Abstract
Persistent high-risk human papillomavirus (HR-HPV) infection is the strongest risk factor for high-grade cervical precancer. We performed a systematic review and meta-analysis of HPV persistence patterns worldwide. Medline and ISI Web of Science were searched through January 1, 2010 for articles estimating HPV persistence or duration of detection. Descriptive and meta-regression techniques were used to summarize variability and the influence of study definitions and characteristics on duration and persistence of cervical HPV infections in women. Among 86 studies providing data on over 100,000 women, 73% defined persistence as HPV positivity at a minimum of two time points. Persistence varied notably across studies and was largely mediated by study region and HPV type, with HPV-16, 31, 33 and 52 being most persistent. Weighted median duration of any-HPV detection was 9.8 months. HR-HPV (9.3 months) persisted longer than low-risk HPV (8.4 months), and HPV-16 (12.4 months) persisted longer than HPV-18 (9.8 months). Among populations of HPV positive women with normal cytology, the median duration of any-HPV detection was 11.5 and HR-HPV detection was10.9 months. In conclusion, we estimated that approximately half of HPV infections persist past 6–12 months. Repeat HPV testing at 12 month intervals could identify women at increased risk of high-grade cervical precancer due to persistent HPV infections.
Keywords: human papillomavirus, HPV, duration, persistence, clearance, natural history, repeat testing, literature review, screening, cervical cancer, meta-analysis
INTRODUCTION
Infection with high-risk human papillomavirus (HR-HPV) genotypes is considered necessary for the development of invasive cervical cancer (ICC)1–4. Despite the high prevalence of HR-HPV infections among women, the incidence of ICC is comparatively low, which highlights the importance of factors that may mediate progression to invasive cancer. Persistent HR-HPV infection has been consistently and strongly associated with cervical intraepithelial neoplasia (CIN) of grades 2 and 35 and is considered essential for the progression of cervical precancer to ICC6. The higher sensitivity of HPV testing but lower specificity, as compared with cytology for the detection of CIN2–37–9, suggest that co-testing with cytology and HPV could enhance accuracy in identifying women at high risk of cervical precancer and cancer5, 10. Recent ASC/ASCCP/ASCP guidelines for cervical cancer screening in women older than 30 years recommend that one option for women with normal cytology and a positive HPV testing is rescreening in one year with Pap smear and HPV co-testing11. Implementation of repeat HPV testing in screening requires a clinically relevant definition of HPV persistence and a better understanding of HPV persistence duration.
There has been wide variation in definitions of HPV persistence used in the literature. Given that aspects of the definition of persistence, such as the frequency and length of testing intervals, may affect the estimated risk of cervical precancer associated with HPV persistence5, persistence and duration of HPV should be better understood to operationalize inclusion of repeat testing into future cervical cancer screening programs and for use as an endpoint in HPV vaccine trials and efficacy modeling. To date, there is no summary of the literature that examines definitions and estimates of HPV persistence across studies. Thus, we performed a systematic review and meta-analysis to determine the influence of HPV persistence definitions and study characteristics on estimated duration of HPV infection and proportion of women with persistently detectable HPV DNA over time.
METHODS
Eligibility and data abstraction
Studies published through January 1, 2010 were identified by searching ISI Web of Science, MEDLINE via PubMed, and the reference lists from eligible articles and relevant review articles, with no language restrictions. Only original peer-reviewed journal articles were included. Broad search term categories included HPV (e.g. HPV, human papillomavirus) and persistence (e.g. persistence, clearance, duration) (see online supplement for full set of terms).
To be included in this review, eligible articles had to present one or more measures of HPV persistence over time, regardless of study design. The overall prevalence of cervical abnormalities, as measured and defined by each study (generally atypical squamous cells of undetermined significance (ASCUS) or greater), at baseline in the study population had to be less than 15% to approximately reflect the characteristics of populations of average risk12. However, population-based studies that did not explicitly state the prevalence of cervical abnormalities were included. Only human immunodeficiency virus (HIV) negative study populations were included; articles that did not state HIV serostatus were assumed to be HIV negative and were included. Studies that tested for cervical or cervicovaginal HPV infections using polymerase chain reaction (PCR) or Hybrid Capture (HC; QIAGEN Gaithersburg, Inc.) DNA detection methods were included. We excluded serology-, male- and low-risk HPV (LR-HPV)-only studies, post-treatment studies, studies with less than three months of total study follow-up, and studies that included only buccal, nasopharyngeal, anal, vulvar or labial specimens. All search results were independently reviewed to ensure that no pertinent articles were omitted.
Abstracted persistence data included (i) proportion of HPV-positive (incident or prevalent) women with persistent infections and the standard error or confidence interval, (ii) median and/or mean duration of HPV infection, and (iii) HPV persistence estimates extracted from Kaplan-Meier survival curves. Variables used to define persistence were abstracted, which included HPV testing interval, number of HPV positive tests, minimum duration of HPV infection to be considered persistent, whether persistence was based on detection of the same HPV type at visit ‘v’ and ‘v+1’ (type-specific persistence) or detection of any HPV type at consecutive visits (non-type-specific), and HPV type (e.g., HPV-16) or HPV grouping (e.g., HR-HPV positivity)). Data were abstracted on population characteristics and study methods, including geographical region, study design, sample size, population and HPV detection method. All data were independently double-abstracted to ensure accuracy. Study authors were contacted if clarification of published information on HPV persistence was needed.
Selection of Persistence Estimates
Many articles that met the inclusion criteria were based on the same study population (28 articles from 11 populations). To maintain the independence of study results, the article from a given study population was chosen that used the most sensitive detection method (e.g., PGMY09/11 over MY09/11, GP5+/6+ over GP5/6)13–15. If HPV methods were similar, we chose the article with more women in the persistence analysis, or the most recent, if study sample sizes did not vary. If multiple articles from the same study population could contribute to separate analyses (e.g., persistence by baseline HPV status and persistence by HPV type), all relevant articles were included.
Most articles (n=57) presented multiple estimates for the proportion persistent or median duration of infection. A set of decision rules was applied to select one result for each meta-regression or meta-regression stratum: (i) choose the HR-group result first, then any-HPV, then single HR-HPV types based on worldwide HPV prevalence in ICC16: 16, 18, 33, 45, 31, 58, 52, 35; (ii) choose the type-specific result over the non-type-specific result; (iii) choose the result with the greatest number of visits used to define persistence unless substantially fewer (30%) women were tested at that visit; (iv) choose the result with mixed incident and prevalent HPV infections first, then incident, then prevalent; (v) if results are presented by age categories, choose the overall estimate first, then choose youngest to oldest; (vi) if results are presented by HPV type variants, choose the estimate for all variants combined or else the largest sample size; (vii) if results are presented by cytology method (e.g. thin-prep, conventional), choose the overall estimate, then the largest sample size.
Descriptive analyses
The number and proportion of persistence estimates were calculated for each category of a given study characteristic. If an article reported multiple results that fell into different categories of study characteristics, the article was included in all relevant categories so results could add to more than 100%. The median duration of HPV infection could not be formally analyzed by meta-regression because most studies did not provide a measure of random error. Instead, estimates of median HPV duration were graphed on a forest plot for HPV groups and HPV types with at least three estimates of median duration. The pooled duration for each HPV group and type was then calculated as the average, weighted by the number of women that were included in each result. Plotting symbols were sized in proportion to the number of women included in the calculation of median duration for each study. Articles that presented Kaplan-Meier curves or reported the proportion persistent at two or more time points were included in a figure to examine patterns of HPV persistence within and across HPV groups. To examine the effect of age on HPV persistence within study populations, any-HPV or else HR-HPV estimates were plotted by age. The mid-range of each reported age category was used to plot persistence, where grayscale symbols indicates the category of the reported mid-point. All figures were created using R version 2.10.1.
Statistical analyses
The proportion persistent was calculated in different ways in the literature: using either women or infections as the unit of analysis, and including all women or only women with infections in the denominator. To standardize the persistence results for inclusion in the same analyses, the total number of women with an HPV infection at baseline (prevalent persistence) and the total number of women who acquired an incident infection (incident persistence) was used as the denominator to calculate the proportion persistent. If both prevalent and incident infections were included in the persistence estimate, both were included in the denominator. If a persistence result was stratified or limited to a subset of the sample (e.g., women with a given HPV type), the number of women with an infection in that particular stratum was used as the denominator. For studies that did not report a standard error for the proportion persistent, it was calculated as the square root of (p*(1−p))/n, where p was the observed proportion persistent and n was the sample size. In cases where the proportion persistent was 0% or 100% and the standard error was undefined, the following adjustment17 was made to calculate the standard error: the overall meta-regression model was first fitted without study characteristics using all studies with defined standard errors. Based on the summary estimate of 40% persistence at 6 months, the undefined standard errors were then estimated by adding 0.4 to the number of women who persisted and 0.6 to the number of women who did not persist (i.e. +0.4/+0.6 adjustment). If the standard error was not reported and could not be calculated, the result was not included in meta-regression analyses.
Random-effects meta-regression and stratification was used to formally compare differences in proportion persistent estimates across study characteristic categories (i.e., difference between estimates in each category compared to a common referent), with the among-study variance estimated by restricted maximum likelihood18. Stratified summary estimates allowed descriptive comparisons across individual categories of study characteristics (i.e., summary estimates and 95% confidence intervals for each category). Variation between estimates was evaluated by comparing Cochran’s Q two-sided P-value with a 0.1 significance level19. The mean length of the testing interval multiplied by the number of HPV testing intervals used to define persistence was included in models, centered at 6 months, as a means to control for the time over which persistence was measured. If the mean testing interval was not reported, the interval specified in the study protocol or the minimum time required to meet the authors' definition of persistence was used. For these analyses, at least three study estimates in each stratum were required. Studies were allowed to contribute to more than one category to reduce the influence of the decision rules on the distribution of study and population characteristics. When multiple results from the same study population were included an indicator variable for that study population was included in the model to account for the lack of independence. Methods and results for sensitivity analyses on adjustment method and choosing rules are presented in an online supplement. Meta-regression analysis was conducted in STATA version 11 (StataCorp, College Station, TX).
RESULTS
Descriptive results
Eligible studies
Of the 4,203 abstracts identified, 86 studies met the study inclusion criteria and reported non-duplicate results. These studies provided estimates of HPV persistence on over 100,000 women. Most studies were conducted in Europe (40%) and North America (29%), with few from each of the other world regions (Central or South America (20%), Asia (5%), Africa (3%) and Australia (1%)) (Table 1). Over half of the results were among women with an average age of 30 years or older, although average age was not reported in 14% of studies. Most studies (65%) were of populations where 100% of women had normal cytology at baseline and were screening-based cohort studies that employed PCR-based detection methods. MY09/11 alone or MY09/11 in combination with other methods was the most frequently used HPV laboratory detection protocol (43%).
Table 1.
