Abstract
Background
Although seasonal variation in tuberculosis (TB) incidence has been described in many countries, it remains unknown in China.
Methods
A time series decomposition analysis (X-12-ARIMA) was performed to examine the seasonal variation in active TB cases nationwide from 2005 through 2012 in China. Seasonal amplitude was calculated for the evaluation of TB seasonal variation.
Results
A total of 7.78 million active TB cases were reported over a period of 8 years. A spring peak (April) was observed with seasonal amplitude of 46.3%, compared with the winter trough (February). Most cases in provinces with subtropical and tropical monsoon climate showed lower amplitudes than those in temperate continental, plateau and mountain climate regions. The magnitude of seasonality varied inversely with annual average temperature, r (95% CI) = -0.71 (-0.79, -0.61). The seasonal amplitudes were 56.7, 60.5, 40.6, 46.4 and 50.9% for patients aged ≤14, 15–24, 25–44, 45–64, and ≥65 years, respectively. Students demonstrated greater seasonal amplitude than peasants, migrant workers and workers (115.3% vs. 43.5, 41.6 and 48.1%). Patients with pulmonary TB had lower amplitude compared to patients with pleural and other extra-pulmonary TB (EPTB) (45.9% vs. 52.0 and 56.3%). Relapse cases with sputum smear positive TB (SS+ TB) had significantly higher seasonal amplitude compared to new cases with sputum smear positive TB (52.2% vs. 41.6%).
Conclusions
TB is a seasonal disease in China. The peak and trough of TB transmission actually are in winter and in autumn respectively after factors of delay are removed. Higher amplitudes of TB seasonality are more likely to happen in temperate continental, plateau and mountain climate regions and regions with lower annual average temperature, and young person, students, patients with EPTB and relapse cases with SS+ TB are more likely to be affected by TB seasonality.
Background
Previous studies conducted in India, Japan, Mongolia, Netherlands, Russia, Spain, United Kingdom, United States, etc. between 1992 and 2012 have evaluated the seasonality of presentation for suspected tuberculosis (TB), [1] TB notification and prevalence,[2]–[13] childhood TB incidence [14] and TB cases in migrants, [15] and explored correlation of seasonal variations and vitamin D status. [3], [16], [17] Several methods have been used to evaluate TB seasonality, including Fourier analysis, [7] cosinor analysis, [11], [16] sinusoidal harmonic model, [15], [18] spectral analysis, [12] seasonal autoregressive integrated moving average model (SARIMA), [7], [17] time-series decomposition method (X-11), [8] and others.
Studies conducted in countries in the northern hemisphere have identified peak months of TB notification in spring (March to May),[6]–[8], [15], [17], late spring and early summer (April to June), [10], [13] or summer (June to August), [5], [11], [18] and trough months in the fall (September to November), [7] late fall and early winter (October to December), [6], [8], [10], [13] or winter (January to February). [18] Undoubtedly, differences of TB seasonality exist among countries, which indicate that the mechanisms underlying the seasonal variation of TB are complex and multifactoral, and need intensive studies.
China’s total population was 1.37 billion in 2011 and 15% of the world’s notified cases of TB in 2010 occurred within China’s borders. [19] China has an area of 9.6 million square kilometers with various different climate zones, including temperate, tropical or subtropical, and frigid climate zones. Given this diversity, the studies for seasonality of TB notification in China will provide important evidence also for other countries.
There are few studies that have evaluated the seasonality of TB in China. A study conducted in Hong Kong demonstrated seasonality of TB cases reported in 2005, [18] and SEIR (susceptible, exposed, infected and resistant) TB models with seasonality were developed to simulate the seasonal variation of the reported cases of active TB in China. [20], [21].
The Chinese Center for Disease Control and Prevention (China CDC) has been conducting national surveillance for TB annually since 2002, leading to the establishment of the National Center for TB Control and Prevention (NCTB) in March 2002, and the National TB Information Management System (NTIMS) based on the internet in January of 2005. [22] These measures made the nationwide TB surveillance a reality and also made the research on TB seasonality possible on a nationwide scale. For this study, we utilized information on TB cases notified in the NTIMS between 2005 and 2012 to evaluate TB seasonality.
Materials and Methods
Data Source
The monthly notification for all forms of active TB cases from all 31 provinces from 2005 to 2012 in the mainland of China, who were directly notified to the NTIMS by all county-level TB dispensaries and monitored by NCTB immediately through the NTIMS, were analyzed.
Time Series Analysis
Monthly raw case counts were analyzed using the X-12-ARIMA seasonal adjustment program (X-12-ARIMA program) that is an enhanced version of the X-11 Variant of the Census Method II seasonal adjustment program developed by the US Census Bureau. [23] In the X-12-ARIMA program, the original time series is decomposed into three basic components: trend-cycle, seasonal and irregular. The trend-cycle is the long-term and medium-to-long term movement of the series, including consequential turning points; the seasonal component is within-year fluctuations about the trend that recur in a very similar way in the same month or quarter each year; and the irregular component is the residual component that remains after trend-cycle and seasonal component are removed from the series.
