Abstract
Background
Lithium is a mood stabilizer with both antidepressant and antimanic properties, though its mechanism of action is unclear. Identifying the genetic factors that influence lithium's therapeutic actions will be an important step to assist in identifying such mechanisms. We previously reported that lithium treatment of male mice has antidepressant-like effects in the C57BL/6J strain but that such effects were absent in the BALB/cJ strain.
Objectives
To assess the roles of both genetic, and non-genetic factors such as sex and non-shared environmental factors that may mediate differential behavioral responses to lithium.
Methods
Mice were treated with lithium for ten days and then tested in the forced swim test followed by lithium discontinuation and retesting to assess effects of lithium withdrawal. We also assessed effects of sex and cross-fostering on lithium response between the C57BL/6J and BALB/cJ strains, and antidepressant-like effects of lithium in the hybrid CB6F1/J strain that is derived from C57BL/6J and BALB/cJ parental strains.
Results
Neither sex nor maternal care significantly influenced the differential antidepressant-like profile of lithium. Withdrawal from lithium treatment reversed antidepressant-like effects in the C57BL/6J strain, but had no effects in BALB/cJ mice. Lithium treatment did not result in antidepressant-like effects in the CB6F1/J strain.
Conclusions
Genetic factors are likely primarily responsible for differential antidepressant-like effects of lithium in the C57BL/6J and BALB/cJ strains. Future studies identifying such genetic factors may help to elucidate the neurobiological mechanisms of lithium's therapeutic actions.
Keywords: Lithium, Depression, Bipolar disorder, Inbred strains, Forced swim test, Maternal care, Mice
Lithium is a widely used mood stabilizing drug with efficacy in the treatment of the manic phases of bipolar disorder and which is also effective in reducing suicidal behavior (Baldessarini et al. 2003; Geddes et al. 2004; Tondo et al. 2001). Lithium is also commonly used as an adjunct antidepressant and in some cases as a monotherapy agent for the treatment of depression (Bauer et al. 2010; Smoller and Finn 2003; Watanabe et al. 1975). However, the therapeutic effects of lithium are limited to only some bipolar disorder and depressed patients, and clinical and epidemiological evidence indicate that lithium responsive patients constitute a subpopulation that is probably genetically distinct from the others (Alda et al. 2005; Grof et al. 2002; McCarthy et al. 2010; Smeraldi et al. 1984). While clinical evidence overwhelmingly supports the therapeutic efficacy of lithium, understanding of the underlying genetics that predict lithium response is insufficient to assist with clinical care. Identifying the genetic determinants of lithium action in validated animal models will assist in isolating the relevant therapeutic mechanisms of lithium that can be translated to humans to help discern the genetic variations that underlie human response.
Recently, we tested male mice from a panel of mouse strains for their antidepressant-like responses to lithium (Can et al. 2011). Our results revealed that both acute and chronic administration of lithium results in antidepressant-like effects in the C57BL/6J strain. However, in the BALB/cJ strain, lithium did not have any antidepressant-like effects, as assessed by both the forced swim test (FST) and the tail suspension test (Can et al. 2011). One of our long-term goals is to conduct quantitative trait loci (QTL) studies to identify genetic loci that co segregate with lithium response. However, prior to initiation of these studies, we aimed to first address non-genetic factors that may influence differential response to lithium. We here assessed whether sex or maternal care modify antidepressant like response to lithium in the C57BL/6J and BALB/cJ mouse strains. We also report the effects of lithium withdraw in these two strains, as well as effects of lithium treatment in the C57BL/6J × BALBc/J hybrid strain CB6F1/J.
Methods
Mice
Mice of BALB/cJ, C57BL/6J, and CB6F1/J (#100007; F1 generation hybrids from breeding of BALB/cJ females and C57BL/6J males) strains seven weeks old at the time of arrival were obtained from the Jackson Laboratories (Bar Harbor, Maine). Female and male mice were separately housed as four animals per cage in an animal room with a constant temperature (22 +/− 1°C) and a 12-h light/dark cycle (lights on/off at 07:00/19:00), with free access to food and water. All experimental procedures were approved by the University of Maryland, Baltimore Animal Care and Use Committee, and were conducted in full accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Cross-fostering
11–12 week old BALB/cJ and C57BL/6J female mice (The Jackson Laboratories, Bar Harbor, ME) were mated with 11–12 week old males of the same strain. These females had been used in another experiment previously that involved the FST; however, mating began at least ten days after the completion of that experiment. All animals were sexually naïve. In each mating cage, one male was cohabitated with 3–4 females. After 12 days of cohabitation with the male, we observed females daily for signs of pregnancy for a period of one week. Pregnant females were removed from mating cages and housed individually. Individually housed females were checked in the morning and evening for parturition. Litters were cross-fostered to another dam from the opposite strain or in-fostered to another dam from the same strain within 36 hours of birth. All pups were either cross-fostered or in-fostered to a dam other than their own biological mother. Litter sizes were kept constant at between five-seven pups. Subjects were weaned from their adopted mothers at postnatal day 21 and four to five mice from the same sex were housed together. Both male and female mice were tested.
