm, and the mean Breslow in our study was 4.5 mm.
Dermoscopy is a non-invasive technique that could be a useful tool in distinguishing CMMM.15,16 Schulz12 found that the prevalence of four out of the 24 features studied (vascular and saccular patterns, light-brown halo and peripheral erythema) was significantly different between the 30 CMMMs and the 250 tumours of the control group, being depicted as malignant features of the lesions. Bono et al.4 analyzed the dermoscopic structures of 130 cutaneous melanoma metastases observed in 32 patients and compared them with 350 melanomas, 150 common naevi, 40 blue naevi, 40 haemangiomas and 50 basal cell carcinomas. The homogeneous, saccular and vascular patterns (showing aneurysms and winding vessels), together with pigmented halo and peripheral grey spots, seem to be the most significant elements suggestive of CMMM.
In contrast to the study of Bono et al. that analysed dermoscopy structures, our study focused on the analyses of dermoscopic patterns, describing six dermoscopic patterns for CMMMs [blue nevus-like, angioma-like, nevus-like (globular and non globular), vascular and unspecific patterns], obtaining a good inter-observer correlation (k between 0.625 and 0.7), except for one observer who obtained a result classified as reasonable (k = 0.562) that we believe could be due to the difference in dermoscopy experience of the authors (ranging from more than 15 years to less than 2 years). These differences in the inter-observer agreement also highlight the value of experience in identifying CMMM by dermoscopy pattern analyses.
Concerning each type of pattern evaluated, we obtained an excellent correlation among the researchers and authors in blue nevus-like patterns, nevus-like (considering globular and non globular together) and angioma-like (more than 80%) and a good correlation for vascular and non-specific patterns (60 to 80%) proving the usefulness of these patterns in classifying CMMM.
CMMM may present in different clinical forms, and differential diagnosis involves common nevus, blue nevus, primary melanoma, angiomas as well as others lesions with vascular patterns, such as basal cell carcinomas, squamous cell carcinomas, psoriasis, pyogenic granulomas and dermal nevus.4,7 In the present study, the ability to detect CMMM amid other lesions was good taking into consideration that, without the patient’s history and with just the dermoscopy image, it could be very difficult to distinguish CMMM, especially from blue nevus-like pattern which are virtually indistinguishable.8 Some authors describe that the homogeneous pattern is peculiar to blue nevus, where the blue colour predominates, whilst the presence of a vascular pattern was more associated with CMMMs.4 These findings were not corroborated in our study, as in most cases for both types of lesions (blue nevus and blue nevus-like CMMM) there were no vascular structures within the homogeneous blue pattern, likewise in a small proportion of both types of lesion, vessels were present and nonspecific. Furthermore, some congenital acral nevi have a homogeneous blue pattern as reported by Garrido-Ríos et al.17 Samimi et al.18 reported that ultrasonography is a reproducible tool that can assist in the differential diagnosis between blue nevus and melanoma metastases but there should be a clinical suspicion present.
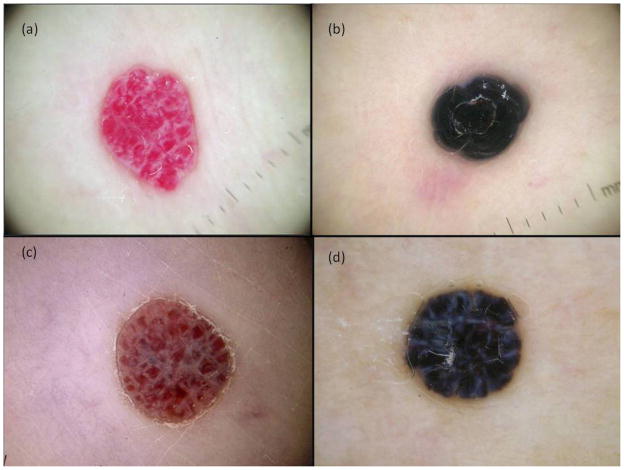
Although the dermoscopic features of haemangiomas and angioma-like CMMMs are similar (Fig. 4), the former consists of blood-filled vascular spaces separated by fibrous tracts while the latter represent nests of atypical melanocytes, which are observed to not be clearly separated by fibrous tracts-like.4,12,19 Jaimes et al.13 also reported lesions with angioma-like lacunae in amelanotic cutaneous melanoma metastases, where most of them had hairpin and serpentine vessels within these structures. Nevertheless, as proposed by the latter author, the presence of red to purple, ill-defined, lacunar structures with (or without) atypical vessels inside, facilitated the diagnosis of angioma-like CMMM, confirmed in our study.