Statistical Steps and Methods
In the first step, the X-12-ARIMA program was applied to the raw monthly case counts. The time series of total active TB cases was decomposed into trend cycle, seasonal and irregular components. A decomposition of monthly case counts was obtained for groups of interest, according to time period, sex, age, occupation, form of TB, sputum smear test and province, which all were calculated as mean peak month, mean trough month, and annual seasonal amplitude with 95% confidence intervals (CI) for the years 2005–2012, if they had identifiable seasonality assessed by the X-12-ARIMA program. Annual seasonal amplitude was calculated from isolated seasonal factors and was defined as a fraction with the numerator being the peak-to-trough difference between the months with the highest and the lowest case counts and with the denominator being mean case counts for that year.
In the second step, the amplitudes of seasonal fluctuation were compared within groups. Local Getis-Ord Gi* was used to examine the local level of spatial autocorrelation and determine locations of clusters or hotspots. A calculated Z score of Gi* >1.96 indicates that the province and its neighboring provinces have a seasonal amplitude statistically significantly higher than other provinces, and Z score<-1.96 indicates the significantly lower seasonal amplitude. Linear correlation was used to demonstrate the correlation of amplitude of seasonal fluctuation and annual average temperature by province. The Student’s t-test for two independent samples was used to compare seasonal amplitudes of two subgroups. The Student-Newman-Keuls method for one way analysis of variance was used to compare all pairwise seasonal amplitudes of three or more subgroups. P-values <0.05 were considered statistically significant.
Statistics Software
The Sigma Plot statistical package (SigmaPlot 11.0; Systat Software, Inc., Chicago, IL, USA) was used for graphing and correlation analysis; ArcGIS (ArcGIS 10.0; ESRI Inc., Redlands, CA, USA) was used for mapping and spatial analysis; and the Statistical Analysis System (SAS 9.2; SAS Institute Inc., Cary, NC, USA) was used for statistical comparisons of seasonal indicators within groups.
Results
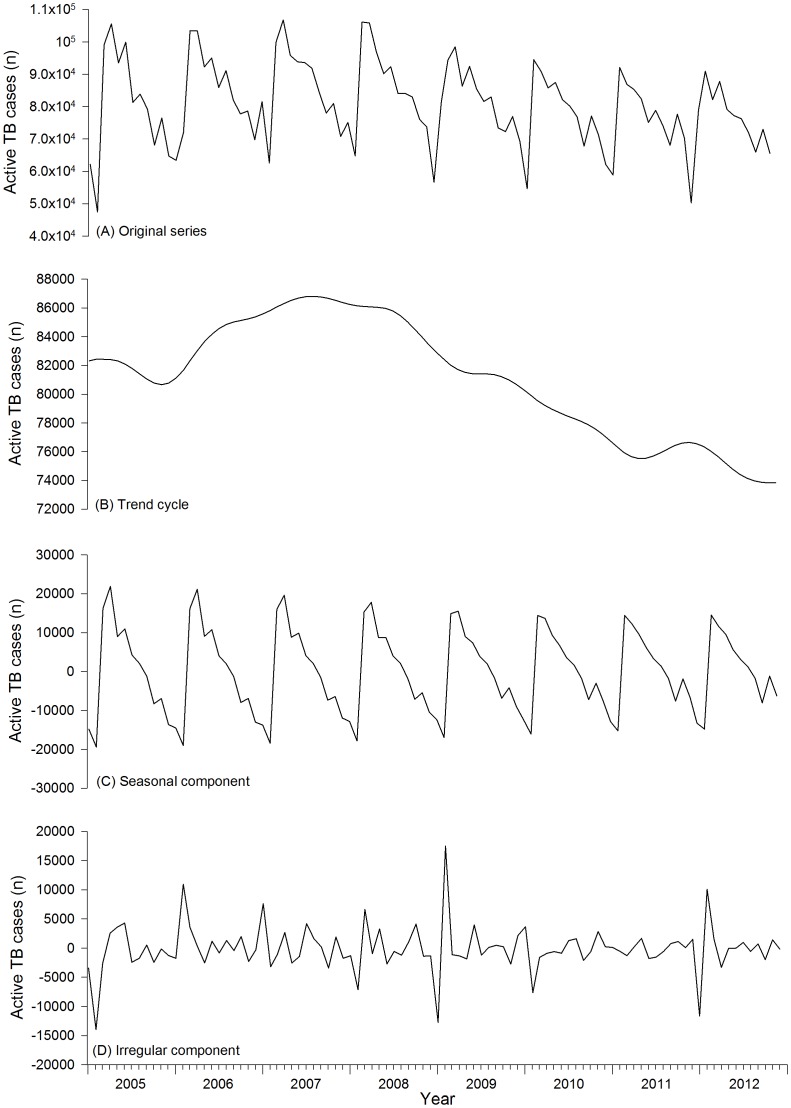
From 2005 to 2012, there were about 7.78 million active TB cases notified by all county-level TB dispensaries in the mainland of China (Table 1). Figure 1A showed original series of active TB case with the X-12-ARIMA seasonal decomposition of the isolated trend cycle (Figure 1B), seasonal (Figure 1C), and irregular (Figure 1D) components. The raw counts showed remarkably consistent seasonal fluctuation. There was a downward trend in 2005, then an upward trend from 2006 to 2007, and then a steadily decreasing trend from 2008 to 2012. From the isolated seasonal component it was found that seasonal amplitude decreased each year from 2005 to 2012.