Lithium Administration
Regular food was removed from all cages and replaced with either lithium chow containing 4 g/kg LiCl or control chow (both from Custom Animal Diets LLC, Bangor, PA). In all experimental groups, animals were provided with both regular water and 0.9% saline, which was provided to reduce ion imbalances that may be caused by chronic lithium administration through food. After ten days of feeding with control or lithium chow diet, all animals were tested in the FST. A ten days treatment period was selected since we had previously shown that lithium levels and behavioral effects of lithium in the FST are present at this time point (Gould et al. 2008). In Experiment 1, mice that were treated with lithium containing food were switched to control food on the next day after the first FST. In this experiment control mice did not experience a change in their chow. Ten days later all mice were tested again in the FST. Our earlier results showed that chronic lithium administration using this paradigm results in brain lithium levels of 0.95±0.03 and 0.93±0.03 mmol/kg±SEM in the BALB/cJ and C57BL/6J mice respectively (Can et al. 2011). These levels were within the human therapeutic range (0.6–1.3 mM). Since, the CB6F1 strain was being treated with LiCl containing chow for the first time in our laboratory, we also determined brain lithium concentration levels in this strain following the end of testing using the established procedures (Can et al. 2011). Briefly, snap frozen brains were weighted and homogenized with a polytron homogenizer (Kinematica AG, Model PT-MR 2100, Littau, Switzerland) with three volumes of 0.5 N trichloroacetic acid. Lithium levels in the brain (mmol/kg, wet weight) were measured with a flame photometer (Cole-Palmer Model 2655-00, Chicago, IL, USA) (Hamburger-Bar et al. 1986). Brain lithium levels in the CB6F1/J strain (n=12) were 0.86±0.019 mmol/kg±SEM.
Forced swim test
The FST is a validated behavioral test to assess the potential efficacy of antidepressant treatments. Antidepressant medications increase the overall struggling movements and lower the total time spent immobile and floating on the surface of the water (Can et al. 2012; Hascoët and Bourin 2009; Porsolt et al. 1977). FST sessions were conducted during the first three hours of the light cycle according to established procedures (Can et al. 2012). Subjects were placed in Plexiglas cylinders (30 cm height × 20 cm diameters) filled with 15 cm of tap water at a temperature of 24 ± 1°C for six minutes and video recorded. The last four minutes of each session were scored by a trained observer who was blind to the group assignments. Mobility was defined as any movements other than those necessary to maintain the head above the water.
Statistical Analyses
All statistical analyses were performed using either GraphPad v.5.02 (San Diego, CA) or SPSS v.17 (Chicago, IL) software. We analyzed the results of Experiment 1 using a two-way repeated measures ANOVA followed by pairwise comparisons between treatment groups using Tukey's multiple comparison test. In the Experiment 2, we analyzed the data using a four-way analysis of variance (ANOVA) followed by post-hoc comparisons between treatment groups made with Tukey's multiple comparison test. In Experiment 3, we analyzed the data using a two-way ANOVA followed by Tukey's multiple comparison tests. Body weights data are provided in the Supplementary Online Information, and statistical comparisons regarding body weight data were made with unpaired t-tests. All statistical tests were conducted as two-tailed and p<0.05 was considered statistically significant.
Results
Experiment 1: Sex differences in the antidepressant-like effects of lithium
We tested whether lithium treatment has the same differential antidepressant-like effects in female mice from the C57BL/6J and BALB/cJ strains as their male counterparts. We also have tested the effects of withdrawal from lithium treatment. Female and male mice from both strains were given lithium-containing or control chow for 10 days and then tested in the FST (Figure 1). After this test, to assess withdraw effects, mice in lithium treatment groups were provided control chow identical to the control mice. The control groups did not experience any changes in their treatment. All mice were tested again in the FST ten days later. The data from these tests were analyzed separately for each strain/sex combination with a two-way repeated measures ANOVA. Since mice from each sex/strain combination were tested on a different day we have not made statistical comparisons across these variables.
