Abstract
We previously reported that dibenzo[a,l]pyrene (DB[a,l]P), the most potent known environmental carcinogen among polycyclic aromatic hydrocarbons (PAH) congeners, is carcinogenic in the oral tissues of mice. We have now developed a new mouse model which employs the oral application of the fjord region diol epoxide, (±)-anti-11,12-dihydroxy-13,14-epoxy-11,12,13,14-tetrahydrodibenzo[a,l]pyrene (DB[a,l]PDE), a metabolite of the tobacco smoke constituent DB[a,l]P, and we show its specific induction of oral squamous cell carcinoma (OSCC) in both tongue and other oral tissues. Groups of B6C3F1 mice (20/group) received 6 or 3 nmol of (±)-anti-DB[a,l]PDE administered into the oral cavity; 3 times per week for 38 weeks. Additional groups received the vehicle alone or were left untreated. Mice were sacrificed 42 weeks after the first carcinogen administration. The high dose induced 74 % and 100 % OSCC in the tongue and other oral tissues, respectively; the corresponding values at the lower dose were 45 % and 89 %. Using immunohistochemistry, we showed that DB[a,l]PDE resulted in overexpression of p53 and COX-2 proteins in malignant tissues when compared to normal oral tissues and tongues. Consistent with the carcinogenicity, we demonstrated powerful mutagenicity in cII gene in B6C3F1 (Big Blue) mouse tongue. The mutational profile in lacI reporter gene is similar to those detected in human head and neck cancer, and p53 mutations were observed in mouse oral tumor tissues. Taken together, we conclude that the formation of diol epoxides plays a major role among the mechanisms by which DB[a,l]P exerts its oral mutagenicity and tumorigenicity.
Keywords: oral cancer, carcinogenesis, mutagenesis, p53, COX-2
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide, representing a major international health problem. The five-year survival rate for patients with HNSCC is about 50% 1, and it has improved only slightly over the past decade. In the United States, over 52,000 cases and about 11,000 deaths from HNSCC occur annually 2. Oral cancer is the most common neoplasm of the head and neck. Worldwide, the annual incidence of new cases exceeds 270,000 3. Oral cancer progresses from hyperplasic lesions, through dysplasia and carcinoma in situ to invasive carcinoma4, 5. Although patients with early lesions have a high cure rate of their primary tumors, second primaries represent a significant problem; and continued exposure to a carcinogenic insult (such as tobacco consumption) and potential promoters can accelerate the development of a second primary 6-8. These factors contribute to the poor prognosis of this disease 6-10.
Tobacco smoking is the most important etiological factor in the development of oral cancer 11; however, the prevention of HNSCC by the cessation of tobacco use appears unattainable due to the addictive power of nicotine and difficulties in altering lifestyles. Other approaches such as chemoprevention and new treatment modalities are being pursued; but progress in these areas has been hampered due to the lack of appropriate animal models that would accurately reflect human etiology of the disease.
The induction of oral cancer by the synthetic carcinogens 4-nitroquinoline-N-oxide (NQO) in rats and mice, and 7,12-dimethylbenz[a]anthracene (DMBA) in the hamster have served as in vivo models to examine the etiology of the disease and to identify agents that suppress oral carcinogenesis 12. However, NQO and DMBA are synthetic chemicals, not found in the human environment. Also, DMBA induces a high percentage of ras mutations (60%), which were found in only 3% of oral tumors in the U.S. and many other countries 12. Although DMBA has been used to induce oral cancer in the hamster cheek pouch 13, there is no analogous pouch in the human mouth, and thus the relevance of this model can be questioned. The development of xenograft or genetically defined animal models has been reported but such models suffer from certain limitations, including lack of tumor heterogeneity 12, 14 and the fact that they do not model initiation of carcinogenesis.
Polycyclic aromatic hydrocarbons (PAH) and tobacco-specific nitrosamines (TSNA) are recognized potential etiological agents for oral cancer 15. DB[a,l]P is the most potent known environmental carcinogen among PAH congeners 16. Although DB[a,l]P is found at lower levels in environmental sources and in cigarette smoke than the most extensively studied PAH (B[a]P), its remarkable carcinogenicity in animal models suggests it can pose a cancer risk to humans 16. PAHs are enzymatically metabolized to a variety of metabolites, among which, diol-epoxides are often the major ultimate carcinogens 16. Syn- and anti- dibenzo[a,l]pyrene-11,12-dihydrodiol-13,14-epoxides (DB[a,l]PDE) bind covalently to DNA, and are potent mutagens in both prokaryotic and eukaryotic in vitro systems, and DB[a,l]P is a potent lung, liver, and mammary carcinogen in rodents 16, 17.
Recently, we reported the induction of oral squamous cell carcinomas by DB[a,l]P in B6C3F1 mice 18. In the current report, we tested the hypothesis that DB[a,l]PDE is the ultimate mutagenic/carcinogenic metabolite of DB[a,l]P in vivo; we further hypothesized that DNA damage-induced by DB[a,l]PDE would result in p53 mutations in mouse oral tissues. To further explore the molecular mechanisms of oral carcinogenesis induced by the parent compound of DB[a,l]PDE (DB[a,l]P), a cancer pathway gene expression array analysis was performed.
Materials and Methods
Chemicals
DB[a,l]P and (±)-anti-DB[a,l]PDE were prepared according to a published method by our group 19. These chemicals were characterized on the basis of NMR and high-resolution mass spectral data and their purity (≥99%) was determined by HPLC.
