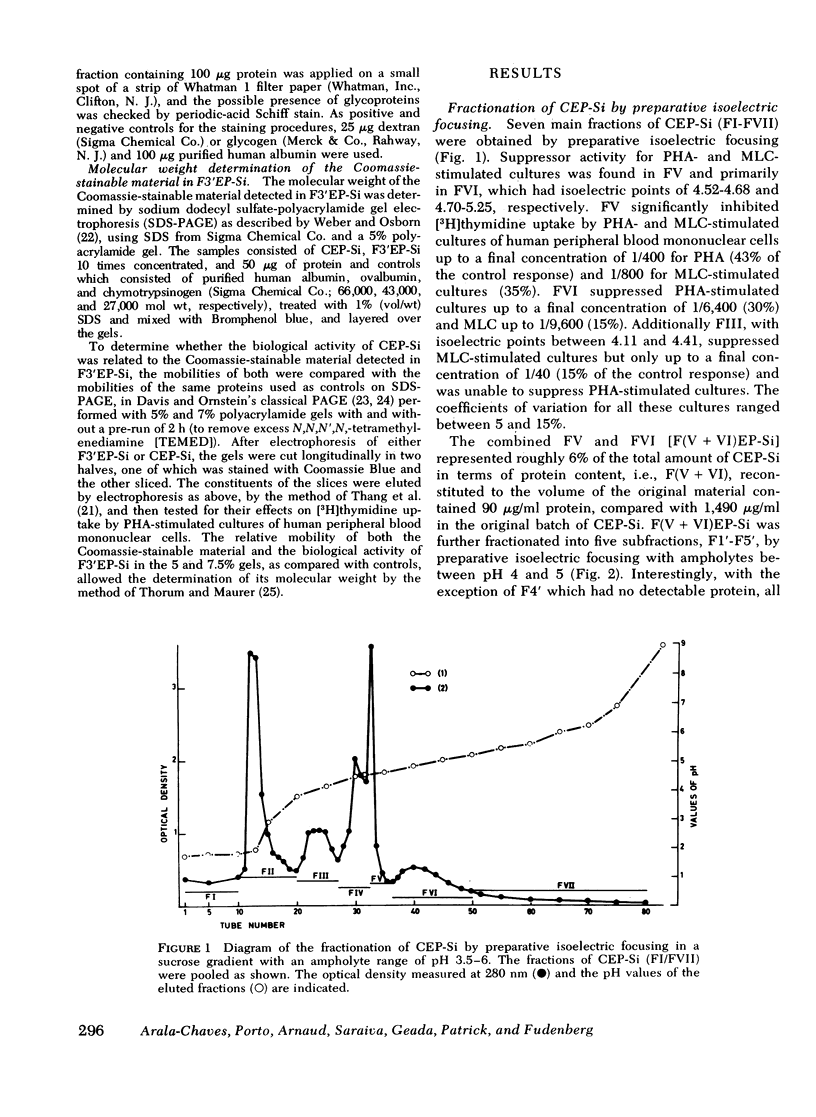
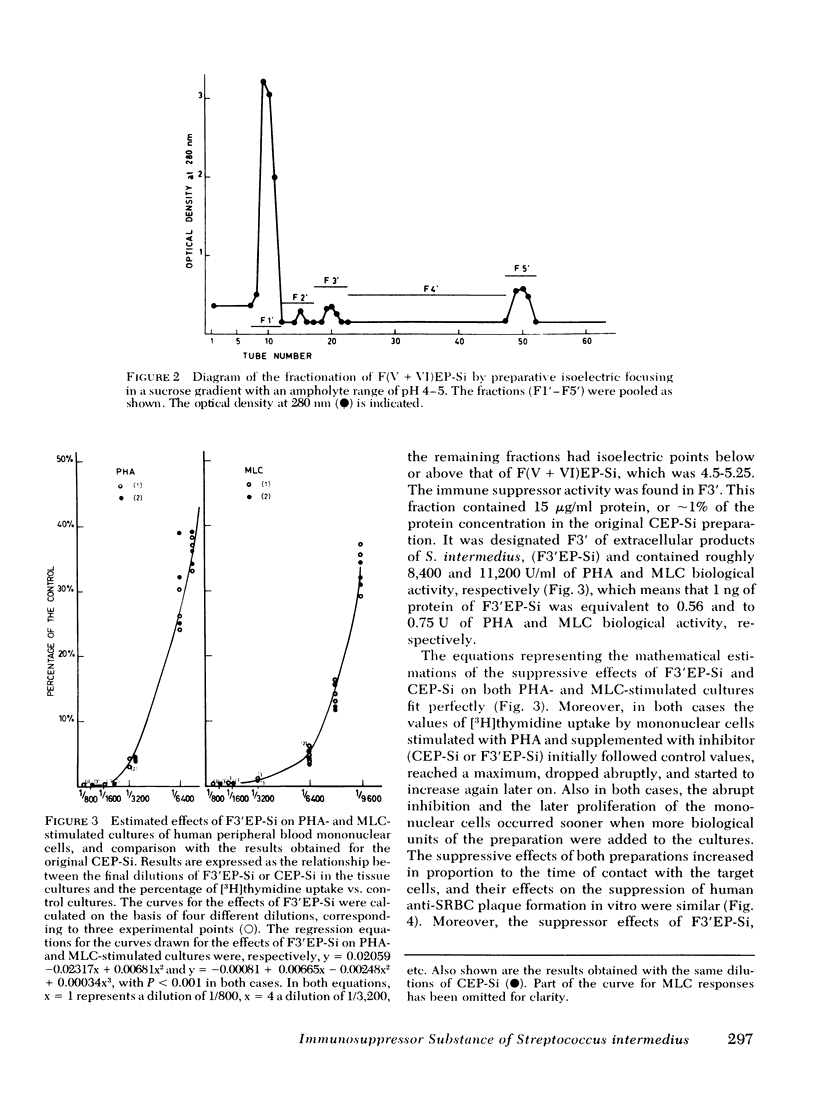
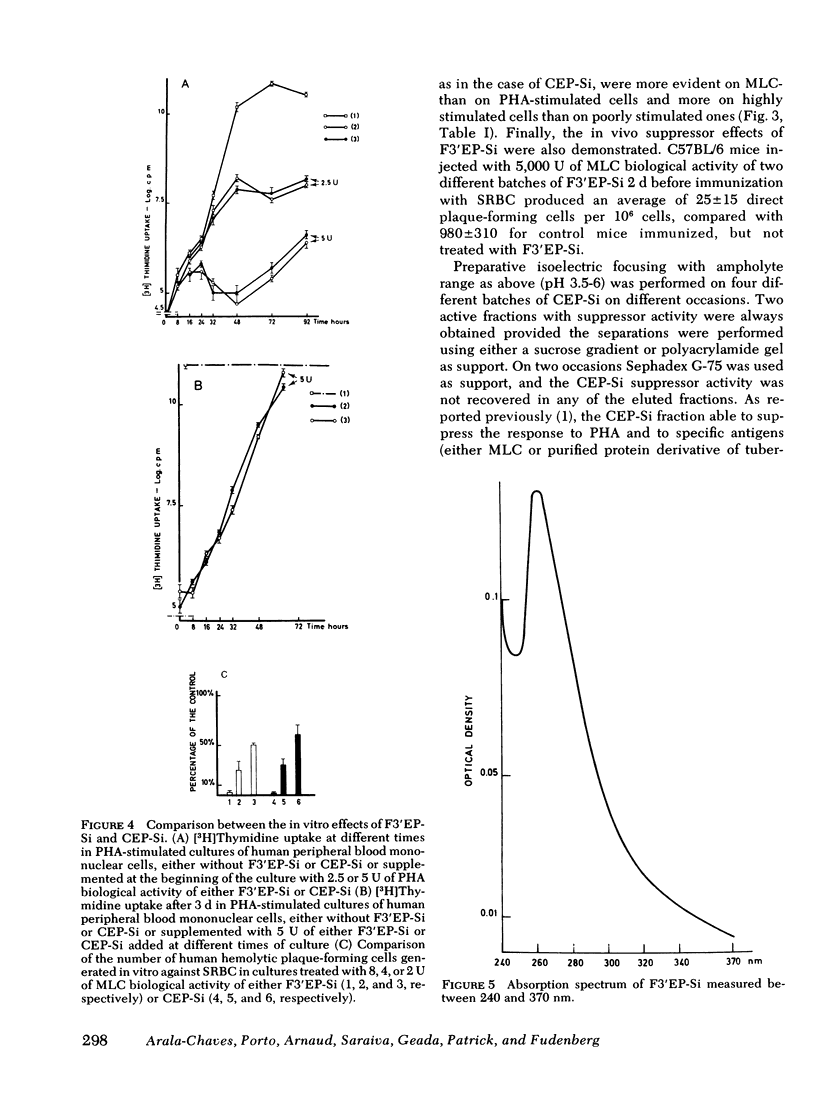
Abstract
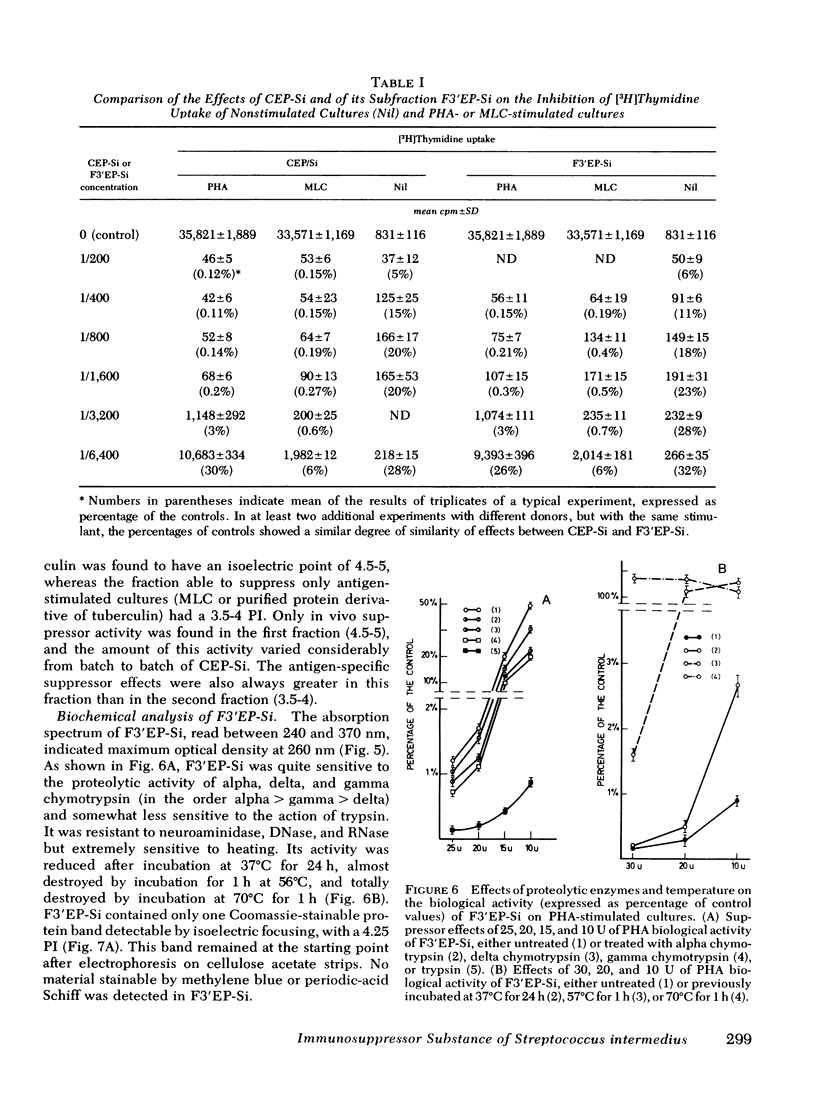
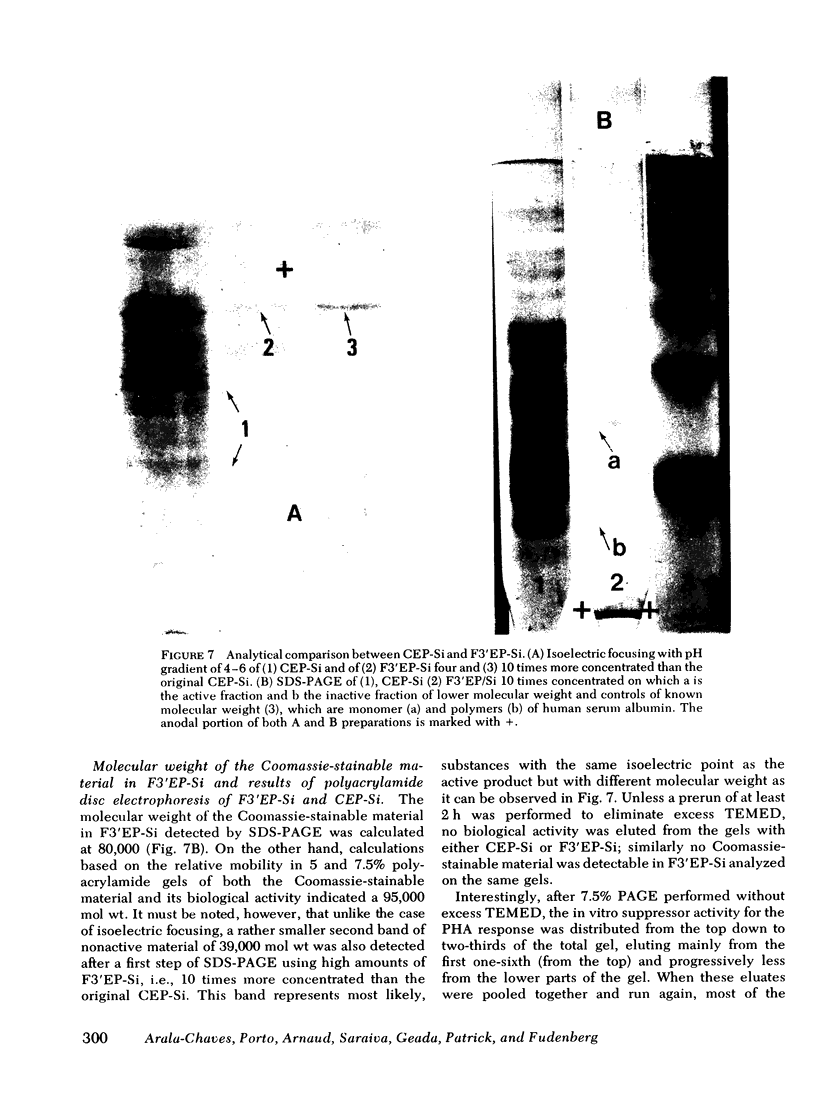
The noncytotoxic immunosuppressive substance detected in crude extracellular products of Streptococcus intermedius (CEP-SI) was fractionated by two steps of preparative isoelectric focusing in sucrose gradients using ampholytes of pH range from 3.5 to 6 and 4 to 5, respectively. The in vitro and in vivo suppressor effects of the most highly purified fraction of CEP-Si, designated fraction 3' (F3'EP-Si), corresponded well with those of the original CEP-Si. F3'EP-Si was sensitive to the effects of alpha, gamma, and delta chymotrypsin, trypsin, and heating. It contained approximately 1% of the total amount of protein found in the original CEP-Si, corresponding to a single band on analytical isoelectric focusing, stainable by Coomassie Blue and of isoelectric point of 4.25. The absorption spectrum of F3'EP-Si had a maximum at 260 nm but its biological