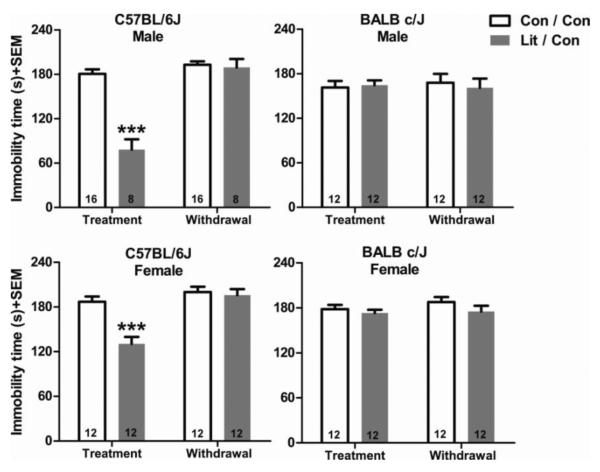
Fig. 1. Immobility time in the first FST after ten days of lithium treatment and following ten days of withdrawal in male and female C57BL/6J and BALB/cJ mice.
Con/Con refers to the groups that received control food in both phases of the experiment; Lit/Con refers to the groups that received lithium chloride containing food in the first ten days of the experiment and then received control food in the second ten days of the experiment. ***p<0.001 denotes a significant difference compared to control group of the treatment phase. Data are expressed as mean±SEM. n=8–16 per group as indicated in the lower portions of the bars.
In the male mice of the C57BL/6J strain the main effect of lithium treatment was significant (F(1,22)=29.77, p<0.001), along with a significant effect of test session F(1,22)=99.28, p<0.001 and the interaction between them (F(1,22)=63.94, p<0.001 respectively). Tukey's multiple comparison test revealed that male mice of the C57BL/6J strain that were treated with lithium spent less time immobile during the first FST compared to all the other treatment groups in both the first and second FSTs (p<0.001). There were no significant differences between any of the other treatment groups (p>0.05). We observed the same response profile in the female C57BL/6J mice. The main effect of lithium treatment was significant (F(1,22)=9.46, p<0.01), along with test session and the interaction between them (F(1,22)=50.22, p<0.001 and F(1,22)=22.23, p<0.001 respectively). As shown by Tukey's multiple comparison test, lithium treatment significantly reduced immobility (p<0.001, lithium treated group in the treatment phase compared to all the other groups), but there were no statistically significant differences between any other groups. As was the case in the male C57BL/6J mice, no significant difference was evident after the lithium treated mice were withdrawn from the treatment for ten days (p>0.05).
In the BALB/cJ mice, neither males nor in females, did we observe an effect of lithium treatment or an effect of withdrawal from lithium. In the male BALB/cJ mice, a two-way repeated ANOVA analysis revealed that none of the main effects of interactions were significant (F(1,22)<1 for all). In the female BALB/cJ mice the main effect of lithium treatment (F(1,22)=1.92, p>0.05), and the main effect of testing (F(1,22)<1), along with the interaction (F(1,22)<1) were not significant.
We consistently observed a 10–15% weight loss in the lithium treated mice regardless of strain or sex and this is in agreement with previous findings (Bersudsky et al. 2007; Can et al. 2011; Kovacsics and Gould 2010). The body weight data and associated statistical comparisons are given in the Supplementary Online Information.
