Abstract
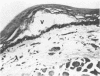
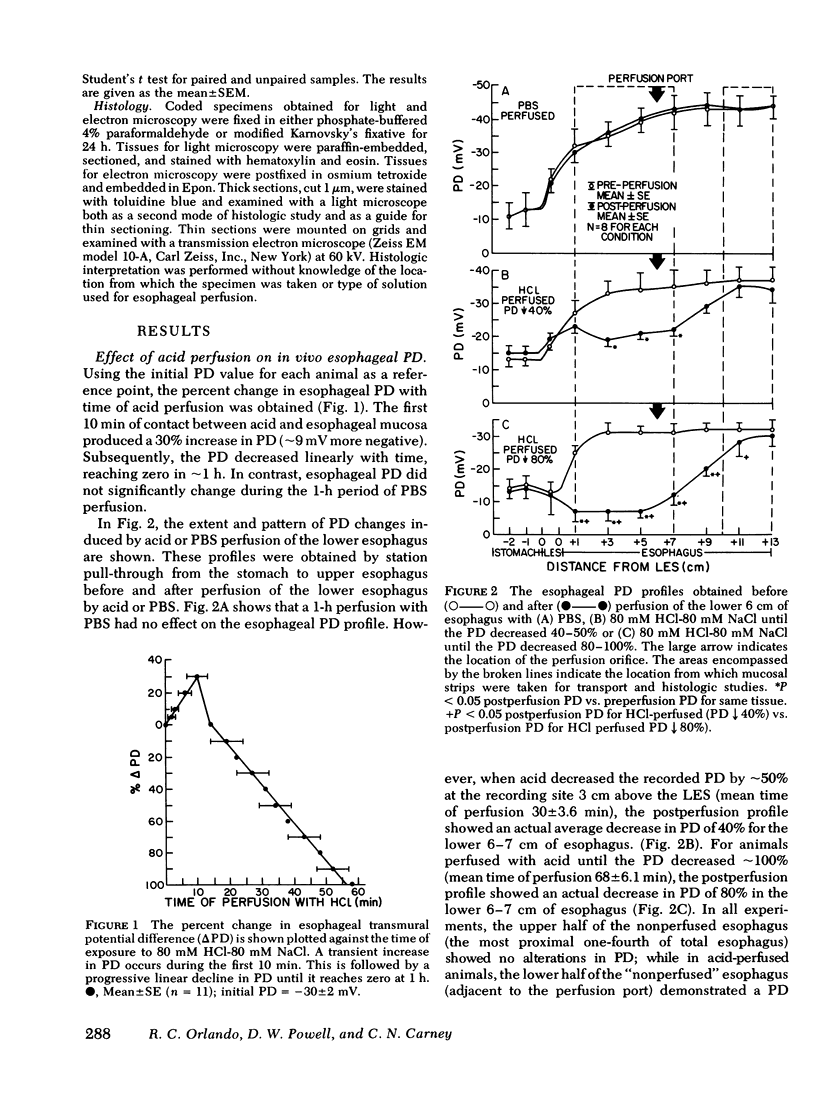
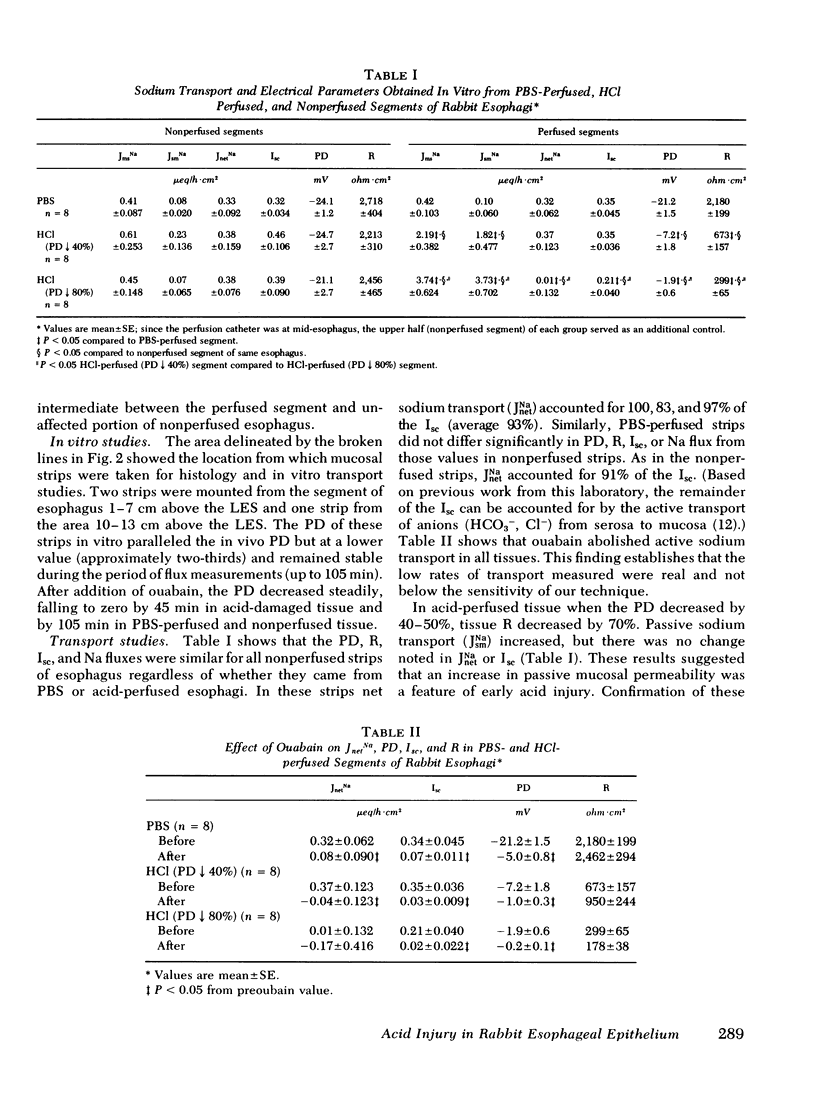
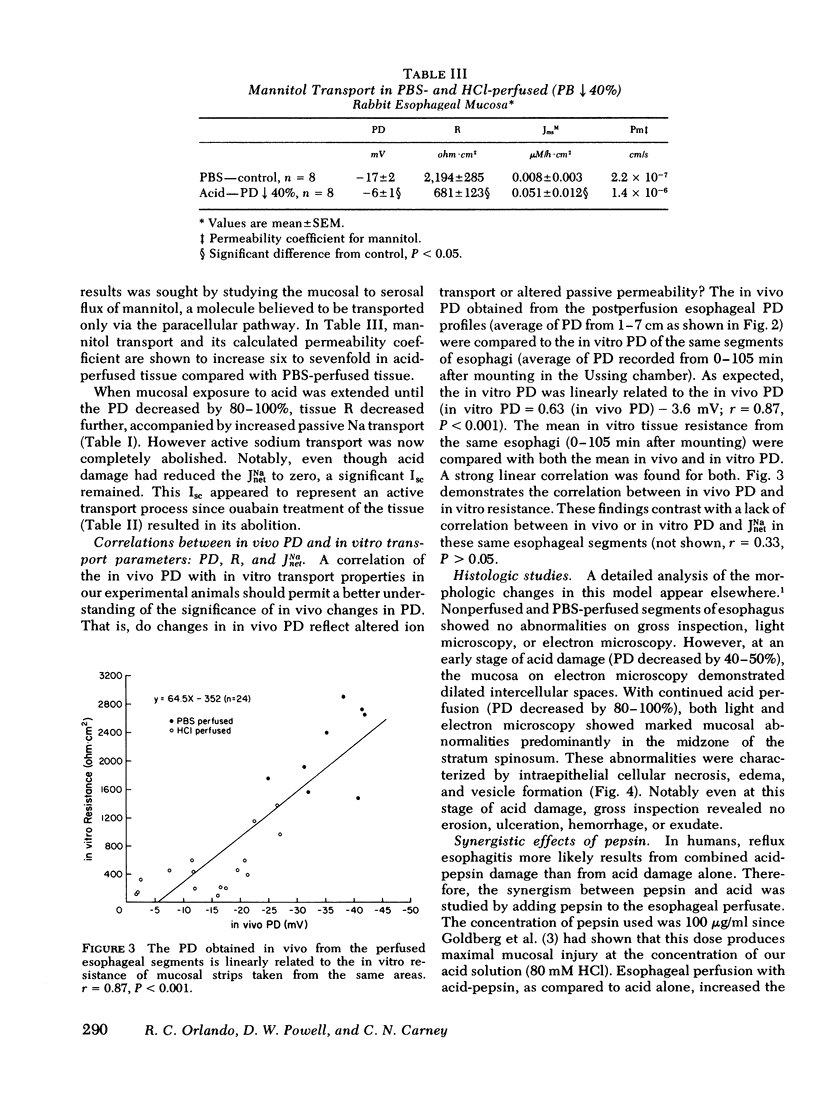
To increase our understanding of the pathophysiology of reflux esophagitis, we sought the early sequence of changes in mucosal structure and function in acutely acid-damage rabbit esophagus. Using a perfused catheter technique esophageal potential difference (PD) profiles were obtained in anesthetized rabbits before, during, and after perfusion of the lower one-half of the esophagus with phosphate-buffered saline or 80 mM NaCl. When acid perfusion reduced the lower esophageal PD by 40-50% or 80-100% of the initial values, the esophagus was removed, sectioned, and the mucosa studied with light microscopy, transmission electron microscopy, and Ussing chamber technique for evaluation of sodium and mannitol transport. The earlier stage of acid damage (PD 40-50%) was associated with reduced mucosal resistance fom 2,180 +/- 199 to 673 +/- 157 ohm cm2 and increased passive transport of sodium (0.10 +/- 0.06 to 1.82 +/- 0.48 microeq/h.cm2) and mannitol (0.008 +/- 0.003 to 0.051 +/- 0.012 microM/h.cm2) (p less than 0.05). There was no significant change in shirt circuit current (0.35 +/- 0.05 to 0.35 +/- 0.04) or net sodium transport (0.32 +/- 0.06 to 0.37 +/- 0.12) at this stage, and the only morphologic finding was dilated intercellular spaces on electron microscopy. The later stage of acid damage (PD 80-100%) exhibited a further reduction in resistance to 299 +/- 65 ohm.cm2 (p less than 0.05), a finding now accompanied by a reduction in short circuit current (0.35 +/- 0.05 to 0.21 +/- 0.04 microeq/h.cm2) and complete inhibition of net sodium transport (0.32 +/- 0.06 to 0.01 +/- 0.13) (p less than 0.05). Morphologic studies at this time revealed cellular necrosis, edema, and vesicle formation in the stratum spinosum. Both gross mucosal changes and transmural necrosis were notably absent. When esophageal perfusion was performed with a combination of acid (80 mM HCl-80 mM NaCl) and pepsin (100 microgram/ml), the