Characteristics of Human Papillomavirus (HPV) Persistence Studies and Results from Published Studies through January 1st, 2010
| No. of results |
% of results |
References | |
|---|---|---|---|
| Study region | |||
| Europe | 34 | 39.5 | 24, 27–29, 34, 36, 39, 43, 51–76 |
| North America | 25 | 29.1 | 21–23, 26, 33, 35, 38, 41, 42, 77–92 |
| Central/South America | 17 | 19.8 | 20, 25, 30–32, 37, 44, 93–102 |
| Africa, Asia, Australiaa | 8 | 9.3 | 40, 103–109 |
| Multicenterb | 2 | 2.3 | 110, 111 |
| Mean age of women | |||
| < 30.0 years | 33 | 38.4 | 23, 24, 27, 28, 30, 33–39, 41–44, 51, 57, 58, 62, 63, 67, 70, 78, 80, 82, 83, 86, 89, 98, 106, 110, 111 |
| ≥ 30.0 years | 50 | 58.1 | 20, 21, 25, 26, 29–40, 53–57, 59–61, 64–66, 68, 69, 71–76, 79, 87, 90–93, 96, 99, 101–104, 107, 108, 112 |
| Not stated | 12 | 14.0 | 22, 52, 81, 84, 85, 88, 94, 95, 97, 100, 105, 109 |
| Baseline abnormal cytologyc | |||
| 0% abnormal | 56 | 65.1 | 21, 23, 24, 26–31, 33, 34, 36, 38–40, 42–44, 51, 52, 54–61, 63, 65–71, 73–76, 78, 79, 81, 82, 84, 88–91, 93, 94, 104, 105, 107, 110, 111 |
| 1–15% abnormal | 8 | 9.3 | 53, 59, 62, 65, 77, 83, 106, 109 |
| Not stated | 24 | 27.9 | 20, 22, 25, 32, 35, 37, 41, 64, 72, 80, 85–87, 92, 95–103, 108 |
| HPV DNA detection method | |||
| MY09/11 ± other primers | 37 | 43.0 | 20, 21, 23, 25, 26, 32, 33, 37, 38, 41, 42, 51, 53, 61, 62, 73, 78, 79, 81, 82, 85, 87–91, 96–103, 108, 109, 112 |
| SPF10, PGMY09/11, GP5+/6+ | 19 | 22.1 | 30, 31, 39, 40, 43, 44, 54, 57, 58, 64, 65, 67–69, 75, 80, 86, 110, 111 |
| pU1M/pU2R, L1C1/L1C2, GP5/6 ± type-specific, other L1 primers | 7 | 8.1 | 27, 36, 66, 71, 95, 105, 106 |
| Type-specific primers | 10 | 11.6 | 22, 24, 28, 39, 52, 72, 83, 84, 92, 104 |
| Hybrid Capture 1/ 2 | 14 | 16.3 | 29, 34, 35, 55, 56, 59, 60, 63, 70, 74, 76, 93, 94, 107 |
| HPV typed | |||
| Any-HPV | 49 | 57.0 | 20–23, 25–27, 30, 32, 33, 37–44, 53, 56, 57, 61, 62, 67, 72, 73, 75, 80–82, 84, 86, 87, 89–92, 96–103, 106, 108, 109, 112 |
| HR-HPV | 56 | 65.1 | 20, 23–25, 29–38, 40, 41, 44, 51, 54–60, 63–65, 67–70, 73–76, 79, 80, 82, 85–87, 91–95, 99–102, 104, 106, 107, 110, 111 |
| HPV-16 | 39 | 45.3 | 20, 22, 24, 25, 28, 30–33, 37–44, 52, 54, 57, 58, 66, 71, 72, 78, 80, 83, 84, 88, 89, 91, 95, 96, 100, 105, 106, 108, 110, 112 |
| HPV-18 | 26 | 30.2 | 20, 22, 25, 30–33, 39–43, 54, 57, 58, 72, 80, 82, 84, 89, 91, 100, 106, 108, 110, 112 |
| LR-HPV | 20 | 23.3 | 20, 23, 25, 30–32, 37, 38, 40, 41, 44, 56, 57, 80, 82, 86, 87, 91, 101, 102 |
| HPV persistence definitione | |||
| ≥2 positive visits | 63 | 73.3 | 20, 23–29, 33, 34, 36–39, 41, 51, 55–62, 64–67, 69, 70, 72–76, 78, 80–85, 87–90, 92–94, 96–101, 103, 104, 106, 109–112 |
| ≥3 positive visits | 12 | 14.0 | 36, 42, 52, 56, 71, 79, 83, 97, 99, 105, 111 |
| Clearance or duration | 19 | 22.1 | 22, 30, 31, 35, 40–44, 53, 54, 63, 68, 86, 91, 93, 95, 108, 112 |
| Persistent pairs | 2 | 2.3 | 21, 102 |
| Minimum HPV persistence duration | |||
| < 6 months | 27 | 31.4 | 20, 23, 25, 28, 32–34, 55, 56, 60, 64, 68, 74, 78, 80, 83, 86, 87, 89, 91, 98, 99, 101, 102, 104, 107, 108 |
| 6 to <12 months | 38 | 44.2 | 21, 22, 27, 30, 31, 34–38, 40–42, 44, 51, 53, 54, 59, 62, 67, 70–72, 75, 82–85, 90, 92, 94, 95, 99, 105, 109–112 |
| ≥12 months | 27 | 31.4 | 24, 26, 29, 34, 36, 38, 39, 52, 56–58, 61, 63, 65, 66, 69, 73, 76, 79, 81, 93, 97, 99, 100, 103, 106, 111 |
| Not stated | 3 | 3.5 | 43, 88, 96 |
| Mean testing interval | |||
| < 6 months | 20 | 23.3 | 20, 25, 28, 32, 33, 56, 68, 78, 80, 83, 86, 89, 91, 99, 101, 102, 104, 105, 107, 108 |
| 6 to <12 months | 35 | 40.7 | 21–23, 27, 30, 31, 34, 38, 40–44, 51, 54, 59, 60, 64, 70–72, 74, 75, 79, 84, 85, 87, 90, 94, 95, 98, 109–112 |
| ≥12 months | 30 | 34.9 | 24, 26, 29, 35–37, 39, 52, 53, 55, 57, 58, 61–63, 65–67, 69, 73, 76, 81, 82, 88, 92, 93, 97, 100, 103, 106 |
| Not stated | 1 | 1.2 | 96 |
| Type-specific HPV persistence | |||
| Type-specific | 62 | 72.1 | 20–25, 27, 28, 30–33, 37–44, 51, 52, 54, 57, 58, 61, 62, 64, 66–69, 71–73, 78–80, 82–89, 91, 92, 95–98, 100–102, 105, 106, 108, 110–112 |
| Non-type-specific | 36 | 41.9 | 24, 26, 29, 34–36, 38–40, 53, 55–57, 59, 60, 63, 65, 67, 70, 73–76, 81, 90, 93, 94, 98, 99, 101, 103, 104, 106, 107, 109, 112 |
| Baseline HPV infection status | |||
| Prevalent | 60 | 69.8 | 23, 27–29, 32–41, 44, 53–71, 73–76, 81, 82, 84, 86–88, 90, 92–97, 100, 101, 103–109 |
| Mixed prevalent and incident | 13 | 15.1 | 22, 23, 28, 30, 34, 41–43, 78, 83, 89, 90, 110 |
| Incident | 19 | 22.1 | 20, 21, 26, 28, 31, 32, 51, 52, 72, 79, 80, 85, 90, 91, 98, 99, 102, 111, 112 |
| Not stated | 2 | 2.3 | 24, 25 |
HPV: human papillomavirus; LR: Low-risk; HR: High-risk
Multicenter: North/South/Central America110, 111 Australia, Belgium, Brazil, Canada, Finland, Germany, Italy, Mexico, Philippines, Spain, Taiwan, Thailand, UK, and USA
Classification based on individual study definitions of abnormal cytology but generally included ASCUS or greater
Any-HPV: any and all HPV types detected; HR-HPV: HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68; LR-HPV: all other genital HPV types.
Persistent pairs: any pair of two consecutive positive visits (v, v+1)
Definitions and characteristics of HPV persistence
HPV persistence was most commonly defined as two or more HPV DNA positive time points (73%; Table 1), whereas other definitions included a minimum of three positive tests, infection duration, and persistent pairs (positive at any pair of visits, v and v+1). Consecutive HPV positive visits were generally required for HPV persistence, but intervening HPV DNA negative visits were allowed in 12 (14%) of the studies20–27. Minimum duration of HPV persistence, defined as the shortest time period of HPV positivity for a woman to be considered persistent, was 6 to <12 months for most studies (41%). The median time between HPV tests (i.e. the testing interval) was 6 months, although there was a wide range of testing intervals, from 1.328 to 117.629 months. Most studies reported type-specific HPV persistence (72%) and persistence among HPV infections that were prevalent at baseline (70%).