Experiment 2: Effects of cross-fostering
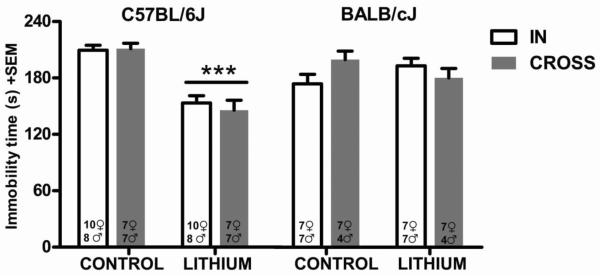
While strain differences within inbred mice are generally considered genetic in origin, they could also be due to environmental differences unique to each strain. We cross-fostered newborn pups of both the C57BL/6J and BALB/cJ strain to either a dam of the same strain (in-fostered) or to the other strain (cross-fostered). We did not make an attempt to determine the sex of pups at the time of fostering. Available male and female mice were randomly assigned to control or lithium chow as adults in equal numbers, and following ten days of treatment were tested in the FST. We analyzed the effects of strain, sex, treatment with lithium, and fostering type on the immobility time in the FST with a four-way ANOVA. The main effect of lithium treatment was statistically significant (F(1,98)=22.47, p < 0.001). The main effects of strain (F(1,98)=1.39, p=0.24), sex (F(1,98)=0.04, p=0.83), or fostering type (F(1,98)=0.16, p=0.68) were not statistically significant. Among interaction effects, strain by treatment was statistically significant (F(1,98)=26.95, p< 0.001). None of the other possible interactions among these four factors were significant. Tukey's multiple comparison test revealed that lithium treatment significantly reduced immobility time in the FST of the C57BL/6J strain in both in-fostered and cross-fostered mice (p<0.001 for all), but no other comparisons were statistically significant. In the BALB/cJ strain, there was no significant effect of lithium on immobility time (p > 0.05 for all possible pairwise comparisons).
Experiment 3: Effects of lithium in the F1 hybrid progeny of BALB/cJ and C57BL/6J strains
We compared the effects of ten days lithium treatment on FST performance in male mice from the CB6F1/J and C57Bl/6J strains. A two-way ANOVA revealed that the main effect of strain was not statistically significant (F(1,44)=2.65, p=0.11). However, the main effect of treatment was statistically significant (F(1,44)=24.67, p < 0.001). The interaction between strain and treatment was statistically significant as well (F(1,44)=7.78, p < 0.01). Tukey's multiple comparison test revealed that lithium treated C57BL/6J mice had significantly less immobility compared to both C57BL/6J and CB6F1/J control groups (p < 0.001). However, the lithium treated CB6F1/J mice did not differ from C57BL/6J and CB6F1/J control groups (p > 0.05) (Figure 3). While we found that there was no significant difference in immobility times between the control chow treated groups from each strain (p > 0.05) the lithium treated CB6F1/J mice manifested less mobility compared to the lithium treated C57BL/6J mice (p < 0.05).
Fig. 3. Immobility time in the FST after ten days of lithium treatment in male C57BL/6J and CBF1/J mice.
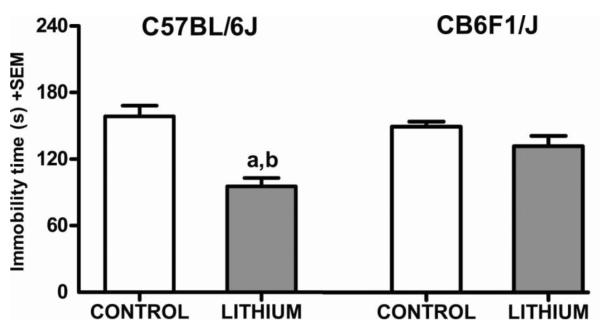
“a” denotes a significant difference compared to the C57BL/6J and CB6F1/J control groups, p<0.001. “b” denotes a significant difference compared to the CB6F1/J lithium group, p<0.05. Data are expressed as mean±SEM. n=12 per group.
Discussion
Inbred strains of rodents are commonly utilized to compare effects of biological and genetic variations in behavioral models. These behavioral patterns present in different mouse strains have long assisted in the characterization of essential neural processes that are influenced by strain-dependent inheritable traits. Comparing strains is also useful to help elucidate the molecular, cellular, and in particular genetic underpinnings that underlie response to pharmacological agents. Such comparisons been successfully used to assess the responses to various antidepressant treatments (Bai et al. 2001; Can et al. 2011; Cervo et al. 2005; Crowley et al. 2005; David et al. 2003; Dubocovich et al. 1990; Dulawa et al. 2004; Liu and Gershenfeld 2001; Lucki et al. 2001; Nomura et al. 1991; Porsolt et al. 1978; Ripoll et al. 2003; van der Heyden et al. 1987), as well as the antimanic-like effects of lithium (Gould et al. 2007; Gould et al. 2001; Hamburger-Bar et al. 1986), and effects of lithium on pre-pulse inhibition (O'Neill et al. 2003; Ong et al. 2005) in the past. However, identification of inbred strains that differ in terms of the behavioral effects of a particular drug treatment is only the first step toward elucidating underlying genetic mechanisms. After identifying the initial mouse strains, the approximate locations of responsible genetic factors in the mouse genome can be identified by using QTL methods which requires interbreeding classically between the original strains, and testing the F2 progeny of these strains for the trait under study.