activity was resistant to deoxyribonuclease and ribonuclease A and it did not contain material stainable by methylene blue. It was also resistant to neuraminidase and did not contain material stainable by periodic acid schiff. We conclude that the substance responsible for the suppressor activity of CEP-Si is a protein of molecular weight approximately 90,000, which adheres to Sephadex of cellulose acetate and forms complexes with other, nonactive constituents of CEP-Si.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud P., Chapuis-Cellier C., Vittoz P., Fudenberg H. H. Genetic polymorphism of serum alpha-1-protease inhibitor (alpha-1-antitrypsin): Pi i, a deficient allele of the Pi system. J Lab Clin Med. 1978 Aug;92(2):177–184. [PubMed] [Google Scholar]
- Böyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7–7. [PubMed] [Google Scholar]
- Carpenter C. B., Milton J. D., Mowbray J. F., Butterworth A. E. Immunosuppressive properties of a ribonuclease-containing fraction from bacterial cultures. Immunology. 1972 Aug;23(2):171–182. [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Du Bois M. G., Huismans D. R., Schellekens P. T., Eijsvoogel V. P. Investigation and standardization of the conditions for micro-lymphocyte cultures. Tissue Antigens. 1973;3(5):402–409. doi: 10.1111/j.1399-0039.1973.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Evans R., Booth C. G. Inhibition of 125IUdR incorporation by supernatants from macrophage and lymphocyte cultures: a cautionary note. Cell Immunol. 1976 Sep;26(1):120–126. doi: 10.1016/0008-8749(76)90354-3. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Activation of human B lymphocytes. I. Direct plaque-forming cell assay for the measurement of polyclonal activation and antigenic stimulation of human B lymphocytes. J Exp Med. 1976 Sep 1;144(3):674–684. doi: 10.1084/jem.144.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Polyclonal activation of bone-marrow-derived lymphocytes from human peripheral blood measured by a direct plaque-forming cell assay. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3676–3679. doi: 10.1073/pnas.73.10.