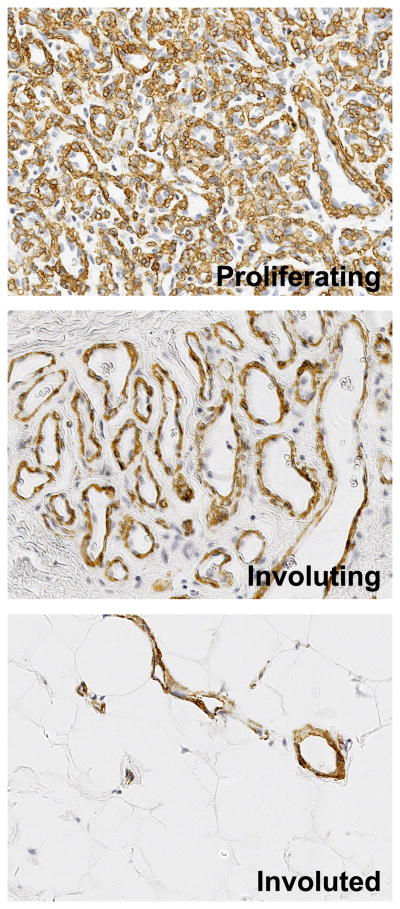
Hemangioma is a vascular tumor of infancy that is well-known for its rapid growth during the first weeks to months of a child’s life followed by a spontaneous but slow involution. During the proliferative phase, the vessels are disorganized and composed of immature endothelial cells but are not leaky 1, perhaps due to the abundance of α-smooth muscle actin (α-SMA)-positive perivascular that circumscribe the vessels (Figure 1). When the tumor involutes, the vessels mature and enlarge but are reduced in number. Fat, fibroblasts and connective tissue replace the vascular tissue, with few large feeding and draining vessels evident.
Figure 1.
α-Smooth muscle actin (α-SMA) highlights vessel maturation and regression during the life cycle of IH. Paraffin-embedded sections from proliferating, involuting and involuted phase IH were immunostained with anti-α-SMA, images taken at 40X.
Both angiogenesis 2 and vasculogenesis 3 have been proposed as mechanisms contributing to the neovascularization in hemangioma tumors. Angiogenesis is defined as the growth of new vessels from pre-existing vessels, requiring degradation of the basement membrane, migration of endothelial cells and tubulogenesis, followed by recruitment of perivascular cells. Vasculogenesis is the de novo formation of blood vessels from stem or progenitor cells. In recent years, several of the “building blocks”, the cells comprising the hemangioma, have been isolated. Among them are hemangioma progenitor/stem cells (HemSC), endothelial cells (HemEC) and pericytes (HemPericytes). This review will focus on these cell types, as well as molecular pathways within these cells that have been implicated in driving the pathogenesis of IH.
Cellular Components of IH
Stem Cells
The concept that IH arises from a dysregulated differentiation of embryonic cells has been considered for several decades4–6. Further, Smoller and Apfelberg speculated that primitive cells within interstitial regions of the tumor are capable of differentiating into endothelial cells and pericytes7. In 2008, our group isolated a primitive mesenchymal cell with these properties from proliferating phase IH 8 using anti-CD133-coated magnetic beads (CD133 is a cell surface membrane glycoprotein expressed on many types of human stem and progenitor cells.) The CD133+ cells are rare, comprising between 0.1–1% of the cells in proliferating phase IH. We call the CD133-selected cells hemangioma stem cells (HemSC) based on their ability to self-renew and undergo multi-lineage differentiation, two essential properties of stem cells. When implanted sub-cutaneously into immune-deficient (nude) mice, the HemSC form GLUT1+ vessels (a specific marker of IH) within 7–14 days. Using Green Fluorescent Protein (GFP)-labeled HemSC we showed that the cells differentiate into endothelium, adipocytes 8 and into pericytes9 in mice and in culture dishes. Two other groups have isolated HemSC using similar techniques and have reported similar vasculogenic properties10,11. Others have found evidence for primitive mesodermal cells in IH 12–14. That HemSC form vessels de novo strongly implicates vasculogenesis as an important mechanism underlying hemangioma-genesis15.
Endothelial Cells
In the proliferative phase of hemangioma, the vessels are small with lumens that are sometimes difficult to see by histology. The endothelium is plump and appears metabolically active, suggesting an immature phenotype 16. North and colleagues showed that glucose transporter-1 (GLUT1) is consistently expressed on hemangioma endothelium, and indeed provides a definitive immunohistochemical test for distinguishing IH from other types of vascular tumors and vascular malformations17 Beginning in 1982 18 Mulliken and colleagues showed that hemangioma-derived endothelial cells (HemEC) proliferate readily in the culture dish and form capillary-like tubes – a phenomenon called “in vitro angiogenesis” 19. Subsequent in vitro studies revealed that HemEC resemble fetal endothelial cells more than neonatal endothelial cells20. The HemEC and fetal endothelial cells displayed a spindle-shaped morphology and the endothelial adhesion molecule CD31/PECAM-1 was detected on intracellular membranes, suggesting it had not reach the cell surface, where it resides in mature endothelium. Boye and colleagues provided evidence that HemEC are clonal, suggesting they arise from a common precursor21. The contribution of HemEC to the total non-hematapoietic (CD45-) cell population in proliferating phase hemangioma ranges from 24% when VEGFR-2/KDR is used to quantify endothelial cells 22 and up to 30% when a cocktail of antibodies against endothelial markers (CD31, VE-cadherin, CD34 and VEGFR-2) are used to quantify the endothelial compartment (Lan Huang, unpublished data). A small percentage (0.1–2%) of these endothelial cells co-express the human stem cell marker CD133, suggesting that the endothelial cells may be transitioning from an immature to mature phenotype22.