morphologic and physiologic findings were essentially the same as with acid alone; however, the time of perfusion to reach either the 50 or 100% reduction in PD was shortened. The findings in this model can be explained on an initial increase in cellular and/or paracellular permeability followed by inhibition of active sodium transport. The resulting loss of osmolar regulation leads to cell necrosis in the stratum spinosum.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dodds W. J., Hogan W. J., Miller W. N. Reflux esophagitis. Am J Dig Dis. 1976 Jan;21(1):49–67. doi: 10.1007/BF01074140. [DOI] [PubMed] [Google Scholar]
- Eckardt V. F., Adami B. Esophageal transmural potential difference in patients with symptomatic gastroesophageal reflux. Klin Wochenschr. 1980 Mar 17;58(6):293–297. doi: 10.1007/BF01476571. [DOI] [PubMed] [Google Scholar]
- Elias P. M., McNutt N. S., Friend D. S. Membrane alterations during cornification of mammalian squamous epithelia: a freeze-fracture, tracer, and thin-section study. Anat Rec. 1977 Dec;189(4):577–594. doi: 10.1002/ar.1091890404. [DOI] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. FUNCTIONAL ORGANIZATION OF AMPHIBIAN SKIN. Proc Natl Acad Sci U S A. 1964 Apr;51:569–577. doi: 10.1073/pnas.51.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON D. J., SANCHEZ-PALOMERA E., SAKO Y., CLATWORTHY H. W., Jr, TOON R. W., WANGENSTEEN O. H. Studies on experimental esophagitis. Surgery. 1950 Dec;28(6):1022–1039. [PubMed] [Google Scholar]
- Fischbarg J., Whittembury G. The effect of external pH on osmotic permeability, ion and fluid transport across isolated frog skin. J Physiol. 1978 Feb;275:403–417. doi: 10.1113/jphysiol.1978.sp012197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell R. T., Stacy B. D. Effects of ruminal hyperosmolality on the ultrastructure of ruminal epithelium and their relevance to sodium transport. Q J Exp Physiol Cogn Med Sci. 1973 Oct;58(4):315–323. doi: 10.1113/expphysiol.1973.sp002225. [DOI] [PubMed] [Google Scholar]