Table 1. Active tuberculosis cases in the mainland of China, 2005–2012.
| Group | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | Total | |
| All active TB cases | 96.2 | 101.5 | 104.0 | 103.2 | 98.2 | 93.8 | 91.2 | 89.9 | 778.0 | |
| Sex | Male | 66.8 | 70.2 | 72.4 | 72.2 | 68.2 | 65.1 | 63.7 | 62.5 | 541.2 |
| Female | 29.3 | 31.3 | 31.6 | 31.1 | 30.0 | 28.6 | 27.5 | 27.4 | 236.8 | |
| Age (years) | 0–14 | 1.5 | 1.2 | 1.0 | 0.9 | 0.9 | 0.7 | 0.6 | 0.6 | 7.4 |
| 15–24 | 14.9 | 16.6 | 16.8 | 17.4 | 17.1 | 16.3 | 15.7 | 14.5 | 129.3 | |
| 25–44 | 32.0 | 33.8 | 33.5 | 32.5 | 30.3 | 28.6 | 27.6 | 26.4 | 244.8 | |
| 45–64 | 29.5 | 30.5 | 32.0 | 32.3 | 31.1 | 30.3 | 30.0 | 30.2 | 245.9 | |
| 65+ | 18.2 | 19.4 | 20.6 | 20.1 | 18.8 | 17.9 | 17.3 | 18.1 | 150.6 | |
| Occupation | Peasant | 64.9 | 67.2 | 68.8 | 68.2 | 63.7 | 60.7 | 59.4 | 59.2 | 512.0 |
| Migrant worker | 3.1 | 3.7 | 4.1 | 4.1 | 3.8 | 3.9 | 3.1 | 2.5 | 28.3 | |
| Worker | 5.5 | 5.8 | 5.9 | 5.9 | 6.1 | 6.1 | 5.4 | 4.9 | 45.6 | |
| Student | 5.2 | 5.5 | 5.3 | 5.2 | 5.4 | 4.5 | 4.0 | 3.7 | 38.9 | |
| Others | 17.5 | 19.2 | 19.9 | 19.9 | 19.4 | 18.6 | 19.3 | 19.5 | 153.3 | |
| Form of disease | Pulmonary TB | 93.1 | 98.3 | 101.0 | 99.9 | 94.8 | 90.7 | 88.1 | 86.8 | 752.6 |
| Pleural TB | 2.3 | 2.4 | 2.1 | 2.3 | 2.6 | 2.5 | 2.5 | 2.5 | 19.1 | |
| Other extra-pulmonary TB | 0.8 | 0.8 | 0.9 | 1.0 | 0.8 | 0.7 | 0.7 | 0.6 | 6.2 | |
| Sputum smear microscopy | Positive TB | 55.8 | 54.7 | 53.2 | 52.8 | 50.9 | 48.4 | 42.4 | 35.5 | 393.8 |
| Negative TB | 35.4 | 42.2 | 46.5 | 46.5 | 43.5 | 42.0 | 45.5 | 51.0 | 352.6 | |
| Sputum smear positive TB | New case | 47.3 | 47.2 | 46.4 | 46.4 | 44.9 | 43.0 | 37.7 | 31.5 | 344.4 |
| Relapse case | 8.5 | 7.6 | 6.8 | 6.4 | 6.0 | 5.4 | 4.7 | 4.1 | 49.4 |
(Unit: 10 thousand).
Figure 1. Seasonal decomposition of active tuberculosis cases per month in the mainland of China, 2005–2012.
Original series (A) with trend cycle (B), seasonal component (C), and irregular component (D).