Pericytes
The perivascular cells surrounding the nascent vessels in the proliferative phase express the pericyte markers α-SMA23, neural glial antigen-2 (NG2), platelet-derived growth factor receptor-β (PDGFRβ), calponin, and smooth muscle myosin heavy chain9. NOTCH3, typically associated with smooth muscle cells, is also detected in the perivascular layer24, with colocalized expression of the NOTCH target gene HEYL15. Thus - the phenotype of the perivascular cells in hemangioma is typical of both pericytes and smooth muscle cells. They have also been shown to have characteristics of mesenchymal stem cells 25. For simplicity, we will refer to the perivascular cells in infantile hemangioma as pericytes.
The pericytes are abundant in the proliferating phase and appear to undergo a maturation process concurrently with the endothelial cells (Figure 1). Recently, pericytes were isolated from proliferating phase (n=4) and involuting phase (n=4) IH specimens from different patients26. In vitro, the hemangioma-derived pericytes were found to express all of the markers detected in cells surrounding hemangioma vessels in histological sections(e.g. NG-2, PDGFRβ, calponin, αSMA, NOTCH3), consistently over several in vitro passages. When hemangioma pericytes were combined with endothelial cells and implanted in mice, the cells assembled into vessels that connected with murine vessels within 7 days. When compared to normal human pericytes isolated from retina or placenta, hemangioma pericytes proliferated more rapidly, expressed more VEGF-A, but expressed reduced levels of angiopoietin-1 (ANGPT1). In co-culture, hemangioma pericytes showed a reduced ability to suppress the proliferation and migration of normal human endothelial cells. Taken together, the increased VEGF-A, decreased ANGPT1, increased proliferation, increased vessel formation in vivo, and decreased ability to suppress proliferation and migration of endothelial cells indicates that hemangioma pericytes are pro-angiogenic 26.
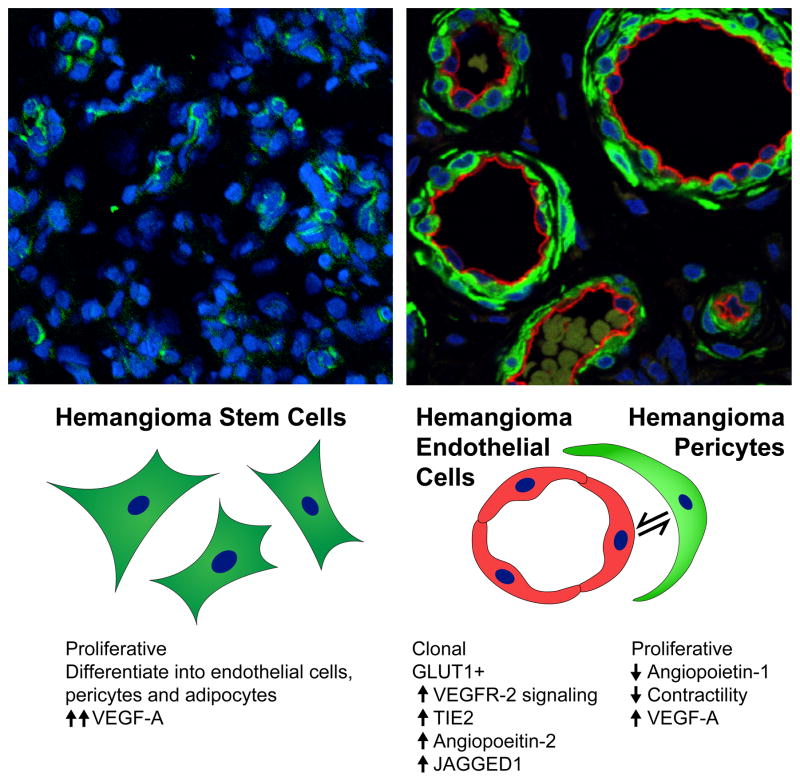
Figure 2 depicts the key cellular features of HemSC, HemEC, and HemPericytes.
Figure 2.
Cellular Components of IH. Left panel shows a proliferative phase IH tumor section stained for the human stem/progenitor cell marker CD133 (CD133+ cells, green; nuclei, stained blue). Staining and image provided by Dr. Arnaud Picard. CD133+ cells are sparse and not associated with well-formed luminal structures. Right panel shows IH tumor section double-stained for the endothelial marker CD31 (red) and the smooth muscle marker calponin (green). Nuclei are stained blue with DAPI. Lumens are lined with plump endothelial cells surrounded by perivascular cells. Staining and image provided by Dr. Elisa Boscolo. The schematic below depicts 1) the mesenchymal morphology of HemSC and 2) the interaction between HemEC and HemPericytes and 3) cellular and molecular features of each cell type.