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görg A., Postel W., Westermeier R. Ultrathin-layer isoelectric focusing in polyacrylamide gels on cellophane. Anal Biochem. 1978 Aug 15;89(1):60–70. doi: 10.1016/0003-2697(78)90726-1. [DOI] [PubMed] [Google Scholar]
- HJERTEN S. "Molecular sieve" chromatography on polyacrylamide gels, prepared according to a simplified method. Arch Biochem Biophys. 1962 Sep;Suppl 1:147–151. [PubMed] [Google Scholar]
- Hersh E. M. Immunosuppressive enzymes. Transplant Proc. 1973 Sep;5(3):1211–1214. [PubMed] [Google Scholar]
- Hersh E. M. L-glutaminase: suppression of lymphocyte blastogenic responses in vitro. Science. 1971 May 14;172(3984):736–738. doi: 10.1126/science.172.3984.736. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Vesole D. H., Goust J. M. Inhibitory effects of extracellular products from oral bacteria on human fibroblasts and stimulated lymphocytes. Infect Immun. 1978 Aug;21(2):567–574. doi: 10.1128/iai.21.2.567-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoessli D. C., Jones A. P., Waksman B. H. Potentiation of the T lymphocyte response to mitogens IV. Serum-free production and testing of macrophage soluble products. Cell Immunol. 1977 May;30(2):310–320. doi: 10.1016/0008-8749(77)90074-0. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller G. A., Urban J., Jackson R. W. Effects of a streptococcal lipoteichoic acid on host responses in mice. Infect Immun. 1976 May;13(5):1408–1417. doi: 10.1128/iai.13.5.1408-1417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjardino J., Hall V. Extraction and purification of rat alpha-fetoprotein. Biochem Med. 1977 Apr;17(2):173–179. doi: 10.1016/0006-2944(77)90021-7. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. II. Preparative isoelectric focusing. Biochim Biophys Acta. 1975 Mar 28;386(1):181–195. doi: 10.1016/0005-2795(75)90258-5. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Dondon L., Godefroy-Colburn T. Degradation of Escherichia coli polynucleotide phosphorylase by E. coli endogenous proteases and by trypsin. Biochimie. 1971;53(3):291–302. doi: 10.1016/s0300-9084(71)80095-0. [DOI] [PubMed] [Google Scholar]