Figure 4.
Dermoscopic images of angioma and angioma-like CMMM. (a) Angioma with blood-filled vascular spaces separated by fibrous tracts, (b) thrombosed angioma, (c) and (d) angioma-like CMMM in which pseudolagoons are nests of atypical melanocytes not observed separated by fibrous tracts-like.
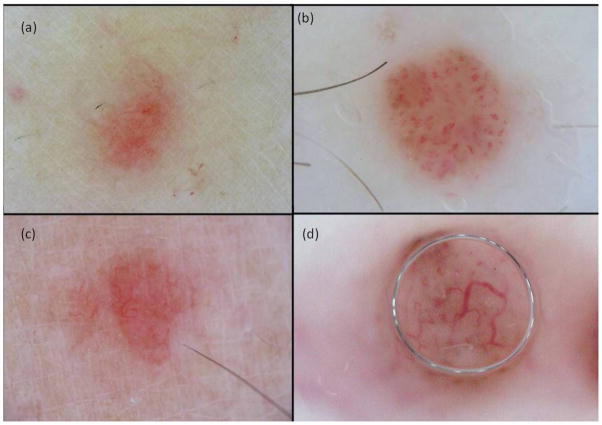
Bono et al.4 reported that a vascular pattern (especially polymorphic atypical vessels and winding vessels) was observed in 53% of CMMM. Minagawa et al.20 also reported dermoscopic findings of polymorphous vessels, including linear irregular vessels, dotted vessels and coiled vessels in CMMM, as well as Jaimes et al.13 who also reported that 100% of amelanotic CMMM had an atypical vascular pattern (with one or more vascular patterns) which we also observed in our cases (Fig. 5). Interestingly, in the present study several CMMM lesions were diagnosed as BCC (Fig. 5c, d) due to the presence of a vascular pattern with arborizing in focus vessels.
Figure 5.
CMMM with vascular pattern. (a) Irregular dotted vessels, (b) cork screw vessels, (c) irregular linear vessels, and (d) arborizing vessels (c) and (d) mimicking a basal cell carcinoma.
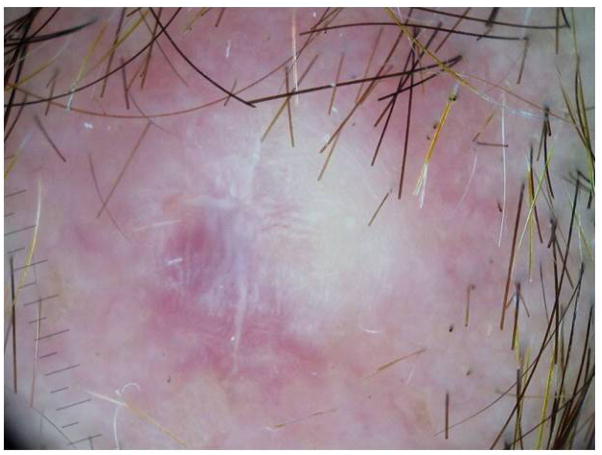
Previously, Pizzichetta et al.21 reported a case of melanoma on the scalp and a further 11 skin metastasis mimicking benign melanocytic nevus (with a globular pattern characterized by greyish-blue globules extending throughout the entire lesion) or primary melanomas (with a multi-component pattern). Lestre et al.22 also reported CMMMs simulating melanocytic nevus or primary cutaneous melanoma. In the present study, CMMMs mimicking benign nevus, globular and non globular, were also found. Among those CMMMs with a globular nevus-like pattern, globules were more heterogeneous in size, colour and distribution than the true benign nevus (Fig. 6). As regards the non-globular nevus-like pattern the majority were correctly classified as nevus-like pattern, but since we found it more confusing to consider them as globular or non-globular, it would be more practical to classify both of them as nevus-like pattern. The presence of milky-red areas with white shiny streaks may be also a clue in the detection of CMMM (Fig. 7).13
Figure 6.