Mast cells
Mast cells are present in IH27,28. Their number predominates in the early to middle involuting phase, whereas lower numbers are seen in the proliferative and the involuted phases1. This difference has led to the hypothesis that mast cells play a role in the regression of IH. However, little is known about these cells. Moreover, it has recently been demonstrated that mast cells are present in comparable numbers in various skin tumors, including basal cell carcinoma, squamous cell carcinoma, melanomas and nevi29. Accordingly, the unique role of mast cells in IH requires further study.
Molecular Basis of IH
Genetics
In contrast to other vascular anomalies, in which a germline or somatic mutations have been identified as a cause 30, the etiology of hemangioma of infancy still remains obscure. It is likely that the origin of IH is multi-factorial, with genetic factors being part of the contributing triggers. In most cases, IH are sporadic. However, autosomal dominant inheritance pattern and a linkage to 5q have been reported in few families 31,11. Recently, in a study of large population database, a 2-fold increased relative risk for hemangiomas among siblings of an affected proband has been shown, further supporting the hypothesis of a genetic cause as a contributing factor32. Somatic mutations leading to uncontrolled proliferation of hemangioma cells have been also proposed. In support of this theory, a clonality of ECs from hemangioma lesions have been shown on a small set of IH, using an X-chromosome inactivation assay21. Evidence for clonality was also found in cells from hemangioma tissue sections, supporting the somatic mutation hypothesis33. Mutations in the integrin-like receptor Tumor Endothelial Marker 8 (TEM8) and in VEGFR2 were identified in a small subset of HemEC and corresponding blood sample from IH patients, using a targeted candidate gene approach 34, identifying these as germline, potentially “risk-factor” mutations, that may contribute to IH.
The existence of IH as part of syndromes such as PHACE (posterior fossa abnormalities, infantile hemangioma, arterial abnormalities, cardiac anomalies, and eye colobomas) 35 or SACRAL (spinal dysraphism, anogenital, cutaneous, renal and urologic anomalies, associated with an angioma of lumbosacral localization) 36 also suggests a germline or somatic mutation. No specific mutation was identified for these syndromes, however genomic copy number variation (CNV) has been found in PHACE syndrome37.
VEGF and VEGF-receptors
Several signaling pathways have been linked with IH pathogenesis, with the VEGF (Vascular endothelial growth factor A) pathway being the key one. VEGF-A is a master regulator of angiogenesis and vasculogenesis38. It is present at higher levels in proliferating phase compared to the involuting phase of IH 1,39,40,41,42 and its level in the serum of IH patients is decreased following systemic steroid therapy 43. The high expression of VEGF might be related to hypoxia, as increased hypoxia inducible factor-1α (HIF-1α) stabilization was reported in patients with proliferating IH 40. We recently found that the corticosteroids dramatically down-regulate VEGF-A secretion by hemangioma stem cells (HemSC). Furthermore, silencing the expression of VEGF-A or VEGF receptor 1 (VEGFR-1) in HemSC by short hairpin RNA (shRNA) was sufficient to block blood vessel formation in vivo 41,44. In contrast, HemEC express and secrete very little VEGF and these low levels are not affected by corticosteroid treatment.
VEGF-A binds to three tyrosine kinase receptors, VEGFR-1, VEGFR-2 and VEGFR-3. VEGFR-2 appears to mediate almost all of the known angiogenic responses to VEGF-A. In contrast, the function and signaling of VEGFR-1, which is present on endothelial and non-endothelial cells, is less understood. VEGFR-1 is considered to be a VEGF-A trap, by virtue of its high binding affinity for VEGF and its relatively low kinase activity45. HemEC46, as well as hemangioma specimens47, express relatively low levels of VEGFR-1. This low expression (i.e., fewer “VEGF traps”) results in increased VEGF-dependent activation of VEGFR-2 and downstream signaling pathways. In summary, several lines of research point to a critical role for VEGF and its receptors in the pathological vasculogenesis and angiogenesis in IH.
Angiopoietins and TIE2
Angiopoietin-1 (ANGPT1) and angiopoietin-2 (ANGPT2) signal through the endothelial membrane receptor TIE2 to regulate distinct steps in vascular remodeling, vessel maturation and vascular inflammation 48,49 TIE2 mutations have been identified in inherited form of venous malformation (VM) 50 and 50% of sporadic VM 51. Similar mutations were not found in IH. However Tie2 mRNA and protein were shown to up-regulated in IH tissues and in HemEC. In accordance, increase in cellular responsiveness to Ang1 was observed52. A related study in a murine endothelioma tumor model has shown that blocking Ang2 function with soluble Tie2 receptor or down-regulating Ang2 pharmacologically inhibited growth of a tumor53. Calicchio and colleagues found ANGP2 mRNA significantly increased in hemangioma endothelium isolated by laser capture micro-dissection 54. This, coupled with the diminished expression of ANGPT1 by hemangioma pericytes, would be permissive for angiogenesis and hinder vessel maturation. Still, confirmatory studies in animal models of IH as well as mechanistic studies are needed to clearly define the role of angiopoietins and TIE2 in the growth and involution of IH.