- Goldberg H. I., Dodds W. J., Gee S., Montgomery C., Zboralske F. F. Role of acid and pepsin in acute experimental esophagitis. Gastroenterology. 1969 Feb;56(2):223–230. [PubMed] [Google Scholar]
- Harrison F. A., Keynes R. D., Rankin J. C., Zurich L. The effect of ouabain on ion transport across isolated sheep rumen epithelium. J Physiol. 1975 Aug;249(3):669–677. doi: 10.1113/jphysiol.1975.sp011036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. D., Mugashe F., Jeejeebhoy K. N., Cullen J., Szczepanski M., Boszko A., Marryatt G. The role of bile and acid in the production of esophagitis and the motor defect of esophagitis. Ann Thorac Surg. 1972 Nov;14(5):465–473. doi: 10.1016/s0003-4975(10)65257-5. [DOI] [PubMed] [Google Scholar]
- Henrikson R. C. Mechanism of sodium transport across ruminal epithelium and histochemical localization of ATPase. Exp Cell Res. 1971 Oct;68(2):456–458. doi: 10.1016/0014-4827(71)90174-1. [DOI] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Khamis B., Kennedy C., Finucane J., Doyle J. S. Transmucosal potential difference; diagnostic value in gastro-oseophageal reflux. Gut. 1978 May;19(5):396–398. doi: 10.1136/gut.19.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivilaakso E., Fromm D., Silen W. Effect of bile salts and related compounds on isolated esophageal mucosa. Surgery. 1980 Mar;87(3):280–285. [PubMed] [Google Scholar]
- LAMBERT R. Relative importance of biliary and pancreatic secretions in the genesis of esophagitis in rats. Am J Dig Dis. 1962 Nov;7:1026–1033. doi: 10.1007/BF02231905. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Diamond J. M. Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol. 1976 Aug 27;28(1):1–40. doi: 10.1007/BF01869689. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A., Erlij D., Bracho H. Localization of permeability barriers in the frog skin epithelium. J Cell Biol. 1971 Aug;50(2):277–287. doi: 10.1083/jcb.50.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. W., Ernst S. A., DiBona D. R. Localization of Na+-pump sites in frog skin. J Cell Biol. 1977 Apr;73(1):88–110. doi: 10.1083/jcb.73.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. H., Reisin I. L., Rodríguez Boulan E., Rotunno C. A., Cereijido M. Barriers to sodium movement across frog skin. J Membr Biol. 1973;11(2):99–115. doi: 10.1007/BF01869815. [DOI] [PubMed] [Google Scholar]