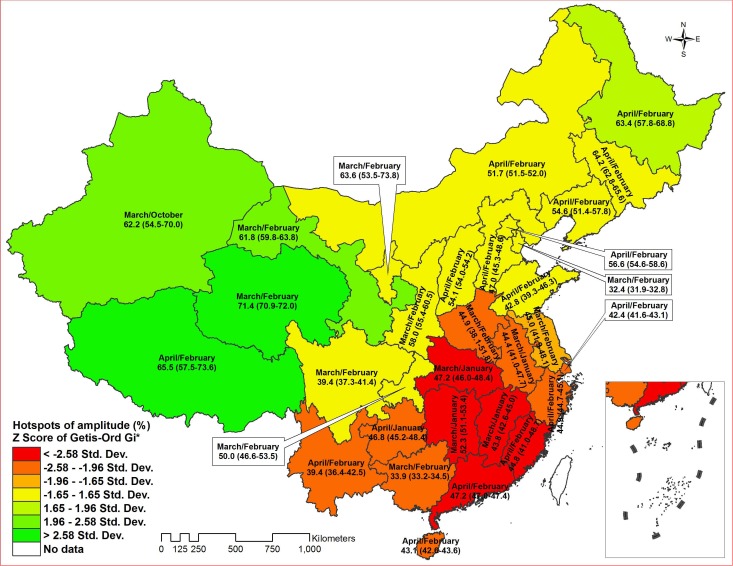
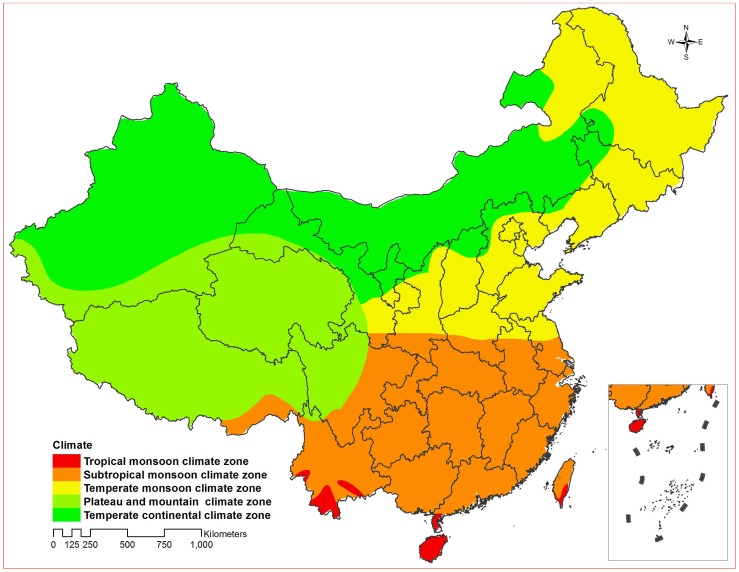
Figure 2 illustrated peak and trough months, seasonal amplitude and clusters (hotspots) of amplitude by province in the mainland of China. Most provinces demonstrated a seasonal peak in March or April, and a trough during January or February. The result of Local Getis-Ord Gi* for spatial autocorrelation showed that there were 2 significant spatial hotspots of amplitude among 31 provinces. One hotspot with Z score >1.96 meant the area of higher amplitude and another with Z score<−1.96 meant the area of lower amplitude, which largely overlapped temperate continental, plateau and mountain climate zones and tropical and subtropical monsoon climate zones, respectively (Figure 2 and Figure 3).
Figure 2. Peak/trough month, seasonal amplitude (%) (95% Confidence Interval) and hotspots of amplitude of active tuberculosis cases by province in the mainland of China, 2005–2012.
Figure 3. Climate zones in China.
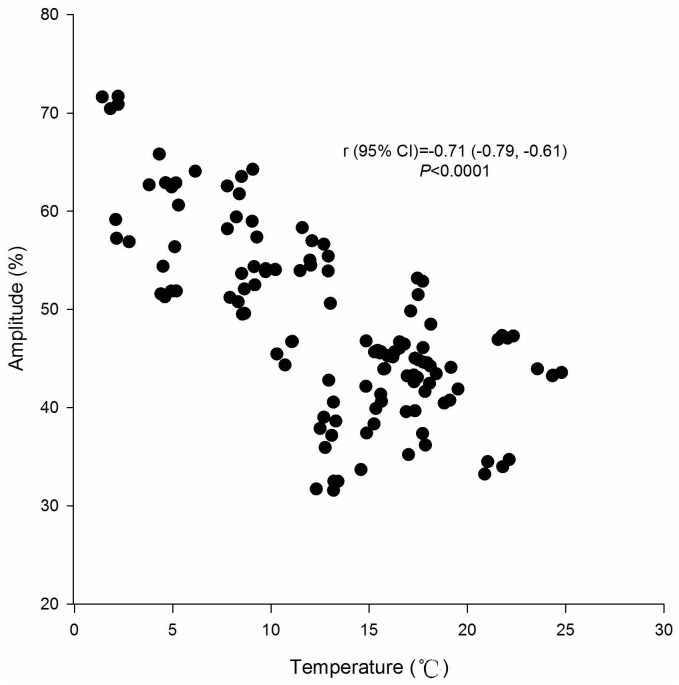
The magnitude of seasonality was inversely correlated with temperature, with seasonal amplitude decreasing with increasing annual average temperature by province (r [95% CI] = −0.71 [−0.79, −0.61], P<0.0001) (Figure 4).