The Notch Pathway
The Notch pathway has also been implicated in hemangiogenesis. This evolutionarily conserved signaling system regulates cell-fate determination during development and in stem cells. In the vascular system, interaction of Notch receptors (Notch1 to Notch4) with their ligands (Delta-like 1, Delta-like 3, Delta-like 4, Jagged-1and Jagged-2) regulates the specification of endothelial cells into arterial and venous phenotypes during development 55. JAGGED1 has been reported to be highly expressed in IH endothelium 24,54 whereas its receptor Notch3 is expressed by HemSC and becomes prominently expressed in the perivascular cells in the involuting phase24. Also, we have recently shown that JAGGED1 directs the differentiation of the HemSC into the “endothelium-coating” cells, the pericytes 56. Based on these observations, it is tempting to speculate that juxtacrine signaling between hemangioma endothelial cells and pericytes is mediated by the Notch pathway.
The mTOR Pathway
Mammalian target of rapamycin complex (mTOR) is a major intersection that translates signals from the extracellular milieu, such as glucose, amino acids and growth factors to corresponding changes in basic intracellular processes including proliferation, protein synthesis and autophagy 57. Rapamycin, an mTOR inhibitor, is known to have an anti-angiogenic effect on ECs in pathological settings 58,59 and has shown efficacy in the treatment of complicated vascular malformations60. We found that rapamycin inhibits the proliferation and the self-renewal activity of the HemSC. This inhibition, in turn, prevented HemSCs, either alone or combined with endothelial cells, from forming blood vessels in vivo 61. Beside its anti-vasculogenic effect on HemSC, rapamycin has anti-angiogenic effect on the HemEC, suppressing their proliferation and leading to regression of pre-existing IH vessels. Rapamycin also decreases expression of HIF-1α, reducing already low levels of VEGF-A in HemEC even further 62.
Other pathways
Various other factors have been found to be differentially expressed in the proliferating phases of IH but their role is less defined. These include MCP-1 63,42, IL-6 and uPAR 42 and Insulin-like growth factor 2 (IGF-2) 64,65. The SKI oncogene, a transcriptional repressor that inhibits expression of TGFβ family members, was found to be highly expressed in the endothelium of proliferating phase IH, but was not detected in several vascular malformation specimens 66, an intriguing suggestion that TGFβ signaling may be suppressed in IH. The cell adhesion molecule E-selectin, normally only expressed in inflamed endothelium, is strongly expressed on vessels in proliferating phase IH 67. E-selectin is also constitutively expressed by proliferating phase HemEC and appears to mediate interactions with HemSC 68.
The Renin-Angiotensin system has recently been suggested to play a role in IH pathogenesis as well as in the response to propranolol 69. Specifically, it was suggested that angiotensin II could drive proliferation of endothelial progenitor cells into mitotically active cells that characterize IH. The hypothesis was based on the clinical observation of a higher incidence of IH in premature babies, female infants and infants of mixed European descent that have been shown to have higher circulating renin activity compared to their age and sex-matched controls 70. Indeed, angiotensin-converting enzyme and angiotensin II receptor-2 were shown to be expressed in IH 69. However, support to this theory from in-vivo studies is lacking. Also against this theory is the low renin activity found in IH patients 71 and the poor effect of the ACE inhibitor captopril on IH 72.
Drug treatment of IH
Corticosteroids have been the first-line treatment for complicated IH for many years 73,74. In recent years, β-adrenergic receptor blockers have emerged as an effective and safe pharmacological treatment of proliferating IH. We have recently published a detailed review of these drugs’ mechanism of action 75. Here, we will focus on new findings regarding the effect of these drugs on the constituent cells and the molecular pathways involved in IH.
Beta blockers
Propranolol, the most commonly used beta-blocker against IH, is an orthosteric antagonist of both β1- and β2-adrenergic receptors. Despite its widespread use, since 2008, propranolol’s mechanism of action remains uncertain. Expression of all three β-adrenergic receptors have been shown in IH tumors 76,77,78. By immunohistochemical analysis, the expression of β2 receptor was located mainly to the endothelial cells 78. However, in-vitro studies show expression of β1 adrenergic receptors in HemEC and β2 adrenergic receptors in HemEC, Hem Pericytes and at low levels in HemSC (Lee, Greenberger and Bischoff, unpublished data). As a rapid change in both the color and the consistency of the tumor is sometimes noticed following the initiation of propranolol, it is reasonable that propranolol exerts its effect via vasoconstriction of the high-flow blood vessels feeding the IH tumor. In the skin, adrenaline-induced vasoconstriction has been shown to be increased by oral propranolol 79. The target cells for this effect might be the tumor’s pericyte.
Additional mechanisms might be responsible for the slower, long-term effect of propranolol. Among them is VEGF suppression. Noradrenaline has been shown to enhance VEGF-A production by several cell types, both normal and cancerous 80,81,82. These effects of the catecholamines are mediated by the β1 and β2 adrenergic receptors and are blocked by propranolol80,81,83 ,82. In IH, Ji and his colleagues have recently shown that the Noradrenaline agonist isoprenaline increased HemECs proliferation via regulation of thee cell-cycle proteins cyclin D1 and its associated kinases, CDK-4 and CDK-6. These effects were reversed by β2-adrenergic receptor antagonists. Furthermore, isoprenaline increased the expression of VEGF-A and the phosphorylation of VEGFR-2 in HemECs in a β-adrenergic receptor- and extracellular-signal-regulated kinase (ERK) -dependent manner.