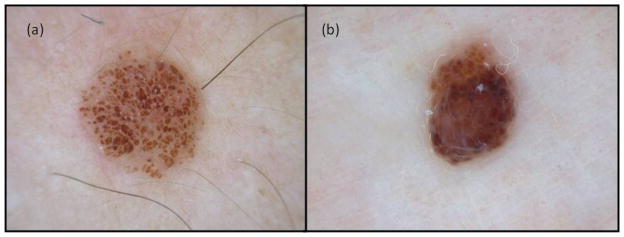
Benign nevus with globular pattern (a) and CMMM with nevus-like pattern (b) where we observe the different appearance of globules in size, color and distribution.
Figure 7.
CMMM with unspecific pattern with milky-red areas and white shiny streaks.
The vast majority of lesions were correctly identified as CMMMs, the dermoscopic patterns achieving a good sensitivity (68%) and specificity (79.8%) in diagnosing CMMM. However, this varied according to experience of the dermoscopist and according to the specific pattern presented by the CMMM. The best agreement was achieved with blue-nevus like, nevus like and angioma like patterns.
The description of the dermoscopy patterns of melanoma metastases may be useful to recognize early metastasis, when, clinically, they are not suspicious, facilitating an early removal and histopathological confirmation. However, patients with locally advanced disease may also benefit from the dermoscopic classification of CMMM that may be of use in the future to better characterize the response to treatments (such as isolated limb perfusion/infusion; electrochemotherapy; or target therapies) or early failure or ulterior relapse.
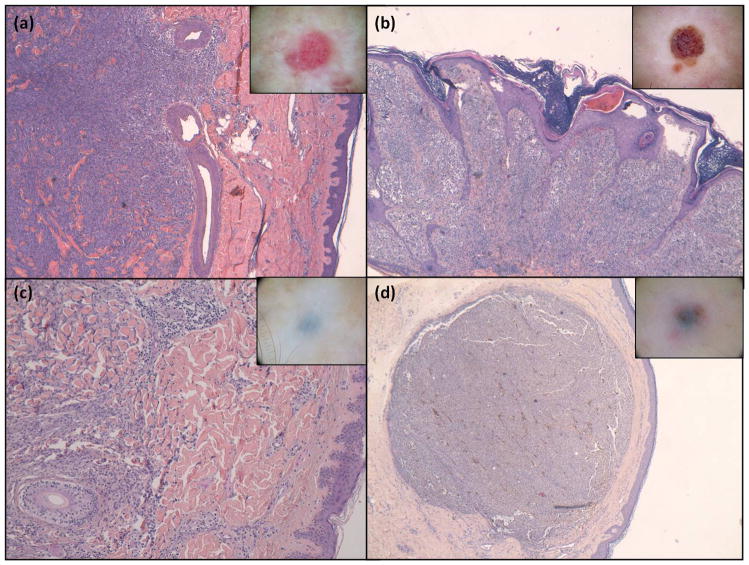
Furthermore, the clinical-dermoscopic-histological correlation is very interesting. Classically, metastases of melanoma are divided into epidermotropic and non epidermotropic8,22 and dermoscopy could also be a useful non-invasive tool to diagnose and distinguish these two types of metastases as suggested by the group of Watanabe et al23. Table 4 showed the histopathological correlation of main M1 patterns described in the present manuscript and Figures 8 and 9 illustrate the histological findings for the four main dermoscopic patterns (blue nevus-like, angioma-like, vascular and nevus-like patterns).
Table 4.
Correlation between dermoscopic patterns and histology findings.
| Dermoscopic pattern | Histology |
|---|---|
| Vascular | Abundant vascularization in the superficial dermis as well as large vessels in the reticular dermis on the metastatic infiltration |
| Angioma-like | Epidermis tracts that get into the superficial and middle dermis between metastatic infiltrating melanocyte nests with neoangiogenesis probably simulating fiber tracts and lagoons in dermoscopy. |
| Blue nevus-like | First type: Metastatic cells between collagen bundles simulating an interstitial pattern Second type: pigmented nodule in middle-deep dermis. |
| Nevus-like | Dermal metastatic cells that are grouped forming nests separated by low or absent stroma, and with a tendency to invade the epidermis also forming nests. |
Figure 8.