Median duration of HPV persistence
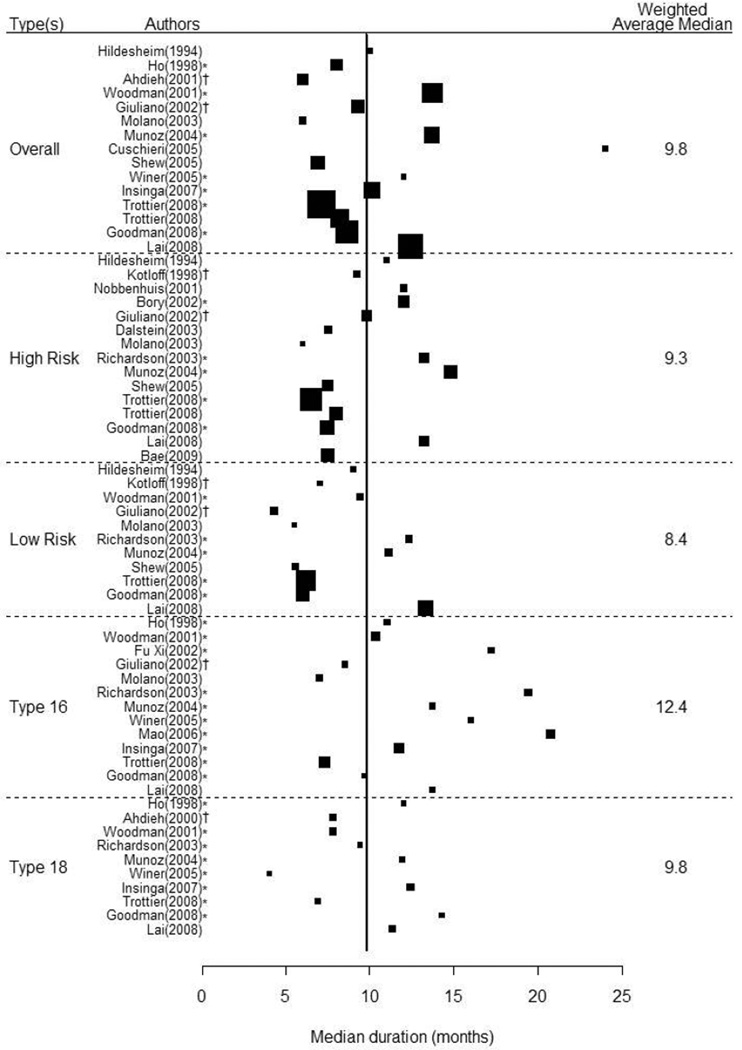
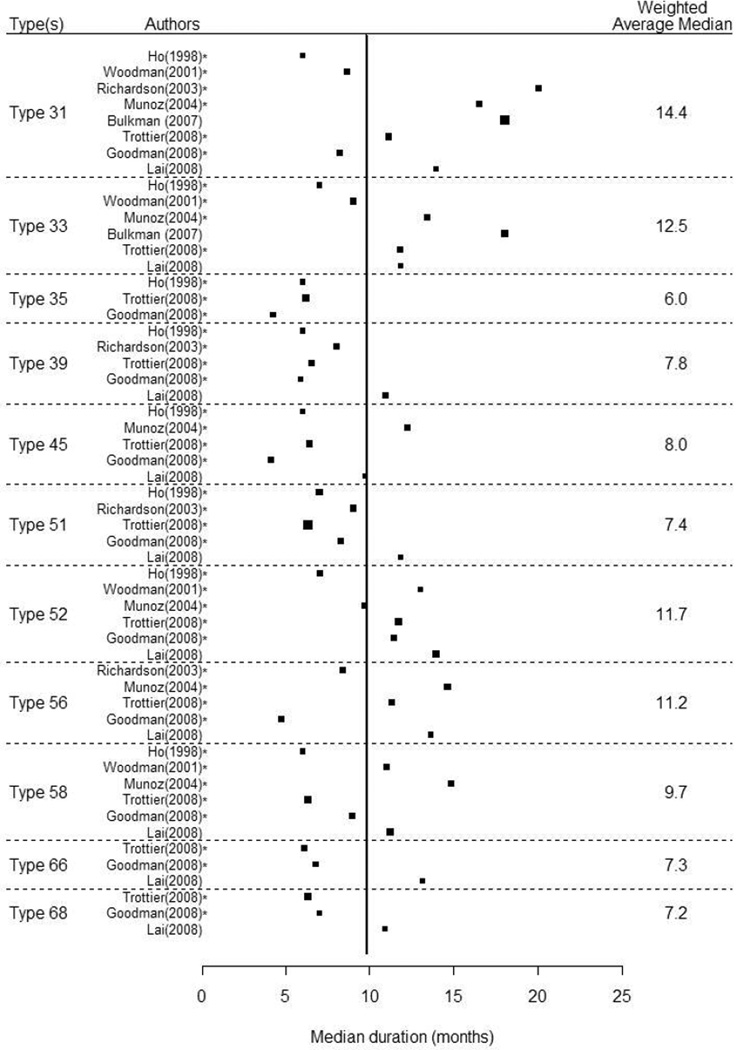
Including all estimates (N=119), regardless of HPV type or group, the average median duration of any-HPV was 9.8 months (Figure 1). There were 15 independent study results for estimation of median duration of any-HPV, which had the widest range estimated median durations, from 6.0 to 24.0 months. Weighted average median duration for any-HPV infection was 9.8 months. HR-HPV (n=15) had a slightly longer median duration at 9.3 months (range: 6.0–14.8) compared to LR-HPV (n=11) at 8.4 months (range: 4.3–13.3). Among the individual HPV-types, median duration was longest for HPV-31, with a weighted average of 14.4 months, followed by HPV-33 at 12.5 months, and HPV-16 at 12.4 months. Median duration of detection of all other high-risk HPV types ranged from 6.0 to 11.7 months. The median durations of any-HPV and HR-HPV from populations of women with 100% normal cytology at baseline were 11.5 and 10.9 months, respectively.
Figure 1.
Forest Plots of the Median Duration of Infection by Human Papillomavirus Group or Type. Estimates are for human papillomavirus infections prevalent at baseline unless otherwise indicated, where * denotes incident infections and † denotes a mixture of prevalent and incident infections. The vertical line represents the overall, weighted median of 9.8 months.
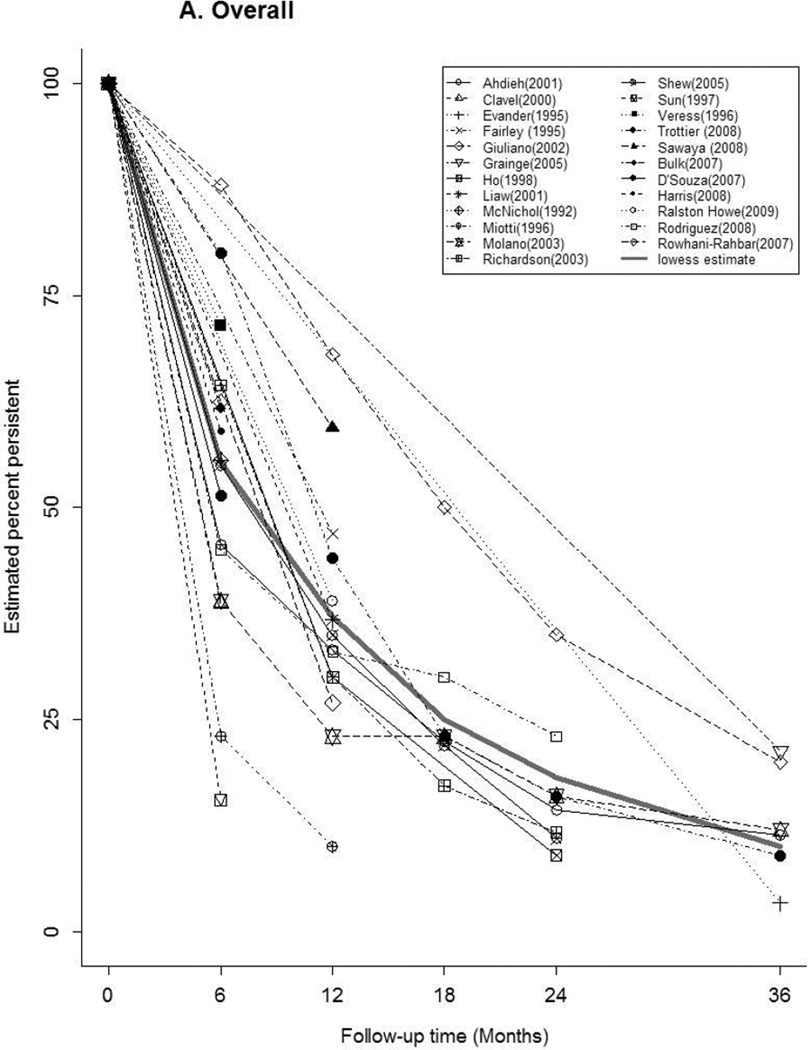
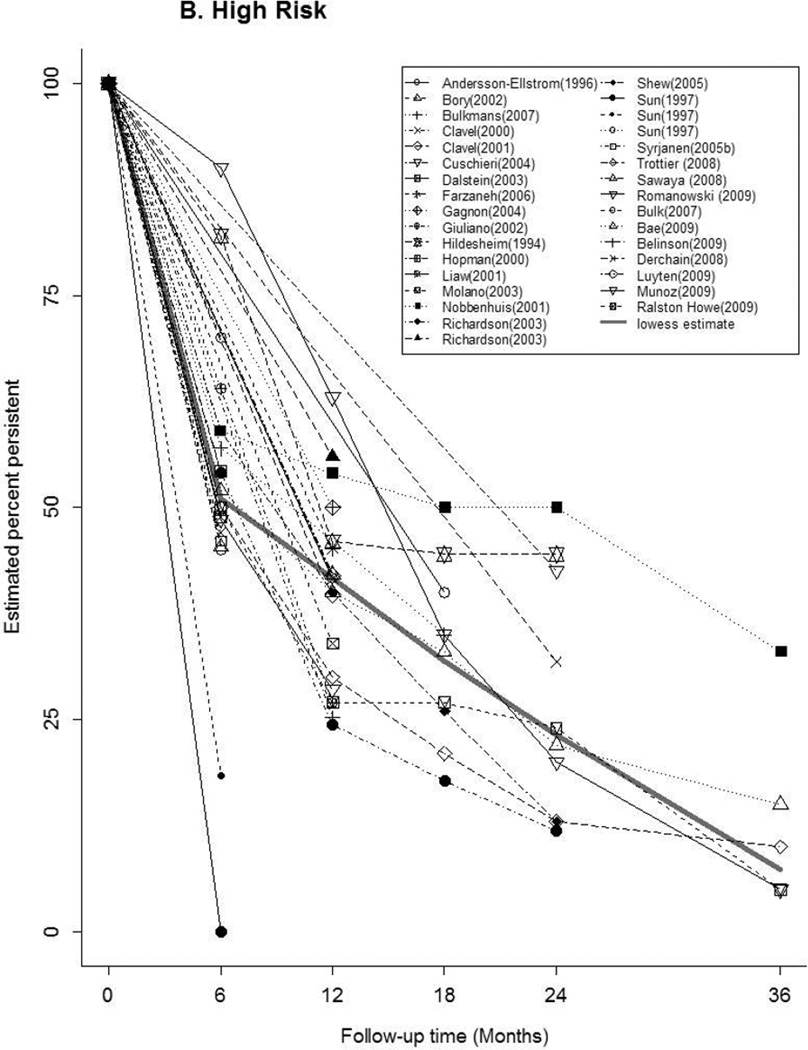
Time-specific HPV persistence
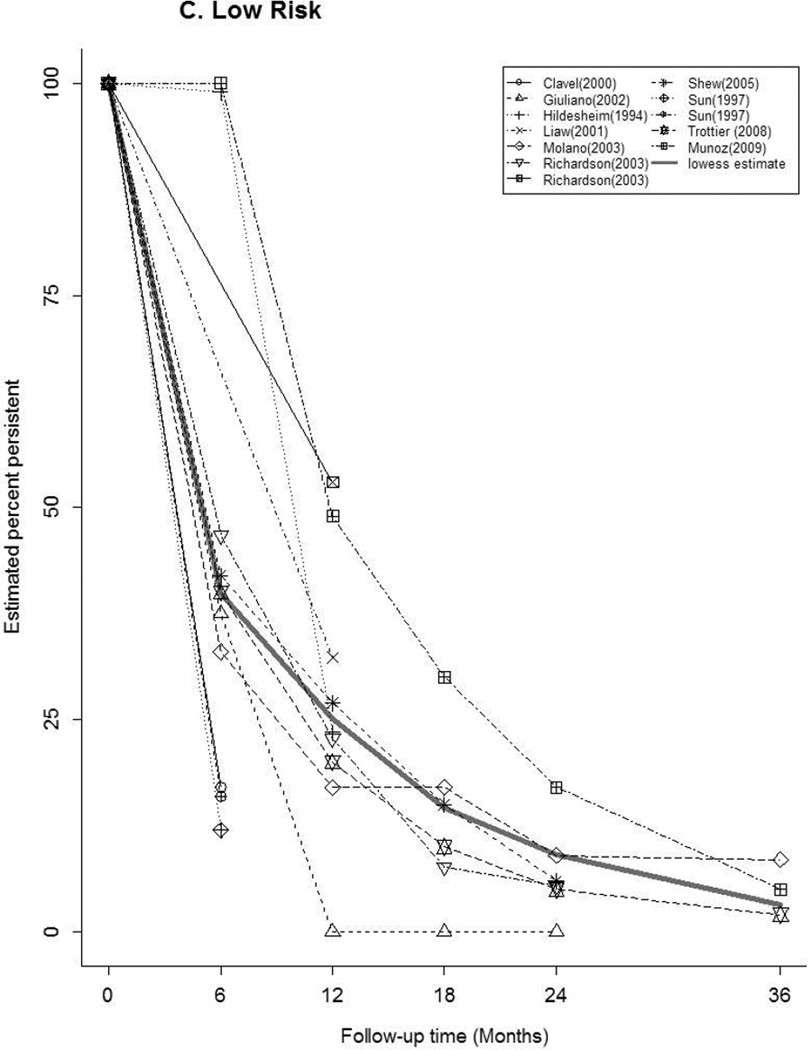
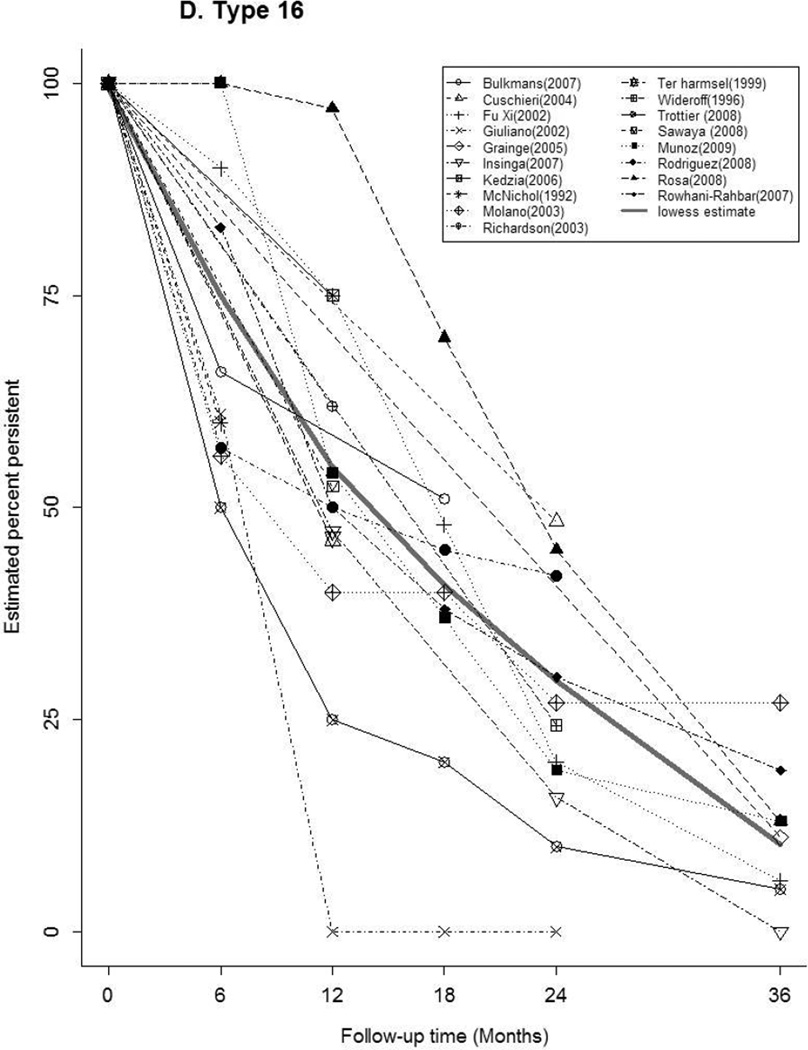
Forty-seven articles reported at least one estimate of HPV persistence at a standard 6 month interval for any-HPV (n=20), HR-HPV (n=22), LR-HPV (n=7), HPV-16 (n=16) (Figure 2). The thick gray curve in each panel represents the weighted summary of all study estimates across time. The curves for any-HPV and LR-HPV were similar, with a rapid decrease in persistence within 6 months, followed by a slow decline through 36 months. There was a relatively slower, more uniform decrease in HR-HPV and HPV-16 persistence over time. Between studies, the variability in estimates differed by HPV type and by time. At 6 months, estimates of the proportion persistent ranged from 16–88% for any-HPV, 18–90% for HR-HPV, and 50–100% for HPV-16. At 12 months, the proportion persistent ranged from 10–68% for any-HPV, 24–63% for HR-HPV, and 0–97% for HPV-16.
Figure 2.
Estimates of the Proportion of Infections Persisting across Time for A) any-HPV, B) high-risk HPV, C) low-risk HPV, D) HPV type 16. The legend in each panel provides the references for articles that contributed data to each figure. The thick gray curve is a locally weighted polynomial regression (lowess) estimate based on data from all studies over all time points.
Age-specific HPV persistence
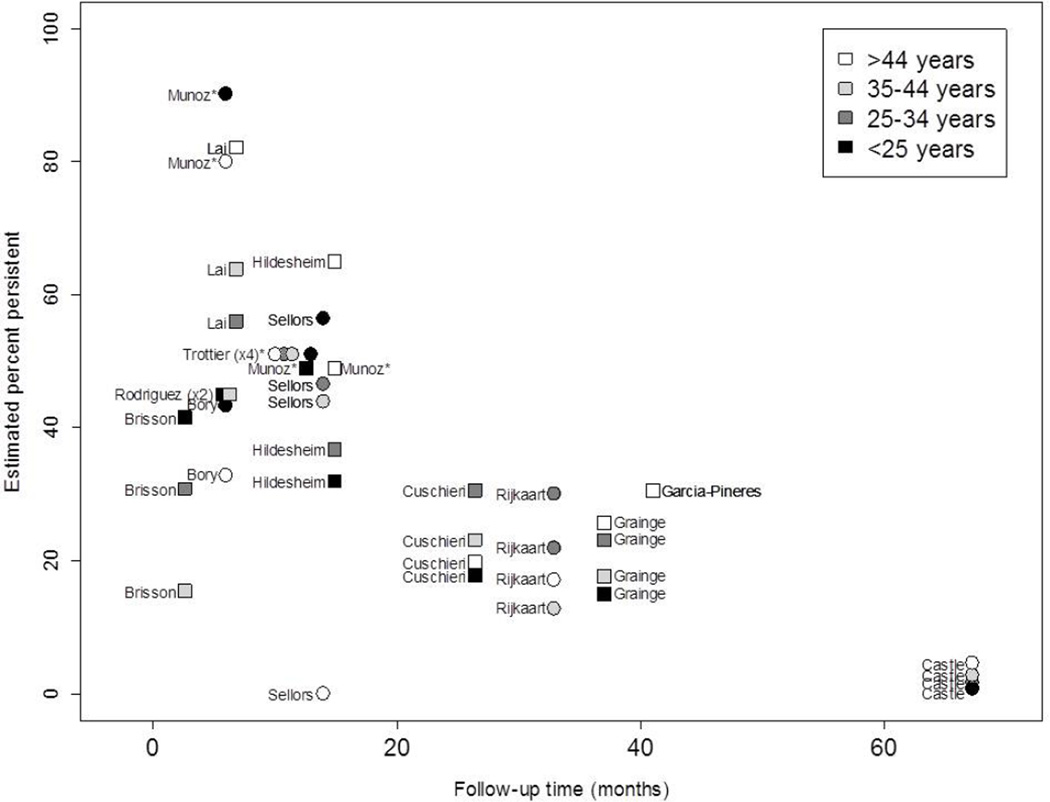