Previously, we tested a panel of both inbred and outbred mouse strains in the FST and the tail suspension tests in order to identify strains that manifest differential antidepressant-like responses to lithium treatment after acute systemic, chronic, and intracerebroventricular administration (Can et al. 2011). We found that while lithium did not have an antidepressant-like effect in the BALB/cJ strain, it did result in antidepressant-like effects in the C57BL/6J strain across all routes of administration. In this previous report we also showed that three weeks of chronic lithium treatment did not significantly affect the general locomotor behavior in a panel of inbred mouse strains including C57BL/6J and BALB/cJ (Can et al. 2011). This previous report was limited only to male mice. However, in the event that sex differences or non-shared environmental differences such as maternal care affect the trait that is tested, the results of genetic strain studies can be difficult to interpret. In addition to the possibility of an interaction between the genetics and sex, the sex differences in baseline performance levels in behavioral tests may also lead to divergent results in terms of responses to the pharmacological agent under study (Simpson and Kelly 2012).
In order to address these issues, we tested both male and female mice from the BALB/cJ and C57BL/6J strains following lithium treatment in the FST. Our results, in addition to replicating our earlier findings with male mice, revealed that the behavioral responses to lithium as assessed in the FST were similarly divergent in female BALB/cJ and C57BL/6J mice. C57BL/6J females manifested an antidepressant-like response to lithium while BALB/cJ females did not. Thus, sex did not alter the antidepressant-like responses to lithium in these two strains. Further, we tested the effect of withdrawal from chronic lithium treatment. The results of a number of clinical studies indicate that withdrawal from lithium treatment exacerbates symptoms in bipolar disorder patient populations (Baldessarini and Tondo 1998; Davis et al. 1999; Margo and McMahon 1982; Moncrieff 1995; Suppes et al. 1991). By testing mice during and after the discontinuation of lithium treatment, our studies revealed that antidepressant-like effect of lithium are fully reversible in C57BL/6J mice, and that the BALB/cJ mice do not manifest any changes in FST behavior following withdraw of lithium treatment.
In a second experiment, we investigated the influence of maternal care on the later adult behavioral response to lithium treatment. There are well-documented differences in terms of quality of maternal care between the BALB/cJ and C57BL/6J strain dams. The dams of the BALB/c strain manifest fewer maternal behaviors such as nursing, licking, and nest building compared to other inbred strains including the C57BL/6 strain (Shoji and Kato 2006). Since the quality of maternal care can modify the behavioral phenotype of pups later in adult life, these differences between maternal care provided by the BALB/cJ and C57BL/6J dams to their pups is an important variable that has the potential to effect the behaviors of their offspring when adults (Brodkin 2007; Francis et al. 2003; Priebe et al. 2005; Sankoorikal et al. 2006). For example, while BALB/c mice manifest higher levels of anxiety-like behaviors and stress reactivity compared to C57BL/6 mice (Anisman et al. 2001; Lepicard et al. 2000), these differences are smaller if BALB/c pups are cross-fostered to C57BL/6 dams (Caldji et al. 2004). The higher stress reactivity of the BALB/c strain, thus, can be transferred to the next generation not only by shared genetics but also through the differences in the quality of maternal care (Calatayud and Belzung 2001). Our results indicate that lithium treatment had an antidepressant-like effect in the C57BL/6J mice as assessed in the FST regardless of the strain of the dam providing maternal care. Similarly, we did not observe a significant effect of cross-fostering on lithium response in BALB/cJ mice. These results suggest that the antidepressant-like effect of lithium is dependent on genetic factors and not affected by differences in maternal care between the two strains. In addition to the postnatal environment, it is possible that strain differences in prenatal conditions can also lead to changes in adult behaviors. A previous report did not show a robust effect of the differences in the intrauterine environments of C57BL/6 and BALB/cJ dams on various anxiety-like behaviors (Francis et al. 2003). However, we cannot exclude the possibility that depression-like behaviors and manifesting an antidepressant-like behavioral response to lithium might be affected by intrauterine environment in our experimental design.