- Pope C. E., 2nd Pathophysiology and diagnosis of reflux esophagitis. Gastroenterology. 1976 Mar;70(3):445–454. [PubMed] [Google Scholar]
- Powell D. W., Binder H. J., Curran P. F. Electrolyte secretion by the guinea pig ileum in vitro. Am J Physiol. 1972 Sep;223(3):531–537. doi: 10.1152/ajplegacy.1972.223.3.531. [DOI] [PubMed] [Google Scholar]
- Powell D. W., Morris S. M., Boyd D. D. Water and electrolyte transport by rabbit esophagus. Am J Physiol. 1975 Aug;229(2):438–443. doi: 10.1152/ajplegacy.1975.229.2.438. [DOI] [PubMed] [Google Scholar]
- Read N. W., Fordtran J. S. The role of intraluminal junction potentials in the generation of the gastric potential difference in man. Gastroenterology. 1979 May;76(5 Pt 1):932–938. [PubMed] [Google Scholar]
- Safaie-Shirazi S., DenBesten L., Zike W. L. Effect of bile salts on the ionic permeability of the esophageal mucosa and their role in the production of esophagitis. Gastroenterology. 1975 Apr;68(4 Pt 1):728–733. [PubMed] [Google Scholar]
- Shirazi S. S., Platz C. E. Effect of alcohol on canine esophageal mucosa. J Surg Res. 1978 Oct;25(4):373–379. doi: 10.1016/0022-4804(78)90133-6. [DOI] [PubMed] [Google Scholar]
- Turner K. S., Powell D. W., Carney C. N., Orlando R. C., Bozymski E. M. Transmural electrical potential difference in the mammalian esophagus in vivo. Gastroenterology. 1978 Aug;75(2):286–291. [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- Vidins E. I., Fox J. A., Beck I. T. Transmural potential difference (PD) in the body of the esophagus in patients with esophagitis, Barrett's epithelium and carcinoma of the esophagus. Am J Dig Dis. 1971 Nov;16(11):991–999. doi: 10.1007/BF02235011. [DOI] [PubMed] [Google Scholar]
- Voûte C. L., Ussing H. H. Some morphological aspects of active sodium transport. The epithelium of the frog skin. J Cell Biol. 1968 Mar;36(3):625–638. doi: 10.1083/jcb.36.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]