Articles that presented estimates of persistence stratified by age were limited (n=14) (Figure 3). Estimates of any-HPV by age were most common (n=8), followed by HR-HPV estimates (n=5). Three of the HPV persistence results were based on incident infections30–32. There was no distinguishable trend of HPV persistence by age. The study with the shortest persistence follow-up (3 months)33 found that women aged <25 years were more likely to persist compared to all older age groups. Three other studies found a similar trend: older women had lower persistence compared to younger women34–36. However, the study with the longest persistence follow-up (67 months)37 found the opposite result: younger women had a lower proportion of persistent infections compared to older women, as did four other studies30, 38–40.
Figure 3.
Age-Specific HPV Persistence. Estimates of the proportion of women with persistent human papillomavirus (HPV) infection by time are presented for any-HPV (squares) or high-risk HPV (circles). All results were based on prevalent infections unless otherwise noted, where* indicates HPV persistence based on incident infections. Some points were dithered slightly to avoid overlap.
Analytic results
Evaluation of study characteristics and persistence definitions
Of the 86 included articles, 4 did not report proportion persistent results (these estimated median duration of infection in Figure 1), thus the number of estimates included in each meta-regression could be more or less than 82 depending on whether estimates from the same study population entered into different study characteristic strata. After applying the inclusion and selection rules, 68 independent estimates produced a summary estimate for persistence at 6 month of 39% (95% confidence interval (CI): 34%, 45%).
The p-value for Cochran’s Q statistic was less than 0.01 for all meta-analyses, indicating a large amount of variation among the proportion persistent estimates included in each meta-regression. Given the relatively small number of studies in each meta-regression stratum and the inclusion of several variables in each model, most meta-regression coefficient estimates were imprecise, as indicated by relatively wide confidence intervals. Compared to Europe, which had the highest proportion persistence at 6 months (49%; 95% CI: 40%, 57%), HPV persistence at 6 months was significantly lower among studies from North America (40%; 95% CI: 31%, 48%) and from Central and South America (28%; 95% CI: 8%, 48%) (Table 2). There was a slight difference in persistence between younger (<30) and older women (≥30) at 40% (95% CI: 32%, 48%) and 48% (95% CI: 41%, 535), respectively. HPV persistence was highest among the few studies that used pU1M/pU2M, L1C1/L1C2, GP5/6 or other L1 primers (56%; 95% CI: 40%, 72%) and Hybrid Capture 1/2 (46%; 95% CI: 36%, 55%).
Table 2.