Dermoscopic-histologic correlation of CMMM patterns. (a) vascular (b) angioma-like and (c),(d) blue nevus-like. In box shows the images corresponding to each dermoscopic pattern. Hematoxylin & Eosin stain x4.
Figure 9.
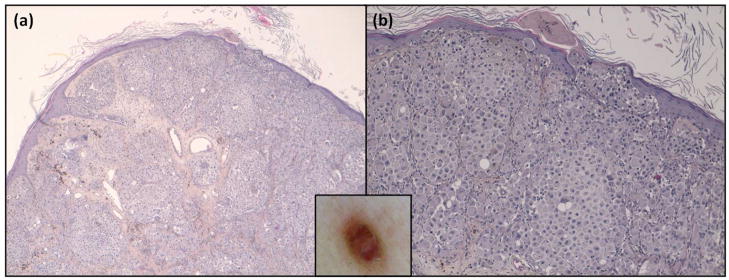
Histology of nevus-like pattern. (a) nests of metastatic cells, which at higher magnification (b) tendency to invade epidermis. In box shows the corresponding dermoscopic image. (a) Hematoxylin & eosin x4 and, (b) Hematoxycilin and eosin x10.
In conclusion, five dermoscopic patterns of CMMM (blue nevus-like, angioma-like, nevus-like, vascular and unspecific) demonstrated a good inter-observer agreement, in addition to good sensitivity, specificity, PPN, NPV and reproducibility. The present study confirms that dermoscopy is a useful tool that may increase the physician’s diagnostic accuracy for CMMM and allow its differentiation from other cutaneous lesions, always taking into account the patient’s medical record.
Vignettes.
What’s already known?
Dermoscopy helps to identify both benign and malignant lesions.
In the literature, several dermoscopic features of cutaneous metastases of malignant melanoma (CMMM) are described.
What does this study add?
We describe five characteristic dermoscopic patterns: blue nevus-like, nevus-like, vascular, angioma-like and unspecific patterns to help classify CMMM obtaining good sensitivity and specificity between different readers.
Acknowledgments
Funding/Support
The research at the Melanoma Unit in Barcelona is partially funded by Grant 09/1393 from Fondo de Investigaciones Sanitarias, Spain; by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain; by the AGAUR 2009 SGR 1337 of the Catalan Government, Spain; by the European Commission under the 6th Framework Programme, Contract no: LSHC-CT-2006-018702 (GenoMEL); and by the National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115).
Role of the Sponsors: The sponsors had no role in the design and conduct of the study. The sponsors had also no role in the collection, analysis and interpretation of data.
We are indebted to all our patients and their families who have always collaborated with us and who are the objective of our work; our colleagues, to our nurses, Pablo Iglesias, Daniel Gabriel and M Eugenia Moliner, who work together on a daily basis and whose effort is not always reflected in research papers. We also thank Helena Kruyer for her help with text edition.
Footnotes
Duties:
Design of the study: J.M. and S.P.
Inclusion of cases: C.C., J.M. and S.P.
Data collection: J.C., G.S., C.C. and S.P.
Supervision of data collection: C.C., J.M. and S.P.
Data analyses: K.O., J.M. and S.P.
Elaboration of the manuscript: J.C., K.O., J.M. and S.P.
Critical review of the manuscript: G.S., V.B. and C.C.