Additional pro-angiogenic effects of noradrenaline blocked by propranolol are up-regulation of HIF-1α protein 82, matrix metalloproteinases (MMPs) 2 and 7 and 9 81,84,85 and IL-6 86. However, these effects have not been studied in IH-derived cells or in IH animal models. Several groups have demonstrated a direct pro-apoptotic or anti-migratory activity of propranolol on HemEC. However, these effects were achieved in high concentrations of propranolol that are not likely to be present in the tumor’s microenvironment87.
Corticosteroids
Corticosteroids affect the growth of IH by modifying the “pro-angiogenic” environment of the tumor, mostly likely by suppressing the high levels of VEGF-A secreted by the HemSC. By using IH animal model, we found that steroids lead to dose-dependent reduction in the tumor’s vascularity. Corticosteroids, including dexamethasone, prednisone, prednisolone and methylprednisolone dramatically down-regulate VEGF-A secretion by HemSC and this suppression was sufficient to block blood vessel formation in vivo41. Several other pro-angiogenic genes were modified as well by steroids. These include Monocyte chemotactic protein-1 (MCP-1), Interleukin-6, matrix metalloproteinase-1 (MMP-1) and urokinase-type plasminogen activator receptor (uPAR)41. These target genes have high expression level in proliferating versus involuting IH tissue. The down-regulation of these pro-angiogenic cytokines, including VEGF-A might be mediated by corticosteroids interference with Nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) activity 42.
Concluding Remarks
Substantial progress has been made on understanding the cells and molecular pathways that are prominent in IH. There remain, however, several unsolved mysteries. What is the nature and origin of the HemSC – is this normal post-natal vasculogenic stem cell perturbed by an external stress or has it acquired a mutation that expands its proliferative and differentiative capacity? Do HemSC arise in situ or do they come from a distant location such as the placenta or bone marrow? Do the hemangioma stem cells, ECs or pericytes exert dominant effects on surrounding vessels, subverting and/or increasing their angiogenic and vasculogenic capability? And finally, what triggers involution? The answers to these questions may help to devise therapies that can stop hemangioma growth in its tracks, providing enormous relief and reassurance to patients and families. Such therapies might consist of combinations of drugs given concurrently or perhaps sequentially to target cells that are most active in the proliferating phase. Another goal would be to identify biomarkers that would predict early in the proliferative phase which tumors should be treated and which could be left untreated to follow the natural cycle of growth and involution.
Acknowledgments
S.G is supported by the March of Dimes, Basil O’Connor Starter Scholar Research Award #5-FY12 -55 and by the The Talpiot Medical Leadership Program, Sheba Medical Center. J.B is supported by NIH grants R01 HL 096384 and P01 AR48564.
References
- 1.Takahashi K, Mulliken JB, Kozakewich HP, et al. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357–64. doi: 10.1172/JCI117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielenberg DR, Bucana CD, Sanchez R, et al. Progressive growth of infantile cutaneous hemangiomas is directly correlated with hyperplasia and angiogenesis of adjacent epidermis and inversely correlated with expression of the endogenous angiogenesis inhibitor, IFN-beta. International journal of oncology. 1999;14:401–8. doi: 10.3892/ijo.14.3.401. [DOI] [PubMed] [Google Scholar]
- 3.Boscolo E, Bischoff J. Vasculogenesis in infantile hemangioma. Angiogenesis. 2009;12:197–207. doi: 10.1007/s10456-009-9148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pack GT, Miller TR. Hemangiomas; classification, diagnosis and treatment. Angiology. 1950;1:405–26. doi: 10.1177/000331975000100504. [DOI] [PubMed] [Google Scholar]
- 5.Malan E. Vascular Malformations (Angiodysplasias) Milan: Carlo Erba Foundation. 1974:4. [Google Scholar]
- 6.Kaplan E. Vascular malformations of extremities. Vol. 17. St. Louis: C.V. Mosby Co; 1983. [Google Scholar]
- 7.Smoller BR, Apfelberg DB. Infantile (juvenile) capillary hemangioma: A tumor of heterogeneous cellular elements. J Cutan Pathol. 1993;20:330–6. doi: 10.1111/j.1600-0560.1993.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 8.Khan ZA, Boscolo E, Picard A, et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–9. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boscolo E, Stewart CL, Greenberger S, et al. JAGGED1 signaling regulates hemangioma stem cell-to-pericyte/vascular smooth muscle cell differentiation. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2181–92. doi: 10.1161/ATVBAHA.111.232934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu D, OTM, Shartava A, et al. Isolation, characterization, and in vitro propagation of infantile hemangioma stem cells and an in vivo mouse model. J Hematol Oncol. 2011;4:54. doi: 10.1186/1756-8722-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mai HM, Zheng JW, Wang YA, et al. CD133 selected stem cells from proliferating infantile hemangioma and establishment of an in vivo mice model of hemangioma. Chinese medical journal. 2013;126:88–94. [PubMed] [Google Scholar]