Meta-Regression Estimates of the Proportion Persistent and Persistence Differences at 6 Months by Study Characteristics
| N | Summary % persistencea (95% CI) |
% persistent differences (95% CI) |
|||
|---|---|---|---|---|---|
| Study region | |||||
| Europe | 31 | 48.6 | 39.9, 57.3 | 0 | 0 |
| North America | 22 | 39.5 | 30.6, 48.4 | −9.1 | −20.9, −2.7 |
| Central/South Americab | 7 | 28.2 | 8.4, 48.0 | −20.4 | −41.5, 0.6 |
| Africa, Asia, Australia | 8 | 45.2 | 32.6, 57.9 | −3.4 | −18.6, 11.8 |
| Mean age of total study population | |||||
| < 30.0 years | 29 | 40.1 | 32.3, 48.0 | 0 | 0 |
| ≥ 30.0 years | 40 | 47.6 | 40.6, 54.6 | 7.5 | −2.3, 17.2 |
| HPV DNA detection method | |||||
| MY09/11 ± other primers | 26 | 40.7 | 31.7, 50.0) | 0 | 0 |
| SPF10, PGMY09/11, GP5+/6+ | 14 | 40.7 | 28.6, 52.7 | −0.04 | −14.4, 14.4 |
| pU1M/pU2R, L1C1/L1C2, GP5/6 ± type-specific, other L1 primers | 6 | 56.4 | 40.4, 72.3 | 15.7 | −2.1, 33.5 |
| Type-specific primers | 10 | 41.2 | 28.5, 53.8 | 0.5 | −14.6, 15.5 |
| Hybrid Capture 1/2 | 14 | 45.6 | 35.8, 55.4 | 4.9 | −7.9, 17.8 |
| HPV type | |||||
| Any-HPV | 35 | 42.5 | 36.9, 48.2 | 0 | 0 |
| LR-HPV | 14 | 32.4 | 23.6, 41.3 | −10.1 | −20.2, −0.08 |
| HR-HPV | 41 | 43 | 37.8, 48.2 | 0.5 | −6.6, 7.5 |
| HPV-16 | 31 | 53.8 | 47.5, 60.0 | 11.2 | 3.3, 19.1 |
| HPV-18 | 21 | 47.7 | 39.6, 55.8 | 5.1 | −4.3, 14.5 |
| HPV-31 | 13 | 43.9 | 33.5, 54.3 | 1.3 | −9.9, 12.6 |
| HPV-33 | 11 | 46.6 | 34.6, 58.7 | 4.1 | −8.7, 16.9 |
| HPV-35 | 9 | 34.1 | 20.3, 47.9 | −8.4 | −22.9, 6.0 |
| HPV-39 | 8 | 42.8 | 30.0, 55.7 | 0.3 | −13.4, 13.9 |
| HPV-45 | 10 | 37.9 | 25.9, 50.0 | −4.6 | −17.3, 8.1 |
| HPV-51 | 9 | 30.5 | 19.0, 42.0 | −12.1 | −24.3, 0.2 |
| HPV-52 | 8 | 43.8 | 31.3, 56.3 | 1.2 | −12.0, 14.4 |
| HPV-56 | 9 | 41.9 | 29.4, 54.5 | −0.6 | −13.9, 12.7 |
| HPV-58 | 9 | 37 | 24.8, 49.3 | −5.5 | −18.6, 7.5 |
| HPV-59 | 7 | 40 | 24.8, 55.2 | −2.6 | −18.4, 13.3 |
| HPV-66 | 7 | 29.4 | 16.1, 42.7 | −13.2 | −27.2, 0.8 |
| HPV-68 | 6 | 33.2 | 18.0, 48.4 | −9.3 | −25.2, 6.5 |
| HPV persistence definition | |||||
| ≥2 positive visits | 51 | 43.1 | 36.9, 49.4 | 0 | 0 |
| ≥3 positive visits | 11 | 49.7 | 36.1, 63.4 | 6.6 | −12.6, 11.9 |
| Clearance or duration | 15 | 42.8 | 32.0, 53.5 | −0.4 | −12.6, 11.9 |
| Persistent pairs | 2 | 52.7 | 25.0, 80.5 | 9.6 | −18.4, 37.6 |
| Minimum HPV persistence duration | |||||
| < 6 months | 20 | 44 | 35.3, 52.6 | 0 | 0 |
| 6 to <12 months | 36 | 46 | 38.9, 53.1 | 2 | −8.9, 13.0 |
| ≥12 months | 25 | 51.7 | 41.2, 62.3 | 7.8 | −5.8, 21.3 |
| Mean testing interval | |||||
| < 6 months | 15 | 43 | 32.3, 53.7 | 0 | 0 |
| 6 to <12 months | 28 | 45.6 | 37.7, 53.5 | 2.6 | −10.7, 15.9 |
| ≥12 months | 26 | 42.3 | 30.7, 53.9 | −0.7 | −16.6, 15.2 |
| Type-specific HPV persistence | |||||
| Type-specific | 47 | 42.7 | 35.8, 49.6 | 0 | 0 |
| Non-type-specific | 33 | 43.6 | 36.1, 51.1 | 0.9 | −7.8, 9.5 |
| Baseline HPV infection status for persistence | |||||
| Prevalent | 52 | 42.6 | 36.2, 49.1 | 0 | 0 |
| Mixed prevalent and incident | 13 | 42.5 | 29.5, 55.4 | −0.2 | −14.0, 13.7 |
| Incident | 13 | 45.1 | 33.2, 57.1 | 2.5 | −10.6, 15.6 |
CI: confidence interval; HPV: human papillomavirus; LR: low-risk; HR: high-risk
Estimates are adjusted for persistence follow-up, which was centered at 6 months, and for study populations that contributed to more than one stratum.
Includes multi-center studies.
In comparison to the summary estimate for any-HPV persistence at 6 months (43%; 95% CI: 37%, 48%), HR-HPV persistence was nearly identical (0.5%; 95% CI: −7%, 8%), whereas LR-HPV persistence was lower (−10%; 95% CI: −20%, – 0.1%). HPV-16 (54%; 95% CI: 48%, 60%), HPV-18 (48%; 95% CI: 40%, 56%) and HPV-33 (47%; 95% CI: 35%, 59%) had the highest proportion persistent. HPV-51 (30%; 95% CI: 19%, 42%) and HPV-66 (29%; 95% CI: 16%, 43%) had the lowest proportion of persistent infections at 6 months. Consistent with the trend observed in Figure 1, HPV-16 persistence (54%; 95% CI: 48%, 60%) was slightly higher than HPV-18 (48%; 95% CI: 40%, 56%).
There was a slight difference in the proportion HPV persistent when persistence was defined by a minimum of two time points (43%; 95% CI: 37%, 49%) compared to a minimum of three time points (50%; 95% CI: 36%, 63%). There was also a slight increase in the proportion HPV persistent as the minimum duration of infection required to be considered persistent increased from 6 months or less (44%; 95% CI: 35%, 53%) compared to 12 months or more (52%95% CI: 41%, 62%). There was little difference between the summary estimates for HPV type-specificity: non-type-specific persistence was 44% (95% CI: 36%, 51%) and type-specific persistence was 43% (95% CI: 36%, 50%). Similarly, there was little difference in estimates of persistence by baseline HPV status: 45% (95% CI: 33%, 57%) for incident infections and 43% (95% CI: 36%, 49%) for infections that were prevalent at baseline.
DISCUSSION
This systematic literature review and meta-analysis of HPV persistence combined data on over 100,000 women from 86 studies to examine study characteristics that might affect the duration and likelihood of persistent HPV infection. We found that the median duration of HPV detection was slightly less than one year overall and among women with normal cytology. Given these findings, repeat HPV testing after one year follow-up is expected to have substantial utility for cervical cancer screening. Although most studies were similar in that they defined HPV persistence as HPV positivity at two or more time points (73%), not all required the same HPV type to be detected at consecutive visits (42% non-type-specific persistence). The proportion persistent at 6 months varied across studies and the heterogeneity in the estimates was largely a function of HPV type. The most persistent types were HPV-16, 31, 33 and 52 in the analysis of median duration, which were notably higher than the least persistent types HPV-35, 51, 66 and 68.
The median duration of any-HPV detection was approximately 10 months, whereas the duration of HR-HPV detection was slightly shorter, and that of LR-HPV infections even shorter. This pattern was consistent with the results from the meta-regression of HPV type. Previous reports comparing the duration of HPV-16 infection and HPV-18 infection have been inconsistent41–44, which is confirmed by the variability in our results. Assuming that heterogeneous results can be combined, the estimated average median duration of HPV-16 detection in this systematic review was 12.4 months, compared to 9.8 months for HPV-18. Meta-regression revealed a similar pattern, where the proportion persistent for HPV-18 was 9% less than that for HPV-16 at 6 months. Among alpha-9 (genotypes (HPV-16, 31, 33, 35, 52, 58)45, HPV-16, 31, 33 and 52 were the most persistent. Among alpha-7 genotypes (HPV-18, 39, 45, 59, 68, 70)45, HPV-18, 39, 45, and 59 were the most persistent. Given the biological importance of persistent HPV infections in HPV-related invasive cervical cancer, the relatively longer persistence for HPV-31, 33 and HPV-52 infections supports their inclusion in next generation prophylactic HPV vaccines.
Although the observed patterns of HPV persistence were generally similar between the analyses of median duration and the proportion persistent, discrepancies in the absolute values were noted. For example, we found that the median duration of HR-HPV persistence, the time at which 50% of women had persistently detectable HR-HPV infections, was on average 9.8 months (N=15), whereas meta-regression indicated that 38% persisted at 6 months (N=41). The studies included in the two analyses differed; however, such discrepancies may also result from a potential underestimation of the proportion persistent in meta-regression analysis. The variable we used in meta-regression to control for the time at which persistence was measured corresponded to the interval between two study visits, unless the mean or median time at which persistence was measured was reported, because persistence is most commonly defined as two or more HPV positive visits. However, based on this common definition of HPV persistence, a woman could actually have had more than 2 positive visits, leading to a potential underestimation of how long her HPV infection actually persisted. This would have affected estimates of the proportion persistent but not estimates of median duration, which would take into account the exact number of HPV positive study visits for each women over study follow-up. Also, an important point regarding testing intervals is that if future studies aim to estimate persistence with the highest degree of accuracy, for example down to the month, it would require sample collection at a minimum of weekly intervals, whereas the current literature tests, on average, every 6 months. Length of testing interval might also explain our observation of a higher proportion of women with persistently detectable HPV infections in studies with intervals of 12 months or more as compared to 6 months or less; more frequent testing internals would be expected to detect transient infections that may have been missed by testing with longer intervals. Furthermore, we found that most studies did not take into account in the definitions of persistence or clearance the potential for HPV latency46, and thus did not permit the differentiation between true biological persistence and persistence as measured by detectable HPV DNA at the mucosal surface.
Given the limited data available for specific age groups, it is difficult to make definitive conclusions about the relation between age and HPV persistence. Although no distinct trend by age was observed across studies that presented age-stratified estimates, the proportion of women with persistently detectable HPV infections was slightly higher in meta-regression analysis among study populations with a mean age of 30 years and older compared to those with a mean age under 30. These data reflect the inconsistent results reported in the literature and suggest that the complex relationship between age and persistence is likely mediated by additional factors such as incident/prevalent infection status, study design, differences in immune response, and population-level characteristics such as number and type of recent and lifetime sexual partners. Recently, a large study found prevalent HPV infections were slower to “clear” (i.e., become non-detectable) in older compared to younger women, but no difference in persistence of incidence infections was reported by age47. Our descriptive figure was predominately comprised of prevalent HPV infections; thus, we were unable to reliably compare persistence of prevalent versus incident infections by age. In addition, although meta-regression did not identify differences between the proportion of incident and prevalent infections that remained persistent, it is important to note that the interpretation of these estimates are different. Prevalent infections are left-censored so true persistence is likely greater than measured persistence, whereas persistence of incident infections is estimated beginning from the first detection during the study.