Figure 4. Correlation of seasonal amplitude of active tuberculosis cases and annual average temperature by province in the mainland of China, 2008–2012.
Source of meteorological data: National Climate Center of China (http://ncc.cma.gov.cn/cn/). Abbreviations: CI, confidence interval.
Table 2 summarized the peak and trough months with seasonal amplitude for subgroups of each group and the comparisons of seasonal amplitudes. An annual mean of 46.3% (95% CI, 42.3%–50.4%) more active TB cases were notified in the peak month (April) compared with the trough month (February) from 2005 to 2012. The seasonal amplitude (53.6%) of year 2005–2008 was significantly higher than that (40.6%) of year 2009–2012.There was no statistical difference in seasonal amplitude between males and females (45.0% vs. 49.5%, P>0.05). There were significant differences in seasonal amplitude by age, with amplitude ranging from 40.6% to 60.6% (P<0.05). There were significant differences in seasonal amplitude by occupation except the comparison between peasants and migrant workers, in which students had a significantly higher seasonal amplitude than workers (115.3% vs. 48.1%, P<0.05), peasants (115.3% vs. 43.5%, P<0.05) and migrant workers (115.3% vs. 41.6%, P<0.05), and workers had a significantly higher seasonal amplitude than peasants (48.1% vs. 43.5%, P<0.05) and migrant workers (48.1% vs. 41.6%, P<0.05). There were significant differences in seasonal amplitude by forms of TB, including pulmonary TB, pleural TB and other extra-pulmonary TB, with amplitude ranging from 45.9% to 56.3% (P<0.05). Patients with sputum smear negative TB had significantly higher seasonal amplitude compared to patients with sputum smear positive TB (53.0% vs. 42.9%, P<0.001). Relapse cases with sputum smear positive TB had significantly higher seasonal amplitude compared to new cases with sputum smear positive TB (52.2% vs. 41.6%, P<0.001). The peak months occurred in the spring (March, April, May) for all subgroups, with April as most common; the trough months for all subgroups occurred in February.
Table 2. The timing and seasonal amplitude of active tuberculosis cases in the mainland of China, 2005–2012.
| Group | Peak/trough month | Seasonal amplitude (%) | SE (%) | 95% CI (%) | P value | |
| All active TB cases | April/February | 46.3 | 1.6 | 42.3–50.4 | ||
| Period | Year 2005–2008 | April/February | 53.6 | 0.4 | 52.4–54.9 | <0.001§ |
| Year 2009–2012 | April/February | 40.6 | 0.1 | 40.2–41.0 | ||
| Sex | Male | April/February | 45.0 | 1.7 | 40.8–49.3 | >0.05§ |
| Female | April/February | 49.5 | 1.5 | 45.9–53.1 | ||
| Age (years) | 0–14 | May/February | 56.7 | 0.2 | 56.2–57.2 | <0.05¶ |
| 15–24 | April/February | 60.5 | 1.1 | 57.8–63.3 | ||
| 25–44 | March/February | 40.6 | 0.8 | 38.6–42.5 | ||
| 45–64 | March/February | 46.4 | 1.3 | 43.1–49.7 | ||
| 65+ | April/February | 50.9 | 2.4 | 45.0–56.7 | ||
| Occupation | Peasant | March/February | 43.5 | 1.3 | 40.4–46.6 | <0.05¶ † |
| Migrant worker | May/February | 41.6 | 0.2 | 41.0–42.1 | ||
| Worker | April/February | 48.1 | 1.7 | 43.9–52.3 | ||
| Student | April/February | 115.3 | 0.2 | 114.9–115.7 | ||
| Form of disease | Pulmonary TB | April/February | 45.9 | 1.6 | 41.9–49.8 | <0.05¶ |
| Pleural TB | April/February | 52.0 | 0.8 | 50.1–54.0 | ||
| Other extra-pulmonary TB | April/February | 56.3 | 1.2 | 53.4–59.3 | ||
| Sputum smear microscopy | Positive TB | March/February | 42.9 | 1.3 | 39.7–46.1 | <0.001§ |
| Negative TB | April/February | 53.0 | 1.3 | 49.9–56.1 | ||
| Sputum smear positive TB | New case | March/February | 41.6 | 1.3 | 38.5–44.8 | <0.001§ |
| Relapse case | March/February | 52.2 | 1.0 | 49.7–54.8 | ||
Abbreviations: SE, standard error; CI, confidence interval.
Two-tailed two independent samples Student’s t-test for difference in seasonal amplitudes.
Student-Newman-Keuls Method of one way analysis of variance for all pairwise multiple comparison among seasonal amplitudes.
P value>0.05 for difference in seasonal amplitudes between subgroup of peasant and subgroup of migrant worker.