We also compared behavioral responses to lithium between mice from the C57BL/6J and CB6F1/J (derived from interbreeding of BALB/cJ females and C57BL/6J males) strains. Like their parent strains, individuals from the F1 generation of two inbred strains are also genetically identical. But unlike inbred mouse strains, F1 hybrids, such as the CB6F1/J, are heterozygous at all genetic loci where the parent strains have different alleles. However, it is not possible estimate the behavioral phenotype of F1 generation from what is known of the parental strains if the trait under study is controlled by multigenic factors (Logue et al. 1997). In a previous study, the antidepressant-like effects of citalopram in an F1 hybrid strain was found be intermediate between as of those parent stains (Crowley et al. 2006). Similarly, previous F1 hybrid studies showed a variety of behavioral phenotypes, such as shock avoidance conditioning, spatial learning, motor activity, are controlled by multigenic factors rather than a dominant single-gene mechanism (Festing 1974; Messeri 1972; Royce 1971). We hypothesized that the antidepressant-like effects of lithium in the CB6F1/J strain would be present, but less pronounced than what we observed in the C57BL/6J mice suggesting the effect of multigenic factors determining the antidepressant-like efficacy of lithium. Lithium treatment did not lead to a statistically significant change in the immobility time in the CB6F1/J strain. Our results revealed that baseline immobility times in the FST did not differ significantly between these two strains. However, the comparison of groups treated with lithium indicated that lithium treatment lowered the immobility time in the C57BL/6J strain to a significantly greater extent than the CB6F1/J hybrids. This shows that the lack of antidepressant-like effect of lithium in the CB6F1/J strain was not related to the baseline differences between the strains but rather to the lack of responding to this effect of lithium. This type of complex multigenic influence on behavior thus suggests the value of a genetic study to dissect those putative multigenic factors determining the differential antidepressant-like efficacy of lithium in these two mouse strains.
Taken together, these results demonstrate that the antidepressant-like efficacy of lithium in two strains that manifest distinct behavioral differences in the FST in response to lithium treatment is not determined by sex or maternal care. We have confirmed our previous finding that chronic lithium treatment does not result in a FST antidepressant-like response in BALB/cJ mice. However, recent studies indicate that lithium has effects to increase neurogenesis in stressed in BALB/cJ mice, and also augments the effects of other antidepressants in this strain (O'Leary et al. 2012; O'Leary et al. 2013). It should be noted that these studies used a different substrain of BALB/c strain and there might be important differences between substrains. Indeed, a variety of behavioral differences were detected between mice of the same strain that were acquired from different breeders in the past (Crawley et al. 1997; Juetten and Einat 2012; Stiedl et al. 1999). Overall our results suggest that the differential efficacy of lithium in the BALB/cJ and C57BL/6J strains is genetically mediated. These may be identified using QTL or other approaches. The results of such a study may aid in understanding of the neurobiological mechanisms by which lithium exerts its effects.
Supplementary Material
Fig. 2. Immobility time in the FST after ten days of lithium treatment in BALB/cJ and C57BL/6J mice cross-fostered to a dam of the same strain (in-fostered) or to the other strain (cross-fostered).
***p<0.001 denotes a significant difference compared to the C57BL/6J control groups. Data are expressed as mean±SEM. n=11–18 per group. In each bar, the female and male numbers for that particular group are indicated.
Acknowledgements
This study was supported by the grants MH084043 and MH091816 to TDG.
References
- Alda M, Grof P, Rouleau GA, Turecki G, Young LT. Investigating responders to lithium prophylaxis as a strategy for mapping susceptibility genes for bipolar disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:1038–1045. doi: 10.1016/j.pnpbp.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. Psychogenic, Neurogenic, and Systemic Stressor Effects on Plasma Corticosterone and Behavior: Mouse Strain-Dependent Outcomes. Behav Neurosci. 2001;115:443–454. [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacology Biochemistry and Behavior. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Tondo L. Recurrence Risk in Bipolar Manic-Depressive Disorders after Discontinuing Lithium Maintenance Treatment: An Overview. Clinical Drug Investigation. 1998;15:337–351. doi: 10.2165/00044011-199815040-00010. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry. 2003;64(Suppl 5):44–52. [PubMed] [Google Scholar]