HPV persistence within strata of individual study characteristics showed notable heterogeneity, potential evidence that the use of broad or heterogeneous categories may mask important associations between study variables. This complexity is not unexpected given that persistence estimates within a stratum may differ by several other characteristics including study region, HPV type, testing interval, or detection method. For example, each HPV detection assay has different sensitivities for the detection of individual HPV types, especially if multiple HPV types are present48, 49. In addition, previous studies suggest that most women with persistent HPV infections actually have type-specific infections even if non-type-specific persistence was measured50, which may explain why we observed no difference between type-specific and non-type-specific HPV persistence. Although it is likely that the relation between such study characteristics and HPV persistence are multi-factorial, data were too sparse to examine several study characteristics simultaneously. Future reviews could consider obtaining individual-level study data for a pooled analysis approach.
This meta-analysis systematically described the patterns and characteristics associated with the persistence and duration of HPV infections in published studies. Our results confirm individual study findings that specific HPV types, particularly HPV-16, are more likely to produce persistent infections with a longer duration of detection than other HR-HPV types. Repeat and possibly type-specific HPV testing would increase specificity over a one-time HPV test, thus improving clinical detection of cervical high-grade precancer by providing a higher sensitivity and similar specificity as compared to cytologic screening5. Current ASC/ASCCP/ASCP guidelines recommend a one-year repeat screening interval for women over 30 years who are HPV-positive with normal cytology. These guidelines are consistent with our systematic review, which indicates that the median duration of HPV detection was less than one year among women with normal cytology for both any-HPV and high-risk type infections.
Supplementary Material
Impact statement.
HPV persistence varied notably across studies and was largely mediated by study region, detection method, and HPV type. We estimated that approximately half of HPV infections persist past 6–12 months. Weighted median duration of any-HPV detection was 9.8 months and HR-HPV was 9.3 months. Repeat HPV testing at 12 month intervals could identify women at increased risk of high-grade cervical precancer due to persistent HPV infections.
Acknowledgments
The authors would like to thank Dr. Ghassan Hamra for reviewing search results and double abstracting the data and Dr. Nick Galwey for his independent review of our statistical methods. All authors participated in designing the study and writing the manuscript, and all analyses were conducted by A. Rositch and M. Hudgens.
Funding: This work was supported by GlaxoSmithKline.
Footnotes
Conflicts of interest statement:
Dr. Anne Rositch was directly supported for this project by GlaxoSmithKline (GSK) from 2006–2008 as a Graduate Student in the Department of Epidemiology at the University of North Carolina and was a Graduate Research Assistant at GSK from 2008–2009; Dr. Jill Koshiol was employed part-time by GSK from 2001–2005; Dr. Michael Hudgens, no conflict; Hilda Razzaghi has worked as a Graduate Research Assistant at both GSK and Merck in the last five years; Dr. Danielle Backes, no conflict; Dr. Jeanne Pimenta is an employee of GSK; Dr. Eduardo Franco has occasionally served on advisory boards at GSK, Merck, Roche, and Gen-Probe; Dr. Charles Poole receives partial salary support and has received lecture honoraria from GSK; Dr. Jennifer Smith has received grants and consulting fees in the last six years from GSK, Merck, Hologic, Qiagen, and Gen-Probe.
Conference presentations:
Rositch, AF, Koshiol, J, Franco, E, Poole, C, Pimenta, J, and Smith, JS. Human papillomavirus persistence patterns in women worldwide: a meta-analysis (Oral presentation). Eurogin, November, 2008. Nice, France.
Fortino, AE, Backes DM, and Smith, JS. Systematic review of human papillomavirus persistence patterns in women worldwide (Poster presentation, PS16-16). 24th International Papillomavirus Society, November, 2007. Beijing, China.
References
- 1.Bosch FX, Lorincz A, Munoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz N. Human papillomavirus and cancer: the epidemiological evidence. Journal of Clinical Virology. 2000;19:1–5. doi: 10.1016/s1386-6532(00)00125-6. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Monograph on human papillomavirusesed. vol. 90: International Agency for Research on Cancer. 2007 [Google Scholar]
- 5.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. American journal of epidemiology. 2008;168:123–137. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 7.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlee F, Franco EL. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. The New England journal of medicine. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 8.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, Radberg T, Strander B, Forslund O, Hansson BG, Rylander E, Dillner J. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. The New England journal of medicine. 2007;357:1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 9.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 10.Castle PE. Invited commentary: is monitoring of human papillomavirus infection for viral persistence ready for use in cervical cancer screening? American journal of epidemiology. 2008;168:138–144. doi: 10.1093/aje/kwn037. discussion 45-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS, Jr, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA: a cancer journal for clinicians. 2012 doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 12.Ting J, Kruzikas DT, Smith JS. A global review of age-specific and overall prevalence of cervical lesions. Int J Gynecol Cancer. 2010;20:1244–1249. doi: 10.1111/igc.0b013e3181f16c5f. [DOI] [PubMed] [Google Scholar]
- 13.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: Comparison by geographic region and with cervical cancer. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1157–1164. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 14.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, Schiffman MH, Scott DR, Apple RJ. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan PK, Cheung TH, Tam AO, Lo KW, Yim SF, Yu MM, To KF, Wong YF, Cheung JL, Chan DP, Hui M, Ip M. Biases in human papillomavirus genotype prevalence assessment associated with commonly used consensus primers. International journal of cancer. 2006;118:243–245. doi: 10.1002/ijc.21299. [DOI] [PubMed] [Google Scholar]
- 16.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International journal of cancer. Journal international du cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 17.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 18.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Statistics in Medicine. 1999;18:2693–2708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Statistics in Medicine. 1998;17:841–856. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Desy M, Rohan TE. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. Journal of Infectious Diseases. 1999;180:1415–1423. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 21.Ahdieh L, Klein RS, Burk R, Cu-Uvin S, Schuman P, Duerr A, Safaeian M, Astemborski J, Daniel R, Shah K. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. Journal of Infectious Diseases. 2001;184:682–690. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 22.Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:709–715. doi: 10.1158/1055-9965.EPI-06-0846. [DOI] [PubMed] [Google Scholar]
- 23.Kotloff KL, Wasserman SS, Russ K, Shapiro S, Daniel R, Brown W, Frost A, Tabara SO, Shah K. Detection of genital human papillomavirus and associated cytological abnormalities among college women. Sexually transmitted diseases. 1998;25:243–250. doi: 10.1097/00007435-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Moberg M, Gustavsson I, Gyllensten U. Type-specific associations of human papillomavirus load with risk of developing cervical carcinoma in situ. International journal of cancer. 2004;112:854–859. doi: 10.1002/ijc.20480. [DOI] [PubMed] [Google Scholar]
- 25.Sichero L, Ferreira S, Trottier H, Duarte-Franco E, Ferenczy A, Franco EL, Villa LL. High grade cervical lesions are caused preferentially by non-European variants of HPVs 16 and 18. International journal of cancer. 2007;120:1763–1768. doi: 10.1002/ijc.22481. [DOI] [PubMed] [Google Scholar]
- 26.Smith EM, Johnson SR, Ritchie JM, Feddersen D, Wang D, Turek LP, Haugen TH. Persistent HPV infection in postmenopausal age women. International Journal of Gynecology & Obstetrics. 2004;87:131–137. doi: 10.1016/j.ijgo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Veress G, CsikyMeszaros T, Konya J, Czegledy J, Gergely L. Follow up of human papillomavirus (HPV) DNA and local anti-HPV antibodies in cytologically normal pregnant women. Medical Microbiology and Immunology. 1996;185:139–144. doi: 10.1007/s004300050023. [DOI] [PubMed] [Google Scholar]
- 28.Schneider A, Kirchhoff T, Meinhardt G, Gissmann L. Repeated evaluation of human papillomavirus 16 status in cervical swabs of young women with a history of normal Papanicolaou smears. Obstetrics and gynecology. 1992;79:683–688. [PubMed] [Google Scholar]
- 29.Kjaer S, Hogdall E, Frederiksen K, Munk C, van den Brule A, Svare E, Meijer C, Lorincz A, Iftner T. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer research. 2006;66:10630–10636. doi: 10.1158/0008-5472.CAN-06-1057. [DOI] [PubMed] [Google Scholar]
- 30.Munoz N, Mendez F, Posso H, Molano M, van den Brule AJC, Ronderos M, Meijer C, Munoz A. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. Journal of Infectious Diseases. 2004;190:2077–2087. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 31.Munoz N, Hernandez-Suarez G, Mendez F, Molano M, Posso H, Moreno V, Murillo R, Ronderos M, Meijer C, Munoz A. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100:1184–1190. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trottier H, Mahmud S, Prado JC, Sobrinho JS, Costa MC, Rohan TE, Villa LL, Franco EL. Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. The Journal of infectious diseases. 2008;197:1436–1447. doi: 10.1086/587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brisson J, Bairati I, Morin C, Fortier M, Bouchard C, Christen A, Bernard P, Roy M, Meisels A. Determinants of persistent detection of human papillomavirus DNA in the uterine cervix. Journal of Infectious Diseases. 1996;173:794–799. doi: 10.1093/infdis/173.4.794. [DOI] [PubMed] [Google Scholar]
- 34.Bory JP, Cucherousset J, Lorenzato M, Gabriel R, Quereux C, Birembaut P, Clavel C. Recurrent human papillomavirus infection detected with the hybrid capture II assay selects women with normal cervical smears at risk for developing high grade cervical lesions: A longitudinal study of 3,091 women. International journal of cancer. 2002;102:519–525. doi: 10.1002/ijc.10735. [DOI] [PubMed] [Google Scholar]