Discussion
The X-12-ARIMA program was used to analyze the TB seasonality in this study. A study indicated that ARIMA model is the most appropriate model for forecasting seasonality pattern of seasonal diseases since it has the more relatively accuracy than models of linear regression, moving average, decomposition, Holt-Winter’s and artificial neural network. [24] Compared with ARIMA, maybe the X-12-ARIMA program is more appropriate model. The chief source of the X-12-ARIMA program is the extensive set of time series model building facilities built into the program for fitting the regARIMA models. These are regression models with ARIMA errors, in which the mean function of the time series (or its logs) is described by a linear combination of regressors, and the covariance structure of the series is that of an ARIMA process. [23].
In this study, we found TB was seasonal disease in China, whose notification figures were lowest between January and February in winter and highest between March and May in spring. The peak months were similar to what had been reported in some countries in the northern hemisphere, such as Kuwait, Mongolia, Netherlands and United States,[6]–[8], [15], [17] and earlier than other countries, such as India, Japan, Spain and United Kingdom. [4], [5], [10], [11], [13] On the contrary, the trough months were later than that of all countries noted above.
There are some delays between TB infection and notification. First, there is a long delay between onset of symptoms and initiation of notification among TB cases. The median delay was 65 days in China, [25] which also was observed in other counties. [26], [27] Additionally, maybe the Spring Festival, a Chinese traditional festival, is a special reason of delay in January or February generally, during which patients often get used to delaying health-seeking when they fall ill because health-seeking or sickness is regarded as an unlucky thing. [18] Second, there is an incubation period between infection and onset of symptoms. It was reported that the geometric mean incubation period of TB was 20.8 weeks. [28] Thus, we might infer that the peak and trough months of TB transmission are in winter and in autumn respectively, corresponding to that of TB notification in spring and in winter in China. Previous studies in many countries in the northern hemisphere also indicated that a seasonal pattern of TB with a mostly predominant peak was seen during the spring and summer seasons, which leads to assume that the risk of TB transmission appears to be the greatest during winter months. [29].
Close contact of indoor winter crowding has to be considered first for TB transmission. In winter the indoor activities are much more common than in a warm climate, which increase the probability of healthy people exposing to tubercle bacilli expelled from the infected persons in a room with closed windows for a longer period of time.[29]–[31] Maybe public transportation during the Spring Festival is another opportunity of close contact crowding in winter in China. Wherever they are, hundreds of millions of people traditionally return hometown to reunite with their family and relatives by public transportation, such as train, bus, plane or ship during this festival every year. An investigation in United States indicated the limited transmission of TB from a potentially highly infectious passenger to other persons during extended train and bus travel. [32] Therefore, we suppose that it is a good opportunity of TB transmission for a great deal of population who make a long journey in the closed coaches in winter, even though we have not direct proofs in this study.
Besides close contact of indoor crowding that is route of transmission, persons with lower Vitamin D level may be susceptible for TB infection in winter. [2], [3], [5], [7], [8], [16]–[18], [29], [31], [33] A systemic review showed that deficiency of Vitamin D impaired host immunological defense with TB infection, and serum Vitamin D concentrations gradually decreasing in autumn and winter; less sunlight exposure because of heavy clothing or indoor activities in winter influenced serum Vitamin D concentrations. [29] In this study, we found seasonal amplitude of TB were higher in temperate continental, plateau and mountain climate zones and lower in tropical and subtropical monsoon climate zones, which tended to increase while annual average temperature by province decreased in China. It was indicated that seasonal amplitudes varied with climate zone and were higher in provinces with cold temperatures for an extended part of the year. Therefore, deficiency of Vitamin D in winter due to reduced sunlight exposure may be another contributor to TB infection in China.
The seasonal pattern of TB is possibly produced by other factors. A wide variety of respiratory infectious diseases both viral and bacterial show the seasonal cycle with a winter peak, [14] which can not cause TB but may accelerate disease manifestation in patients with latent TB or increase susceptibility of individuals to infection through suppressing host immunologic capacity. [8] Seasonal variations in the nutrient intakes and the meal patterns of humans possibly affect immune system functions, and the immune system competency itself also varies periodically through the year, which may be linked with seasonal variability of TB. [29] This study was an analysis of TB surveillance data so that we had not the findings to discuss or support these alternative factors for TB seasonality, which was the main limitation of this study.
Despite the relatively large difference in TB rates, there was no significant difference in the seasonal amplitude between the two sexes in this study, which was consistent with studies conducted in United States, India and Hong Kong. [7], [8], [10], [18] However, the seasonal amplitudes of persons aged ≤24 years old were significantly higher than other persons in this study, which suggested they must have had relatively recent TB infection in winter compared with the elderly who may have been infected many years earlier. These findings were same as the study in United States. [8] The seasonal amplitudes of students were far higher than that of other population in this study maybe because seasonal TB outbreaks happened in schools. Epidemiological survey of TB outbreak in a senior high school in China suggested actually TB transmission among students happened in the closed classrooms in winter even though the outbreak was found in spring or summer. [34] Therefore, TB seasonality of students may be another evidence for recent TB transmission.