- 12.Itinteang T, Tan ST, Brasch H, et al. Primitive mesodermal cells with a neural crest stem cell phenotype predominate proliferating infantile haemangioma. J Clin Pathol. 2010;63:771–6. doi: 10.1136/jcp.2010.079368. [DOI] [PubMed] [Google Scholar]
- 13.Itinteang T, Vishvanath A, Day DJ, et al. Mesenchymal stem cells in infantile haemangioma. J Clin Pathol. 2011;64:232–6. doi: 10.1136/jcp.2010.085209. [DOI] [PubMed] [Google Scholar]
- 14.Itinteang T, Tan ST, Brasch HD, et al. Infantile haemangioma expresses embryonic stem cell markers. J Clin Pathol. 2012;65:394–8. doi: 10.1136/jclinpath-2011-200462. [DOI] [PubMed] [Google Scholar]
- 15.Adepoju O, Wong A, Kitajewski A, et al. Expression of HES and HEY genes in infantile hemangiomas. Vascular cell. 2011;3:19. doi: 10.1186/2045-824X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopel-Kreiner I. Histogenesis of hemangiomas--an ultrastructural study on capillary and cavernous hemangiomas of the skin. Path Res Pract. 1980;170:70. doi: 10.1016/S0344-0338(80)80157-9. [DOI] [PubMed] [Google Scholar]
- 17.North PE, Waner M, Mizeracki A, et al. GLUT1: A newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11–22. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 18.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–22. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–6. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 20.Dosanjh A, Chang J, Bresnick S, et al. In vitro characteristics of neonatal hemangioma endothelial cells: similarities and differences between normal neonatal and fetal endothelial cells. J Cutan Pathol. 2000;27:441–50. doi: 10.1034/j.1600-0560.2000.027009441.x. [DOI] [PubMed] [Google Scholar]
- 21.Boye E, Yu Y, Paranya G, et al. Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest. 2001;107:745–52. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y, Flint AF, Mulliken JB, et al. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103:1373–5. doi: 10.1182/blood-2003-08-2859. [DOI] [PubMed] [Google Scholar]
- 23.Yuan SM, Jiang HQ, Ouyang TX, et al. The distribution and evolution of pericytes in infantile hemangioma. Zhonghua zheng xing wai ke za zhi = Zhonghua zhengxing waike zazhi = Chinese journal of plastic surgery. 2007;23:322–4. [PubMed] [Google Scholar]
- 24.Wu JK, Adepoju O, De Silva D, et al. A switch in Notch gene expression parallels stem cell to endothelial transition in infantile hemangioma. Angiogenesis. 2010;13:15–23. doi: 10.1007/s10456-009-9161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan SM, Chen RL, Shen WM, et al. Mesenchymal stem cells in infantile hemangioma reside in the perivascular region. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2012;15:5–12. doi: 10.2350/11-01-0959-OA.1. [DOI] [PubMed] [Google Scholar]
- 26.Boscolo E, Mulliken JB, Bischoff J. Pericytes from infantile hemangioma display proangiogenic properties and dysregulated angiopoietin-1. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:501–9. doi: 10.1161/ATVBAHA.112.300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glowacki J, Mulliken JB. Mast cells in hemangiomas and vascular malformations. Pediatrics. 1982;70:48–51. [PubMed] [Google Scholar]
- 28.Tan ST, Wallis RA, He Y, et al. Mast cells and hemangioma. Plast Reconstr Surg. 2004;113:999–1011. doi: 10.1097/01.prs.0000105683.10752.a6. [DOI] [PubMed] [Google Scholar]
- 29.Biswas A, Richards JE, Massaro J, et al. Mast cells in cutaneous tumors: innocent bystander or maestro conductor? Int J Dermatol. 2013 doi: 10.1111/j.1365-4632.2012.05745.x. [DOI] [PubMed] [Google Scholar]
- 30.Boon LM, Ballieux F, Vikkula M. Pathogenesis of vascular anomalies. Clin Plast Surg. 2011;38:7–19. doi: 10.1016/j.cps.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter JW, Blei F, Anderson JL, et al. Genetic mapping of a novel familial form of infantile hemangioma. Am J Med Genet. 1999;82:77–83. doi: 10.1002/(sici)1096-8628(19990101)82:1<77::aid-ajmg15>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 32.Grimmer JF, Williams MS, Pimentel R, et al. Familial clustering of hemangiomas. Arch Otolaryngol Head Neck Surg. 2011;137:757–60. doi: 10.1001/archoto.2011.91. [DOI] [PubMed] [Google Scholar]
- 33.Walter JW, North PE, Waner M, et al. Somatic mutation of vascular endothelial growth factor receptors in juvenile hemangioma. Genes Chromosomes Cancer. 2002;33:295–303. doi: 10.1002/gcc.10028. [DOI] [PubMed] [Google Scholar]
- 34.Jinnin M, Medici D, Park L, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nature medicine. 2008;14:1236–46. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol. 1996;132:307–11. doi: 10.1001/archderm.132.3.307. [DOI] [PubMed] [Google Scholar]
- 36.Stockman A, Boralevi F, Taieb A, et al. SACRAL syndrome: spinal dysraphism, anogenital, cutaneous, renal and urologic anomalies, associated with an angioma of lumbosacral localization. Dermatology. 2007;214:40–5. doi: 10.1159/000096911. [DOI] [PubMed] [Google Scholar]