References
- 1.Mohr P, Eggermont AM, Hauschild A, Buzaid A. Staging of cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):14–21. doi: 10.1093/annonc/mdp256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic survey. Melanoma Res. 2009;19:321–6. doi: 10.1097/CMR.0b013e32832ac775. [DOI] [PubMed] [Google Scholar]
- 3.Meier F, Will S, Ellwanger U, et al. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147:62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 4.Bono R, Giampetruzzi AR, Concolino F, et al. Dermoscopic patterns of cutaneous melanoma metastases. Melanoma Res. 2004;14:367–73. doi: 10.1097/00008390-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Brauer JA, Wriston CC, Troxel AB, et al. Characteristics associated with early and late melanoma metastases. Cancer. 2010;116:415–23. doi: 10.1002/cncr.24724. [DOI] [PubMed] [Google Scholar]
- 6.Borgstein PJ, Meijer S, van Diest PJ. Are locoregional cutaneous metastases in melanoma predictable? Ann Surg Oncol. 1999;6:315–21. doi: 10.1007/s10434-999-0315-x. [DOI] [PubMed] [Google Scholar]
- 7.Yu LL, Heenan PJ. The morphological features of locally recurrent melanoma and cutaneous metastases of melanoma. Hum Pathol. 1990;30:551–5. doi: 10.1016/s0046-8177(99)90200-9. [DOI] [PubMed] [Google Scholar]
- 8.Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129–36. doi: 10.1097/DAD.0b013e3181b34a19. [DOI] [PubMed] [Google Scholar]
- 9.Pouryazdanparast P, Newman M, Mafee M, et al. Distinguishing epithelioid blue nevus from blue nevus-like cutaneous melanoma metastasis using fluorescence in situ hybridization. Am J Surg Pathol. 2009;33:1396–400. doi: 10.1097/PAS.0b013e3181a92cbc. [DOI] [PubMed] [Google Scholar]
- 10.Braun RP, Rabinovitz HS, Oliviero M, et al. Dermoscopy of pigmented skin lesions. J Am Acad Dermatol. 2005;52:109–21. doi: 10.1016/j.jaad.2001.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–65. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 12.Schulz H. Epiluminescence microscopy features of cutaneous malignant melanoma metastases. Melanoma Res. 2000;10:273–80. [PubMed] [Google Scholar]
- 13.Jaimes N, Halpern JA, Puig S, Malvehy J, et al. Dermoscopy: An aid to detection of amelanotic cutaneous melanoma metastases. Dermatol surg. 2012 May 15; doi: 10.1111/j.1524-4725.2012.02438.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Botella-Estrada R, Sevila A, Malvehy J. Criterios dermatoscópicos de las metástasis cutáneas de melanoma. In: Malvehy J, Puig S, editors. Principios de Dermatoscopia. Barcelona: CEGE; 2009. pp. 387–92. [Google Scholar]
- 15.Malvehy J, Puig S. Principles of Dermoscopy. Barcelona: CEGE Editors; 2002. [Google Scholar]
- 16.Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679–93. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 17.Garrido-Ríos AA, Carrera C, Puig S, et al. Homogeneous blue pattern in an acral congenital melanocytic nevus. Dermatology. 2008;217:315–7. doi: 10.1159/000151442. [DOI] [PubMed] [Google Scholar]
- 18.Samimi M, Perrinaud A, Naouri M, et al. High-resolution ultrasonography assists the differential diagnosis of blue naevi and cutaneous metastases of melanoma. Br J Dermatol. 2010;163:550–6. doi: 10.1111/j.1365-2133.2010.09903.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari A, Peris K, Piccolo D, Chimenti S. Dermoscopic features of cutaneous local recurrent melanoma. J Am Acad Dermatol. 2000;43:722–4. doi: 10.1067/mjd.2000.107942. [DOI] [PubMed] [Google Scholar]
- 20.Minagawa A, Koga H, Sakaizawa K, et al. Dermoscopic and histopathological findings of polymorphous vessels in amelanotic cutaneous metastasis of pigmented cutaneous melanoma. Br J Dermatol. 2009;160:1134–6. doi: 10.1111/j.1365-2133.2009.09103.x. [DOI] [PubMed] [Google Scholar]
- 21.Pizzichetta MA, Canzonieri V, Gatti A, et al. Skin lesions in melanoma and Kaposi’s sarcoma. Case 2: Dermoscopic features of metastases from cutaneous melanoma mimicking benign nevi and primary melanoma. J Clin Oncol. 2002;20:1412–5. doi: 10.1200/JCO.2002.20.5.1412. [DOI] [PubMed] [Google Scholar]
- 22.Lestre S, Joao A, Ponte P, et al. Intraepidermal epidermotropic metastatic melanoma: a clinical and histopathological mimicker of melanoma in situ occurring in multiplicity. J Cutan Pathol. 2011;38:514–20. doi: 10.1111/j.1600-0560.2011.01694.x. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Higaki H, Yamada N, Yoshida Y, Yamamoto O. Dermoscopic and histopathological findings of epidermotropic metastatic malignant melanoma. Eu J Dermatol. 2011;21:811–3. doi: 10.1684/ejd.2011.1475. [DOI] [PubMed] [Google Scholar]