- 35.Sellors JW, Karwalajtys TL, Kaczorowski J, Mahony JB, Lytwyn A, Chong S, Sparrow J, Lorincz A. Incidence, clearance and predictors of human papillomavirus infection in women. Canadian Medical Association Journal. 2003;168:421–425. [PMC free article] [PubMed] [Google Scholar]
- 36.Rijkaart DC, Bontekoe TR, Korporaal H, Boon ME. Alternating high-risk human papillomavirus infection: consequences of progression to cervical intraepithelial neoplasia. Cancer. 2006;108:475–479. doi: 10.1002/cncr.22305. [DOI] [PubMed] [Google Scholar]
- 37.Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Wacholder S, Tarone R, Burk RD. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. Journal of Infectious Diseases. 2005;191:1808–1816. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 38.Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Zhang T, Scott DR, Rush BB, Lawler P, Sherman ME, Kurman RJ, Manes MM. Persistence of Type-Specific Human Papillomavirus Infection among Cytologically Normal Women. Journal of Infectious Diseases. 1994;169:235–240. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 39.Grainge MJ, Seth R, Guo L, Neal KR, Coupland C, Vryenhoef P, Johnson J, Jenkins D. Cervical human papillomavirus screening among older women. Emerg Infect Dis. 2005;11:1680–1685. doi: 10.3201/eid1111.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai CH, Chao A, Chang CJ, Chao FY, Huang HJ, Hsueh S, Lin CT, Cheng HH, Huang CC, Yang JE, Wu TI, Chou HH, et al. Host and viral factors in relation to clearance of human papillomavirus infection: a cohort study in Taiwan. International journal of cancer. 2008;123:1685–1692. doi: 10.1002/ijc.23679. [DOI] [PubMed] [Google Scholar]
- 41.Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, Coutlee F, Franco EL. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiology Biomarkers & Prevention. 2003;12:485–490. [PubMed] [Google Scholar]
- 42.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. The New England journal of medicine. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 43.Woodman CBJ, Collins S, Winter H, Bailey A, Ellis J, Prior P, Yates M, Rollason TP, Young LS. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 44.Molano M, van den Brule A, Plummer M, Weiderpass E, Posso H, Arslan A, Meijer C, Munoz N, Franceschi S. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: A population-based, 5-year follow-up study. American journal of epidemiology. 2003;158:486–494. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 45.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 46.Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414:153–163. doi: 10.1016/j.virol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maucort-Boulch D, Plummer M, Castle PE, Demuth F, Safaeian M, Wheeler CM, Schiffman M. Predictors of human papillomavirus persistence among women with equivocal or mildly abnormal cytology. International journal of cancer. Journal international du cancer. 2010;126:684–691. doi: 10.1002/ijc.24752. [DOI] [PubMed] [Google Scholar]
- 48.Safaeian M, Herrero R, Hildesheim A, Quint W, Freer E, Van Doorn LJ, Porras C, Silva S, Gonzalez P, Bratti MC, Rodriguez AC, Castle P. Comparison of the SPF10-LiPA system to the Hybrid Capture 2 Assay for detection of carcinogenic human papillomavirus genotypes among 5,683 young women in Guanacaste, Costa Rica. Journal of clinical microbiology. 2007;45:1447–1454. doi: 10.1128/JCM.02580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castle PE, Porras C, Quint WG, Rodriguez AC, Schiffman M, Gravitt PE, Gonzalez P, Katki HA, Silva S, Freer E, Van Doorn LJ, Jimenez S, et al. Comparison of two PCR-based human papillomavirus genotyping methods. Journal of clinical microbiology. 2008;46:3437–3445. doi: 10.1128/JCM.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castle PE, Rodriguez AC, Burk RD, Herrero R, Wacholder S, Alfaro M, Morales J, Guillen D, Sherman ME, Solomon D, Schiffman M. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. Bmj. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.AnderssonEllstrom A, Dillner J, Hagmar B, Schiller J, Sapp M, Forssman L, Milsom I. Comparison of development of serum antibodies to HPV16 and HPV33 and acquisition of cervical HPV DNA among sexually experienced and virginal young girls - A longitudinal cohort study. Sexually transmitted diseases. 1996;23:234–238. doi: 10.1097/00007435-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Beskow AH, Josefsson AM, Gyllensten UB. HLA class II alleles associated with infection by HPV16 in cervical cancer in situ. International journal of cancer. 2001;93:817–822. doi: 10.1002/ijc.1412. [DOI] [PubMed] [Google Scholar]
- 53.Branca M, Garbuglia AR, Benedetto A, Cappiello T, Leoncini L, Migliore G, Agarossi A, Syrjanen K. Factors predicting the persistence of genital human papillomavirus infections and PAP smear abnormality in HIV-positive and HIV-negative women during prospective follow-up. International Journal of Std & Aids. 2003;14:417–425. doi: 10.1258/095646203765371321. [DOI] [PubMed] [Google Scholar]
- 54.Bulkmans NW, Berkhof J, Bulk S, Bleeker MC, van Kemenade FJ, Rozendaal L, Snijders PJ, Meijer CJ. High-risk HPV type-specific clearance rates in cervical screening. Br J Cancer. 2007;96:1419–1424. doi: 10.1038/sj.bjc.6603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clavel C, Masure L, Bory JP, Putaud I, Mangeonjean C, Lorenzato M, Nazeyrollas P, Gabriel R, Quereux C, Birembaut P. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7932 women. British Journal of Cancer. 2001;84:1616–1623. doi: 10.1054/bjoc.2001.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clavel C, Masure M, Levert M, Putaud I, Mangeonjean C, Lorenzato M, Nazeyrollas P, Gabriel R, Quereux C, Birembaut P. Human papillomavirus detection by the hybrid capture II assay: A reliable test to select women with normal cervical smears at risk for developing cervical lesions. Diagnostic Molecular Pathology. 2000;9:145–150. doi: 10.1097/00019606-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Cuschieri KS, Cubie HA, Whitley MW, Gilkison G, Arends MJ, Graham C, McGoogan E. Persistent high risk HPV infection associated with development of cervical neoplasia in a prospective population study. Journal of Clinical Pathology. 2005;58:946–950. doi: 10.1136/jcp.2004.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuschieri KS, Whitley MJ, Cubie HA. Human papillomavirus type specific DNA and RNA persistence - Implications for cervical disease progression and monitoring. Journal of Medical Virology. 2004;73:65–70. doi: 10.1002/jmv.20062. [DOI] [PubMed] [Google Scholar]
- 59.Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, McGoogan E, Menon U, Terry G, Edwards R, Brooks C, Desai M, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871–1876. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 60.Dalstein W, Riethmuller D, Pretet JL, Carval KL, Sautiere JL, Carbillet JP, Kantelip B, Schaal JP, Mougin C. Persistence and load of high-risk hpv are predictors for development of high-grade cervical lesions: A longitudinal French cohort study. International journal of cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 61.Elfgren K, Kalantari M, Moberger B, Hagmar B, Dillner J. A population-based five-year follow-up study of cervical human papillomavirus infection. American Journal of Obstetrics and Gynecology. 2000;183:561–567. doi: 10.1067/mob.2000.106749. [DOI] [PubMed] [Google Scholar]
- 62.Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, Wadell G. Human Papillomavirus Infection Is Transient in Young-Women - a Population-Based Cohort Study. Journal of Infectious Diseases. 1995;171:1026–1030. doi: 10.1093/infdis/171.4.1026. [DOI] [PubMed] [Google Scholar]
- 63.Farzaneh F, Roberts S, Mandal D, Ollier B, Winters U, Kitchener HC, Brabin L. The IL-10-1082G polymorphism is associated with clearance of HPV infection. Bjog. 2006;113:961–964. doi: 10.1111/j.1471-0528.2006.00956.x. [DOI] [PubMed] [Google Scholar]
- 64.Hopman EH, Rozendaal L, Voorhorst FJ, Walboomers JMM, Kenemans P, Helmerhorst TJM. High risk human papillomavirus in women with normal cervical cytology prior to the development of abnormal cytology and colposcopy. British Journal of Obstetrics and Gynaecology. 2000;107:600–604. doi: 10.1111/j.1471-0528.2000.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 65.Hoyer H, Scheungraber C, Kuehne-Heid R, Teller K, Greinke C, Leistritz S, Ludwig B, Durst M, Schneider A. Cumulative 5-year diagnoses of CIN2, CIN3 or cervical cancer after concurrent high-risk HPV and cytology testing in a primary screening setting. International journal of cancer. 2005;116:136–143. doi: 10.1002/ijc.20955. [DOI] [PubMed] [Google Scholar]
- 66.Kedzia W, Olejnik A, Schmidt M, Nawrot R, Gozdzicka-Jozefiak A, Kedzia H, Spaczynski M. The level of antibody against E6 HPV 16 oncoprotein in blood sera of women with chronic HPV 16 infection and cervical cancer. European Journal of Gynaecological Oncology. 2006;27:65–68. [PubMed] [Google Scholar]
- 67.Kjaer SK, van den Brule AJC, Paull G, Svare EI, Sherman ME, Thomsen BL, Suntum M, Bock JE, Poll PA, Meijer C. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. British Medical Journal. 2002;325:572–576. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nobbenhuis MAE, Helmerhorst TJM, van den Brule AJC, Rozendaal L, Voorhorst FJ, Bezemer PD, Verheijen RHM, Meijer C. Cytological regression and clearance of high-risk human papillomavirus in women with an abnormal cervical smear. Lancet. 2001;358:1782–1783. doi: 10.1016/S0140-6736(01)06809-X. [DOI] [PubMed] [Google Scholar]
- 69.Silins I, Ryd W, Strand A, Wadell G, Tornberg S, Hansson BG, Wang X, Arnheim L, Dahl V, Bremell D, Persson K, Dillner J, et al. Chlamydia trachomatis infection and persistence of human papillomavirus. International journal of cancer. 2005;116:110–115. doi: 10.1002/ijc.20970. [DOI] [PubMed] [Google Scholar]
- 70.Syrjanen S, Shabalova I, Petrovichev N, Kozachenko V, Zakharova T, Pajanidi J, Podistov J, Chemeris G, Sozaeva L, Lipova E, Tsidaeva I, Ivanchenko O, et al. Factors predicting persistence of high-risk human papillomavirus (HPV) infections in women prospectively followed-up in three New Independent States (NIS) of the former Soviet Union. European Journal of Gynaecological Oncology. 2005;26:491–498. [PubMed] [Google Scholar]
- 71.ter Harmsel B, Smedts F, Kuijpers J, Van Muyden R, Oosterhuis W, Quint W. Relationship between human papillomavirus type 16 in the cervix and intraepithelial neoplasia. Obstetrics and gynecology. 1999;93:46–50. doi: 10.1016/s0029-7844(98)00306-8. [DOI] [PubMed] [Google Scholar]
- 72.Vandoornum GJJ, Prins M, Juffermans LHJ, Hooykaas C, Vandenhoek JAR, Coutinho RA, Quint WGV. Regional Distribution and Incidence of Human Papillomavirus Infections among Heterosexual Men and Women with Multiple Sexual Partners - a Prospective-Study. Genitourinary Medicine. 1994;70:240–246. doi: 10.1136/sti.70.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallin KL, Wiklund F, Angstrom T, Bergman F, Stendahl U, Wadell G, Hallmans G, Dillner J. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. New England Journal of Medicine. 1999;341:1633–1638. doi: 10.1056/NEJM199911253412201. [DOI] [PubMed] [Google Scholar]