- Bauer M, Adli M, Bschor T, Pilhatsch M, Pfennig A, Sasse J, Schmid R, Lewitzka U. Lithium's Emerging Role in the Treatment of Refractory Major Depressive Episodes: Augmentation of Antidepressants. Neuropsychobiology. 2010;62:36–42. doi: 10.1159/000314308. [DOI] [PubMed] [Google Scholar]
- Bersudsky Y, Shaldubina A, Belmaker RH. Lithium's effect in forced-swim test is blood level dependent but not dependent on weight loss. Behav Pharmacol. 2007;18:77–80. doi: 10.1097/FBP.0b013e32801416ed. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: Low sociability and other phenotypes that may be relevant to autism. Behavioural Brain Research. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Calatayud F, Belzung C. Emotional reactivity in mice, a case of nongenetic heredity? Physiology & Behavior. 2001;74:355–362. doi: 10.1016/s0031-9384(01)00566-2. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal Behavior Regulates Benzodiazepine/GABAA Receptor Subunit Expression in Brain Regions Associated with Fear in BALB/c and C57BL/6 Mice. Neuropsychopharmacology. 2004;29:1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- Can A, Blackwell RA, Piantadosi SC, Dao DT, O'Donnell KC, Gould TD. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes, Brain and Behavior. 2011;10:434–443. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The Mouse Forced Swim Test. Journal of Visualized Experiments. 2012 doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Canetta A, Calcagno E, Burbassi S, Sacchetti G, Caccia S, Fracasso C, Albani D, Forloni G, Invernizzi RW. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–72. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crowley J, Blendy J, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology. 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. Pharmacogenomic Evaluation of the Antidepressant Citalopram in the Mouse Tail Suspension Test. Neuropsychopharmacology. 2006;31:2433–2442. doi: 10.1038/sj.npp.1301065. [DOI] [PubMed] [Google Scholar]
- David DJ, Renard CE, Jolliet P, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology (Berl) 2003;166:373–82. doi: 10.1007/s00213-002-1335-4. [DOI] [PubMed] [Google Scholar]
- Davis JM, Janicak PG, Hogan DM. Mood stabilizers in the prevention of recurrent affective disorders: a meta-analysis. Acta Psychiatrica Scandinavica. 1999;100:406–417. doi: 10.1111/j.1600-0447.1999.tb10890.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Mogilnicka E, Areso PM. Antidepressant-like activity of the melatonin receptor antagonist, luzindole (N-0774), in the mouse behavioral despair test. Eur J Pharmacol. 1990;182:313–25. doi: 10.1016/0014-2999(90)90290-m. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–30. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Festing MFW. Water Escape Learning in Mice. III. A Diallel Study. Behavior Genetics. 1974;4:111–124. doi: 10.1007/BF01065752. [DOI] [PubMed] [Google Scholar]
- Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat Neurosci. 2003;6:445–6. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long-Term Lithium Therapy for Bipolar Disorder: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Psychiatry. 2004;161:217–222. doi: 10.1176/appi.ajp.161.2.217. [DOI] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–87. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–33. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium's reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, O'Donovan C, Alda M. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63:942–7. doi: 10.4088/jcp.v63n1013. [DOI] [PubMed] [Google Scholar]
- Hamburger-Bar R, Robert M, Newman M, Belmaker RH. Interstrain correlation between behavioural effects of lithium and effects on cortical cyclic AMP. Pharmacol Biochem Behav. 1986;24:9–13. doi: 10.1016/0091-3057(86)90036-5. [DOI] [PubMed] [Google Scholar]
- Hascoët M, Bourin M. The Forced Swimming Test in Mice: A Suitable Model to Study Antidepressants. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice (Neuromethods) Humana Press; 2009. pp. 85–118. [Google Scholar]
- Juetten J, Einat H. Behavioral differences in black Swiss mice from separate colonies: implications for modeling domains of mania. Behav Pharmacol. 2012;23:211–214. doi: 10.1097/FBP.0b013e32834f9e4e. 10.1097/FBP.0b013e32834f9e4e. [DOI] [PubMed] [Google Scholar]
- Kovacsics CE, Gould TD. Shock-induced aggression in mice is modified by lithium. Pharmacology Biochemistry and Behavior. 2010;94:380–386. doi: 10.1016/j.pbb.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacology Biochemistry and Behavior. 2000;67:739–748. doi: 10.1016/s0091-3057(00)00419-6. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biological Psychiatry. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: Implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–22. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Margo A, McMahon P. Lithium withdrawal triggers psychosis. Brit J Psychiat. 1982;141:407–410. doi: 10.1192/bjp.141.4.407. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Leckband SG, Kelsoe JR. Pharmacogenetics of lithium response in bipolar disorder. Pharmacogenomics. 2010;11:1439–1465. doi: 10.2217/pgs.10.127. [DOI] [PubMed] [Google Scholar]