Willis, et al. [8] thought that TB resulting from recent transmission was more influenced by season than TB resulting from activation of latent infection but Parrinello, et al. [7] didn’t think so. The opinion of the latter was approved by findings in this study that seasonal amplitudes of relapse sputum smear positive TB cases were higher than that of new sputum smear positive TB cases, which may indicate activation of latent TB infection in winter. We also found that patients with extra-pulmonary TB including TB pleurisy were higher than that of patients with pulmonary TB, which also may support the opinion of activation of latent TB infection because a study of molecular epidemiology showed extra-pulmonary TB was less likely than pulmonary TB to be a result of recent transmission. [35].
This study based on a sample size of nationwide scale for 8 years with substantial information regarding patient-level and province-level characteristics was able to cast light on the potential characteristics and determinants of TB seasonality, while it was unable to illuminate the cause of seasonal variation in disease directly. We found that the seasonal amplitudes of TB notification went down during the years when the number of TB notification decreased, which suggested that there are relationships between TB notification and seasonal amplitude. We hope these findings guide the direction of future research regarding factors related to TB seasonality and targeting time of the year when intervention should be done to cut down the peak of TB transmission.
Acknowledgments
The authors gratefully acknowledge the staff of TB dispensaries at all levels participating in the National TB Information Management System in China, and are grateful to the contributions of Dr. Veronica L. Tallo and Dr. Mari Rose A de los Reyes from Research Institute for Tropical Medicine, Philippines, and Ms. Victoria Paul from WHO Regional Office for the Western Pacific, for reading and commenting on the initial draft.
Funding Statement
The authors have no support or funding to report.
References
- 1. Mabaera B, Naranbat N, Katamba A, Laticevschi D, Lauritsen JM, et al. (2009) Seasonal variation among tuberculosis suspects in four countries. International Health 1: 53–60. [DOI] [PubMed] [Google Scholar]
- 2. Chan TY (1999) Seasonal variations in vitamin-D status and the incidence of tuberculosis in different countries. Respiration 66: 196. [DOI] [PubMed] [Google Scholar]
- 3. Martineau AR, Nhamoyebonde S, Oni T, Rangaka MX, Marais S, et al. (2011) Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A 108: 19013–19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagayama N, Ohmori M (2006) Seasonality in various forms of tuberculosis. Int J Tuberc Lung Dis 10: 1117–1122. [PubMed] [Google Scholar]
- 5. Luquero FJ, Sanchez-Padilla E, Simon-Soria F, Eiros JM, Golub JE (2008) Trend and seasonality of tuberculosis in Spain, 1996–2004. Int J Tuberc Lung Dis 12: 221–224. [PubMed] [Google Scholar]
- 6. Naranbat N, Nymadawa P, Schopfer K, Rieder HL (2009) Seasonality of tuberculosis in an Eastern-Asian country with an extreme continental climate. Eur Respir J 34: 921–925. [DOI] [PubMed] [Google Scholar]
- 7. Parrinello CM, Crossa A, Harris TG (2012) Seasonality of tuberculosis in New York City, 1990–2007. Int J Tuberc Lung Dis 16: 32–37. [DOI] [PubMed] [Google Scholar]
- 8. Willis MD, Winston CA, Heilig CM, Cain KP, Walter ND, et al. (2012) Seasonality of tuberculosis in the United States, 1993–2008. Clin Infect Dis 54: 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ane-Anyangwe IN, Akenji TN, Mbacham WF, Penlap VN, Titanji VP (2006) Seasonal variation and prevalence of tuberculosis among health seekers in the South Western Cameroon. East Afr Med J 83: 588–595. [DOI] [PubMed] [Google Scholar]
- 10. Behera D, Sharma PP (2011) A retrospective study of seasonal variation in the number of cases diagnosed at a tertiary care tuberculosis hospital. Indian J Chest Dis Allied Sci 53: 145–152. [PubMed] [Google Scholar]
- 11. Douglas AS, Strachan DP, Maxwell JD (1996) Seasonality of tuberculosis: the reverse of other respiratory diseases in the UK. Thorax 51: 944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atun RA, Samyshkin YA, Drobniewski F, Kuznetsov SI, Fedorin IM, et al. (2005) Seasonal variation and hospital utilization for tuberculosis in Russia: hospitals as social care institutions. Eur J Public Health 15: 350–354. [DOI] [PubMed] [Google Scholar]
- 13. Thorpe LE, Frieden TR, Laserson KF, Wells C, Khatri GR (2004) Seasonality of tuberculosis in India: is it real and what does it tell us? Lancet 364: 1613–1614. [DOI] [PubMed] [Google Scholar]
- 14. Schaaf HS, Nel ED, Beyers N, Gie RP, Scott F, et al. (1996) A decade of experience with Mycobacterium tuberculosis culture from children: a seasonal influence on incidence of childhood tuberculosis. Tuber Lung Dis 77: 43–46. [DOI] [PubMed] [Google Scholar]
- 15. Akhtar S, Mohammad HG (2008) Seasonality in pulmonary tuberculosis among migrant workers entering Kuwait. BMC Infect Dis 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Douglas AS, Ali S, Bakhshi SS (1998) Does vitamin D deficiency account for ethnic differences in tuberculosis seasonality in the UK? Ethn Health 3: 247–253. [DOI] [PubMed] [Google Scholar]
- 17. Korthals Altes H, Kremer K, Erkens C, van Soolingen D, Wallinga J (2012) Tuberculosis seasonality in the Netherlands differs between natives and non-natives: a role for vitamin D deficiency? Int J Tuberc Lung Dis 16: 639–644. [DOI] [PubMed] [Google Scholar]
- 18. Leung CC, Yew WW, Chan TY, Tam CM, Chan CY, et al. (2005) Seasonal pattern of tuberculosis in Hong Kong. Int J Epidemiol 34: 924–930. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (2011) Global tuberculosis control: WHO report 2011. Geneva, Switzerland: World Health Organization Press. WHO/HTM/TB/2011.16.