- 37.Siegel DH, Shieh JT, Kwon EK, et al. Copy Number Variation Analysis in 98 Individuals with PHACE Syndrome. The Journal of investigative dermatology. 2013;133:677–84. doi: 10.1038/jid.2012.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bautch VL. VEGF-directed blood vessel patterning: from cells to organism. Cold Spring Harbor perspectives in medicine. 2012;2:a006452. doi: 10.1101/cshperspect.a006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang J, Most D, Bresnick S, et al. Proliferative hemangiomas: analysis of cytokine gene expression and angiogenesis. Plastic and reconstructive surgery. 1999;103:1–9. doi: 10.1097/00006534-199901000-00001. discussion 10. [DOI] [PubMed] [Google Scholar]
- 40.Kleinman ME, Greives MR, Churgin SS, et al. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27:2664–70. doi: 10.1161/ATVBAHA.107.150284. [DOI] [PubMed] [Google Scholar]
- 41.Greenberger S, Boscolo E, Adini I, et al. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. The New England journal of medicine. 2010;362:1005–13. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenberger S, Adini I, Boscolo E, et al. Targeting NF-kappaB in infantile hemangioma-derived stem cells reduces VEGF-A expression. Angiogenesis. 2010 doi: 10.1007/s10456-010-9189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Lin X, Wang W, et al. Circulating level of vascular endothelial growth factor in differentiating hemangioma from vascular malformation patients. Plast Reconstr Surg. 2005;116:200–4. doi: 10.1097/01.prs.0000170804.80834.5f. [DOI] [PubMed] [Google Scholar]
- 44.Boscolo E, Mulliken JB, Bischoff J. VEGFR-1 Mediates Endothelial Differentiation and Formation of Blood Vessels in a Murine Model of Infantile Hemangioma. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188–93. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jinnin M, Medici D, Park L, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–46. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picard A, Boscolo E, Khan ZA, et al. IGF-2 and FLT-1/VEGF-R1 mRNA levels reveal distinctions and similarities between congenital and common infantile hemangioma. Pediatric research. 2008;63:263–7. doi: 10.1203/PDR.0b013e318163a243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Augustin HG, Koh GY, Thurston G, et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–77. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 49.Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends in molecular medicine. 2013;19:31–9. doi: 10.1016/j.molmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Wouters V, Limaye N, Uebelhoer M, et al. Hereditary cutaneomucosal venous malformations are caused by TIE2 mutations with widely variable hyper-phosphorylating effects. Eur J Hum Genet. 2010;18:414–20. doi: 10.1038/ejhg.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Limaye N, Wouters V, Uebelhoer M, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nature genetics. 2009;41:118–24. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu Y, Varughese J, Brown LF, et al. Increased Tie2 expression, enhanced response to angiopoietin-1, and dysregulated angiopoietin-2 expression in hemangioma-derived endothelial cells. The American journal of pathology. 2001;159:2271–80. doi: 10.1016/S0002-9440(10)63077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry BN, Govindarajan B, Bhandarkar SS, et al. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. The Journal of investigative dermatology. 2006;126:2316–22. doi: 10.1038/sj.jid.5700413. [DOI] [PubMed] [Google Scholar]
- 54.Calicchio ML, Collins T, Kozakewich HP. Identification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profiling. Am J Pathol. 2009;174:1638–49. doi: 10.2353/ajpath.2009.080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Developmental cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Boscolo E, Stewart CL, Greenberger S, et al. JAGGED1 signaling regulates hemangioma stem cell-to-pericyte/vascular smooth muscle cell differentiation. Arteriosclerosis, thrombosis, and vascular biology. 31:2181–92. doi: 10.1161/ATVBAHA.111.232934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phung TL, Eyiah-Mensah G, O’Donnell RK, et al. Endothelial Akt signaling is rate-limiting for rapamycin inhibition of mouse mammary tumor progression. Cancer Res. 2007;67:5070–5. doi: 10.1158/0008-5472.CAN-06-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 60.Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatric blood & cancer. 2011;57:1018–24. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]
- 61.Greenberger S, Yuan S, Walsh LA, et al. Rapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangioma. The Journal of investigative dermatology. 2011;131:2467–76. doi: 10.1038/jid.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medici D, Olsen BR. Rapamycin inhibits proliferation of hemangioma endothelial cells by reducing HIF-1-dependent expression of VEGF. PloS one. 2012;7:e42913. doi: 10.1371/journal.pone.0042913. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Isik FF, Rand RP, Gruss JS, et al. Monocyte chemoattractant protein-1 mRNA expression in hemangiomas and vascular malformations. J Surg Res. 1996;61:71–6. doi: 10.1006/jsre.1996.0083. [DOI] [PubMed] [Google Scholar]