- Messeri P, Oliverio A, Bovet D. Relations between avoidance and activity: a diallel study in mice. Behavioral Biology. 1972;7:733–742. doi: 10.1016/s0091-6773(72)80080-4. [DOI] [PubMed] [Google Scholar]
- Moncrieff J. Lithium revisited. A re-examination of the placebo-controlled trials of lithium prophylaxis in manic-depressive disorder. The British Journal of Psychiatry. 1995;167:569–73. doi: 10.1192/bjp.167.5.569. [DOI] [PubMed] [Google Scholar]
- Nomura S, Okada H, Naruse R, Yamaoka K. The tail suspension test for screening antidepressant drugs: comparison of movement in ICR and NMRI mice. Jpn J Psychiatry Neurol. 1991;45:113–4. [PubMed] [Google Scholar]
- O'Neill HC, Schmitt MP, Stevens KE. Lithium alters measures of auditory gating in two strains of mice. Biol Psychiatry. 2003;54:847–53. doi: 10.1016/s0006-3223(03)00184-7. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, O'Connor RM, Cryan JF. Lithium-induced effects on adult hippocampal neurogenesis are topographically segregated along the dorso-ventral axis of stressed mice. Neuropharmacology. 2012;62:247–255. doi: 10.1016/j.neuropharm.2011.07.015. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, Zandy S, Dinan TG, Cryan JF. Lithium augmentation of the effects of desipramine in a mouse model of treatment-resistant depression: A role for hippocampal cell proliferation. Neuroscience. 2013;228:36–46. doi: 10.1016/j.neuroscience.2012.09.072. [DOI] [PubMed] [Google Scholar]
- Ong JC, Brody SA, Large CH, Geyer MA. An investigation of the efficacy of mood stabilizers in rodent models of prepulse inhibition. J Pharmacol Exp Ther. 2005;315:1163–71. doi: 10.1124/jpet.105.090845. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–4. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Priebe K, Brake WG, Romeo RD, Sisti HM, Mueller A, McEwen BS, Francis DD. Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: A cross-fostering study. Developmental Psychobiology. 2005;47:398–407. doi: 10.1002/dev.20098. [DOI] [PubMed] [Google Scholar]
- Ripoll N, David DJP, Dailly E, Hascoët M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behavioural Brain Research. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Royce JR, Yeudall LT, Poley W. Diallel analysis of avoidance conditioning in inbred strains of mice. Journal of Comparative and Physiological Psychology. 1971;76:353–358. doi: 10.1037/h0031387. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A Mouse Model System for Genetic Analysis of Sociability: C57BL/6J Versus BALB/cJ Inbred Mouse Strains. Biological Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Shoji H, Kato K. Maternal behavior of primiparous females in inbred strains of mice: A detailed descriptive analysis. Physiology & Behavior. 2006;89:320–328. doi: 10.1016/j.physbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behavioural Brain Research. 2012 doi: 10.1016/j.bbr.2011.12.036. epub Jan, 2012. [DOI] [PubMed] [Google Scholar]
- Smeraldi E, Petroccione A, Gasperini M, Macciardi F, Orsini A, Kidd KK. Outcomes on lithium treatment as a tool for genetic studies in affective disorders. Journal of Affective Disorders. 1984;6:139–151. doi: 10.1016/0165-0327(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Smoller J, W., Finn C, T. Family, twin, and adoption studies of bipolar disorder. American Journal of Medical Genetics. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Radulovic J, Lohmann R, Birkenfeld K, Palve M, Kammermeier J, Sananbenesi F, Spiess J. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behavioural Brain Research. 1999;104:1–12. doi: 10.1016/s0166-4328(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Suppes T, Baldessarini RJ, Faedda GL, Tohen M. Risk of Recurrence Following Discontinuation of Lithium Treatment in Bipolar Disorder. Arch Gen Psychiatry. 1991;48:1082–1088. doi: 10.1001/archpsyc.1991.01810360046007. [DOI] [PubMed] [Google Scholar]
- Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand. 2001;104:163–72. doi: 10.1034/j.1600-0447.2001.00464.x. [DOI] [PubMed] [Google Scholar]
- van der Heyden JA, Molewijk E, Olivier B. Strain differences in response to drugs in the tail suspension test for antidepressant activity. Psychopharmacology (Berl) 1987;92:127–30. doi: 10.1007/BF00215493. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Ishino H, Otsuki S. Double-Blind Comparison of Lithium Carbonate and Imipramine in Treatment of Depression. Arch Gen Psychiatry. 1975;32:659–668. doi: 10.1001/archpsyc.1975.01760230125010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.