- 20. Liu L, Zhao XQ, Zhou Y (2010) A tuberculosis model with seasonality. Bull Math Biol 72: 931–952. [DOI] [PubMed] [Google Scholar]
- 21. Hu X (2012) Threshold dynamics for a tuberculosis model with seasonality. Math Biosci Eng 9: 111–122. [DOI] [PubMed] [Google Scholar]
- 22. Huang F, Du X, Chen W, Cheng S, Wang L (2011) Introduction of tuberculosis information management syetem in China. China Digital Medicine 6: 97–99. [Google Scholar]
- 23.Time Series Staff of Census Bureau’s Statistical Research Division (2011) X-12-ARIMA Reference Manual (Version 0.3). Washington, D.C. Census Bureau website. Available: http://www.census.gov/ts/x12a/v03/x12adocV03.pdf?. Accessed 2013 January 28.
- 24. Permanasari AE, Rambli DR, Dominic PD (2011) Performance of univariate forecasting on seasonal diseases: the case of tuberculosis. Adv Exp Med Biol 696: 171–179. [DOI] [PubMed] [Google Scholar]
- 25. Bai LQ, Xiao SY (2004) [Factors associated with diagnostic delay for patients with smear-positive pulmonary tuberculosis in rural Hunan, China]. Zhonghua Jie He He Hu Xi Za Zhi 27: 617–620. [PubMed] [Google Scholar]
- 26. Tattevin P, Che D, Fraisse P, Gatey C, Guichard C, et al. (2012) Factors associated with patient and health care system delay in the diagnosis of tuberculosis in France. Int J Tuberc Lung Dis 16: 510–515. [DOI] [PubMed] [Google Scholar]
- 27. Belay M, Bjune G, Ameni G, Abebe F (2012) Diagnostic and treatment delay among Tuberculosis patients in Afar Region, Ethiopia: A cross-sectional study. BMC Public Health 12: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ten Asbroek AH, Borgdorff MW, Nagelkerke NJ, Sebek MM, Deville W, et al. (1999) Estimation of serial interval and incubation period of tuberculosis using DNA fingerprinting. Int J Tuberc Lung Dis 3: 414–420. [PubMed] [Google Scholar]
- 29. Fares A (2011) Seasonality of tuberculosis. J Glob Infect Dis 3: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rios M, Garcia JM, Sanchez JA, Perez D (2000) A statistical analysis of the seasonality in pulmonary tuberculosis. Eur J Epidemiol 16: 483–488. [DOI] [PubMed] [Google Scholar]
- 31. Janmeja AK, Mohapatra PR (2005) Seasonality of tuberculosis. Int J Tuberc Lung Dis 9: 704–705. [PubMed] [Google Scholar]
- 32. Moore M, Valway SE, Ihle W, Onorato IM (1999) A train passenger with pulmonary tuberculosis: evidence of limited transmission during travel. Clin Infect Dis 28: 52–56. [DOI] [PubMed] [Google Scholar]
- 33. Davies PD (1997) Seasonality of tuberculosis. Thorax 52: 398. [PubMed] [Google Scholar]
- 34. Chen W, Xia Y, Li X, Zhou L, Li C, et al. (2012) A tuberculosis outbreak among senior high school students in china in 2011. J Int Med Res 40: 1830–1839. [DOI] [PubMed] [Google Scholar]
- 35. Torgersen J, Dorman SE, Baruch N, Hooper N, Cronin W (2006) Molecular epidemiology of pleural and other extrapulmonary tuberculosis: a Maryland state review. Clin Infect Dis 42: 1375–1382. [DOI] [PubMed] [Google Scholar]