- 64.Ritter MR, Dorrell MI, Edmonds J, et al. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7455–60. doi: 10.1073/pnas.102185799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ritter MR, Moreno SK, Dorrell MI, et al. Identifying potential regulators of infantile hemangioma progression through large-scale expression analysis: a possible role for the immune system and indoleamine 2,3 dioxygenase (IDO) during involution. Lymphat Res Biol. 2003;1:291–9. doi: 10.1089/153968503322758094. [DOI] [PubMed] [Google Scholar]
- 66.OTM, Tan M, Tarango M, et al. Differential expression of SKI oncogene protein in hemangiomas. Otolaryngol Head Neck Surg. 2009;141:213–8. doi: 10.1016/j.otohns.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Kraling BM, Razon MJ, Boon LM, et al. E-selectin is present in proliferating endothelial cells in human hemangiomas. The American journal of pathology. 1996;148:1181–91. [PMC free article] [PubMed] [Google Scholar]
- 68.Smadja DM, Mulliken JB, Bischoff J. E-selectin mediates stem cell adhesion and formation of blood vessels in a murine model of infantile hemangioma. The American journal of pathology. 2012;181:2239–47. doi: 10.1016/j.ajpath.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Itinteang T, Brasch HD, Tan ST, et al. Expression of components of the renin-angiotensin system in proliferating infantile haemangioma may account for the propranolol-induced accelerated involution. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2011;64:759–65. doi: 10.1016/j.bjps.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 70.Youmbissi TJ, Tedong F, Fairbank ST, et al. Plasma renin activity studies in a group of African neonates and children. Journal of tropical pediatrics. 1990;36:128–30. doi: 10.1093/tropej/36.3.128. [DOI] [PubMed] [Google Scholar]
- 71.Ma X, Zhao T, Xiao Y, et al. Preliminary experience on treatment of infantile hemangioma with low-dose propranolol in China. European journal of pediatrics. 2013 doi: 10.1007/s00431-012-1928-9. [DOI] [PubMed] [Google Scholar]
- 72.Christou EM, Wargon O. Effect of captopril on infantile haemangiomas: a retrospective case series. The Australasian journal of dermatology. 2012;53:216–8. doi: 10.1111/j.1440-0960.2012.00901.x. [DOI] [PubMed] [Google Scholar]
- 73.Zarem HA, Edgerton MT. Induced resolution of cavernous hemangiomas following prednisolone therapy. Plastic and reconstructive surgery. 1967;39:76–83. doi: 10.1097/00006534-196701000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Cohen SR, Wang CI. Steroid treatment of hemangioma of the head and neck in children. Ann Otol Rhinol Laryngol. 1972;81:584–90. doi: 10.1177/000348947208100419. [DOI] [PubMed] [Google Scholar]
- 75.Greenberger S, Bischoff J. Infantile hemangioma-mechanism(s) of drug action on a vascular tumor. Cold Spring Harbor perspectives in medicine. 2011;1:a006460. doi: 10.1101/cshperspect.a006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossler J, Haubold M, Gilsbach R, et al. beta-Adrenoceptor mRNA level reveals distinctions between infantile hemangioma and vascular malformations. Pediatric research. 2013 doi: 10.1038/pr.2013.16. [DOI] [PubMed] [Google Scholar]
- 77.Chisholm KM, Chang KW, Truong MT, et al. beta-Adrenergic receptor expression in vascular tumors. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:1446–51. doi: 10.1038/modpathol.2012.108. [DOI] [PubMed] [Google Scholar]
- 78.Hadaschik E, Scheiba N, Engstner M, et al. High levels of beta2-adrenoceptors are expressed in infantile capillary hemangiomas and may mediate the therapeutic effect of propranolol. J Cutan Pathol. 2012;39:881–3. doi: 10.1111/j.1600-0560.2012.01937.x. [DOI] [PubMed] [Google Scholar]
- 79.Doshi BS, Kulkarni RD, Dattani KK, et al. Effect of labetalol and propranolol on human cutaneous vasoconstrictor response to adrenaline. Int J Clin Pharmacol Res. 1984;4:25–8. [PubMed] [Google Scholar]
- 80.Fredriksson JM, Lindquist JM, Bronnikov GE, et al. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a beta -adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. J Biol Chem. 2000;275:13802–11. doi: 10.1074/jbc.275.18.13802. [DOI] [PubMed] [Google Scholar]
- 81.Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–21. [PubMed] [Google Scholar]
- 82.Park SY, Kang JH, Jeong KJ, et al. Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. Int J Cancer. doi: 10.1002/ijc.25589. [DOI] [PubMed] [Google Scholar]
- 83.Ji Y, Chen S, Li K, et al. The role of beta-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells. Cell division. 2013;8:1. doi: 10.1186/1747-1028-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo K, Ma Q, Wang L, et al. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep. 2009;22:825–30. doi: 10.3892/or_00000505. [DOI] [PubMed] [Google Scholar]
- 85.Shi M, Liu D, Duan H, et al. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol Cancer. 9:269. doi: 10.1186/1476-4598-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–26. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 87.Wong L, Nation RL, Chiou WL, et al. Plasma concentrations of propranolol and 4-hydroxypropranolol during chronic oral propranolol therapy. British journal of clinical pharmacology. 1979;8:163–7. doi: 10.1111/j.1365-2125.1979.tb05815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]