- 74.Tiews S, Steinberg W, Schneider W, Hanrath C. Determination of the diagnostic accuracy of testing for high-risk (HR) human papillomavirus (HPV) types 16, 18 and 45 in precancerous cervical lesions: preliminary data. J Clin Virol. 2009;46(Suppl 3):S11–S15. doi: 10.1016/S1386-6532(09)70295-1. [DOI] [PubMed] [Google Scholar]
- 75.Bulk S, Bulkmans NW, Berkhof J, Rozendaal L, Boeke AJ, Verheijen RH, Snijders PJ, Meijer CJ. Risk of high-grade cervical intra-epithelial neoplasia based on cytology and high-risk HPV testing at baseline and at 6-months. International journal of cancer. 2007;121:361–367. doi: 10.1002/ijc.22677. [DOI] [PubMed] [Google Scholar]
- 76.Luyten A, Scherbring S, Reinecke-Luthge A, Braun BE, Pietralla M, Theiler K, Petry KU. Risk-adapted primary HPV cervical cancer screening project in Wolfsburg, Germany--experience over 3 years. J Clin Virol. 2009;46(Suppl 3):S5–S10. doi: 10.1016/S1386-6532(09)70294-X. [DOI] [PubMed] [Google Scholar]
- 77.Ahdieh L, Munoz A, Vlahov D, Trimble CL, Timpson LA, Shah K. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. American journal of epidemiology. 2000;151:1148–1157. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 78.Xi LF, Carter JJ, Galloway DA, Kuypers J, Hughes JP, Lee SK, Adam DE, Kiviat NB, Koutsky LA. Acquisition and natural history of human papillomavirus type 16 variant infection among a cohort of female university students. Cancer Epidemiology Biomarkers & Prevention. 2002;11:343–351. [PubMed] [Google Scholar]
- 79.Gagnon S, Hankins C, Tremblay C, Forest P, Pourreaux K, Coutlee F. Viral polymorphism in human papillomavirus types 33 and 35 and persistent and transient infection in the genital tract of women. Journal of Infectious Diseases. 2004;190:1575–1585. doi: 10.1086/424854. [DOI] [PubMed] [Google Scholar]
- 80.Giuliano AR, Harris R, Sedjo RL, Baldwin S, Roe D, Papenfuss MR, Abrahamsen M, Inserra P, Olvera S, Hatch K. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The young women's health study. Journal of Infectious Diseases. 2002;186:462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 81.Liaw KL, Glass AG, Manos MM, Greer CE, Scott DR, Sherman M, Burk RD, Kurman RJ, Wacholder S, Rush BB, Cadell DM, Lawler P, et al. Detection of human papillomavirus DNA in cytologically normal women and subsequent cervical squamous intraepithelial lesions. Journal of the National Cancer Institute. 1999;91:954–960. doi: 10.1093/jnci/91.11.954. [DOI] [PubMed] [Google Scholar]
- 82.Liaw KL, Hildesheim A, Burk RD, Gravitt P, Wacholder S, Manos MM, Scott DR, Sherman ME, Kurman RJ, Glass AG, Anderson SM, Schiffman M. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. Journal of Infectious Diseases. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 83.Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, Alvarez FB, Bautista OM, Jansen KU, Barr E. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia - A Randomized controlled trial. Obstetrics and gynecology. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 84.McNicol P, Guijon F, Brunham R, Gray M, Paraskevas M. Laboratory diagnosis of latent human papillomavirus infection. Diagn Microbiol Infect Dis. 1992;15:679–683. doi: 10.1016/0732-8893(92)90071-z. [DOI] [PubMed] [Google Scholar]
- 85.Minkoff H, Feldman J, DeHovitz J, Landesman S, Burk R. A longitudinal study of human papillomavirus carriage in human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. American Journal of Obstetrics and Gynecology. 1998;178:982–986. doi: 10.1016/s0002-9378(98)70535-6. [DOI] [PubMed] [Google Scholar]
- 86.Shew ML, Fortenberry JD. HPV infection in adolescents: natural history, complications, and. Semin Pediatr Infect Dis. 2005;16:168–174. doi: 10.1053/j.spid.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC. Human papillomavirus infection in women infected with the human immunodeficiency virus. New England Journal of Medicine. 1997;337:1343–1349. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 88.Wideroff L, Schiffman MH, Hoover R, Tarone RE, Nonnenmacher B, Hubbert N, Kirnbauer R, Greer CE, Lorincz AT, Manos MM, Glass AG, Scott DR, et al. Epidemiologic determinants of seroreactivity to human papillomavirus (HPV) type 16 virus-like particles in cervical HPV-16 DNA-positive and -negative women. Journal of Infectious Diseases. 1996;174:937–943. doi: 10.1093/infdis/174.5.937. [DOI] [PubMed] [Google Scholar]
- 89.Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, Koutsky LA. Development and duration of human papillomavirus lesions, after initial infection. Journal of Infectious Diseases. 2005;191:731–738. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 90.Harris TG, Burk RD, Yu H, Minkoff H, Massad LS, Watts DH, Zhong Y, Gange S, Kaplan RC, Anastos K, Levine AM, Moxley M, et al. Insulin-like growth factor axis and oncogenic human papillomavirus natural history. Cancer Epidemiol Biomarkers Prev. 2008;17:245–248. doi: 10.1158/1055-9965.EPI-07-0686. [DOI] [PubMed] [Google Scholar]
- 91.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, Ning L, Killeen J, Kamemoto L, Hernandez BY. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer research. 2008;68:8813–8824. doi: 10.1158/0008-5472.CAN-08-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ralston Howe E, Li Z, McGlennen RC, Hellerstedt WL, Downs LS., Jr Type-specific prevalence and persistence of human papillomavirus in women in the United States who are referred for typing as a component of cervical cancer screening. Am J Obstet Gynecol. 2009;200:245 e1–245 e7. doi: 10.1016/j.ajog.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 93.Belinson JL, Pretorius RG, Enerson C, Garcia F, Cruz EP, Belinson SE, Yeverino Garcia E, Brainard J. The Mexican Cervical Cancer Screening Trial: self-sampling for human papillomavirus with unaided visual inspection as a secondary screen. Int J Gynecol Cancer. 2009;19:27–32. doi: 10.1111/IGC.0b013e318197f479. [DOI] [PubMed] [Google Scholar]
- 94.Derchain SF, Sarian LO, Naud P, Roteli-Martins C, Longatto-Filho A, Tatti S, Branca M, Erzen M, Serpa-Hammes L, Matos J, Gontijo RC, Braganca JF, et al. Safety of screening with Human papillomavirus testing for cervical cancer at three-year intervals in a high-risk population: experience from the LAMS study. J Med Screen. 2008;15:97–104. doi: 10.1258/jms.2008.007061. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, Solomon D, Burk R. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. Journal of the National Cancer Institute. 2008;100:513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosa MI, Fachel JM, Rosa DD, Medeiros LR, Igansi CN, Bozzetti MC. Persistence and clearance of human papillomavirus infection: a prospective cohort study. Am J Obstet Gynecol. 2008;199:617 e1–617 e7. doi: 10.1016/j.ajog.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Pineres AJ, Hildesheim A, Herrero R, Trivett M, Williams M, Atmetlla I, Ramirez M, Villegas M, Schiffman M, Rodriguez AC, Burk RD, Hildesheim M, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer research. 2006;66:11070–11076. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez AC, Burk R, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillen D, Alfaro M, Viscidi R, Morales J, Hutchinson M, et al. The natural history of human papillomavirus infection and cervical intraepithelial neoplasia among young women in the Guanacaste cohort shortly after initiation of sexual life. Sexually transmitted diseases. 2007;34:494–502. doi: 10.1097/01.olq.0000251241.03088.a0. [DOI] [PubMed] [Google Scholar]
- 99.Rousseau MC, Pereira JS, Prado JCM, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. Journal of Infectious Diseases. 2001;184:1508–1517. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 100.Schiffman M, Herrero R, DeSalle R, Hildesheim A, Wacholder S, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Hutchinson M, et al. The carcinogenicity of human papillornavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 101.Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL, Franco EL. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. Jama-Journal of the American Medical Association. 2001;286:3106–3114. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 102.Siegel EM, Craft NE, Duarte-Franco E, Villa LL, Franco EL, Giuliano AR. Associations between serum carotenoids and tocopherols and type-specific HPV persistence: the Ludwig-McGill cohort study. International journal of cancer. 2007;120:672–680. doi: 10.1002/ijc.22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fairley CK, Tabrizi SN, Gourlay SG, Chen SJ, Borg A, Garland SM. A Cohort Study Comparing the Detection of Hpv DNA from Women Who Stop and Continue to Smoke. Australian & New Zealand Journal of Obstetrics & Gynaecology. 1995;35:181–185. doi: 10.1111/j.1479-828x.1995.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 104.Saito J, Sumiyoshi M, Nakatani H, Hoshiai MIH, Noda K. Dysplasia and Hpv Infection Initially Detected by DNA Analysis in Cytomorphologically Normal Cervical Smears. International Journal of Gynecology & Obstetrics. 1995;51:43–48. doi: 10.1016/0020-7292(95)80007-y. [DOI] [PubMed] [Google Scholar]
- 105.Sasagawa T, Yamazaki H, Dong YZ, Satake S, Tateno M, Inoue M. Immunoglobulin-A and -G responses against virus-like particles (VLP) of human papillomavirus type 16 in women with cervical cancer and cervical intra-epithelial lesions. International journal of cancer. 1998;75:529–535. doi: 10.1002/(sici)1097-0215(19980209)75:4<529::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 106.Sawaya GF, Chirenje MZ, Magure MT, Tuveson JL, Ma Y, Shiboski SC, Da Costa MM, Palefsky JM, Moscicki AB, Mutasa RM, Chipato T, Smith-McCune KK. Effect of diaphragm and lubricant gel provision on human papillomavirus infection among women provided with condoms: a randomized controlled trial. Obstetrics and gynecology. 2008;112:990–997. doi: 10.1097/AOG.0b013e318189a8a4. [DOI] [PubMed] [Google Scholar]
- 107.Bae J, Seo SS, Park YS, Dong SM, Kang S, Myung SK, Park SY. Natural history of persistent high-risk human papillomavirus infections in Korean women. Gynecol Oncol. 2009;115:75–80. doi: 10.1016/j.ygyno.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 108.Rowhani-Rahbar A, Hawes SE, Sow PS, Toure P, Feng Q, Dem A, Dembele B, Critchlow CW, N'Doye I, Kiviat NB. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. The Journal of infectious diseases. 2007;196:887–894. doi: 10.1086/520883. [DOI] [PubMed] [Google Scholar]
- 109.Miotti PG, Dallabetta GA, Daniel RW, Canner JK, Chiphangwi JD, Liomba GN, Yang LP, Shah KV. Cervical abnormalities, human papillomavirus, and human immunodeficiency virus infections in women in Malawi. Journal of Infectious Diseases. 1996;173:714–717. doi: 10.1093/infdis/173.3.714. [DOI] [PubMed] [Google Scholar]
- 110.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, Roteli-Martins CM, Teixeira J, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 111.Trottier H, Mahmud SM, Lindsay L, Jenkins D, Quint W, Wieting SL, Schuind A, Franco EL. Persistence of an incident human papillomavirus infection and timing of cervical lesions in previously unexposed young women. Cancer Epidemiol Biomarkers Prev. 2009;18:854–862. doi: 10.1158/1055-9965.EPI-08-1012. [DOI] [PubMed] [Google Scholar]
- 112.Ahdieh L, Munoz A, Vlahov D, Trimble CL, Timpson LA, Shah K. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. American journal of epidemiology. 2000;151:1148–1157. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.