Animals
Female B6C3F1 mice (Jackson Laboratories, Bar Harbor, ME) were used in carcinogenesis and cancer pathway analysis, and Big Blue™ B6C3F1 (lacI) mice (Agilent/Stratagene, La Jolla, CA), were used in mutagenesis study. Mice were quarantined for 1 week; then they were transferred to the bioassay laboratory. All mice were kept on a 12-h light:12-h dark cycle, maintained at 50% relative humidity and 21 ± 2°C, and were fed a semi-purified, modified AIN-93M diet (5% corn oil), and water ad libitum. The bioassay was carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and was approved by Institutional Animal Care and Use Committee.
Carcinogenesis
Three groups of B6C3F1 mice (20/group) at the age of 8 weeks received DB[a,l]PDE in 30 μL DMSO (6 or 3 nmol) or DMSO administered topically into the oral cavity using a micropipette 3 time a week for 38 weeks. Another group was left untreated. Mice were weighed weekly until termination at 42 weeks after the first carcinogen administration. During the progress of the bioassay, mice were culled from the group and sacrificed if we observed a sudden weight loss of more than 20 % or a tumor size exceeding 2 cm in diameter. At termination, mice were sacrificed by CO2 asphyxiation and soft tissues of the oral cavity including tongue, pharynx, and other oral tissues (hard palate, buccal mucosa, and floor of mouth) were collected and fixed in 10% neutral buffered formalin. Tissues were processed in an automated Tissue-Tek VIP processor and paraffin-embedded with a Tissue-Tek TEC embedding station. Sections were cut at 6 μm for routine hematoxylin and eosin (H&E) staining. All tissues were examined by an ACVP diplomate pathologist blinded to treatment and were graded for the presence of hyperplasia, dysplasia, carcinoma in situ (CIS), or invasive squamous cell carcinoma (SCC) according to established criteria 20.
Immunohistochemical Analysis
Bouin's fixed, paraffin-embedded tissue blocks were sectioned at 5 microns. Slides were prepared as described previously (25). After washing, the slides were incubated with 1:50 dilution of the rabbit polyclonal primary antibody, p53 (CM5, Novocastra) or COX-2 (Cayman) for 30 min at room temperature, incubated with Dako Envision+™ anti-rabbit labeled polymer conjugated with HRP for 20 min at room temperature, developed using Dako DAB+™, and counter-stained with Meyer's modified hematoxylin.
The cII Mutagenesis Assay
In the bioassay for mutagenesis study, Big Blue™ B6C3F1 mice (6/group) received 3 nmol treatments of (±)-anti-DB[a,l]PDE in DMSO, or DMSO alone, as described above and mice were sacrificed 36 weeks after the first carcinogen administration. The tongues were excised and stored at -80°C until isolation of DNA.
Big Blue™ B6C3F1 mice contain a lambda shuttle vector that includes the bacterial lacI locus and also the cII gene, which is the target for the mutagenesis studies. As described by our group, DNA was isolated from tongue tissue, the cII mutation assay was performed, and mutant were amplified, purified and sequenced 21.
P53 Mutation
Tumor tissues obtained from the carcinogenesis experiment were used to detect p53 mutations using PCR amplification and direct DNA sequencing. A total of five frozen tumor tissues were homogenized and the isolation of DNA was carried out using a Qiagen genomic DNA isolation procedure as previously described 22, 23. Amplification of evolutionary conserved exons 5-8 of p53 by PCR was conducted with the primers and conditions reported in literatures 24, 25: denaturation at 94°C for 60 s, followed by 35 cycles of amplification (30 s at 94°C, 60 s at 71°C for exons 5, 7, and 8; 60 s at 70°C for exon 6) and an additional extension (70°C or 71°C for 5 min). PCR products were purified with QIAquick PCR purification kit and subjected to direct DNA sequencing on ABI 3130XL capillary sequencer. Both forward and reverse strands were sequenced (2).
The presence of p53 mutations was also confirmed by Single-Strand Conformation Polymorphism (SSCP) using MDE gels. For this assay, PCR products from the reactions described above were treated with stop solution and denatured by heating to 94°C for 5 min, placed on ice to stabilize single strands and then loaded on 1x MDE gel (Lonza, ME) , electrophoresed, and stained with Gelstar fluorescent stain (Lonza, ME) .
Gene expression
The mouse Cancer Pathway Finder PCR array (PAMM-033, SABiosciences™, USA) consisting of 84 genes representative of the six biological pathways involved in transformation and tumorigenesis were employed to help understand at the gene expression level, the tumorigenic effects of DB[a,l]P on mouse oral tissues. In a short-term bioassay, B6C3F1 mice at the age of 8 weeks old received DB[a,l]P (24 nmol) in 30 μL DMSO or DMSO administered topically into the oral cavity using a micropipette 3 time a week for 5 weeks. Mice were sacrificed 48 hr after the last carcinogen treatment. RNA was isolated from pooled oral tissues of 2 mice using an RNAeasy Kit, (Qiagen, Valencia, CA). The quality of isolated RNA was checked with a NanoDrop ND-1000 UV spectrophotometer and an Agilent 2100 Bioanalyzer. The conversion of RNA to cDNA was performed using an RT2 First Strand kit from the SA Biosciences. To each well, cDNA (2 μl from 25 ng RNA), RT2 SYBR® Green qPCR Master Mix (SA biosciences) (12.5 μl) and water (10.5 μl) were added; then, real time PCR arrays were carried out using a Bio-Rad Opticon 2 Real-Time PCR System. ΔCt values (treated vs. control) were obtained relative to the average of five housekeeping genes (Gusb, Hsp90ab1, Hprt1, Gapdh, Actb) present in the arrays. Real time PCR results were analyzed using software provided by the manufacturer. Where sufficient RNA was available arrays were performed in duplicate and the average value is given. Gene pathway analysis was conducted using Gene Network Central Pro (SABiosciences) which is available on http://gncpro.sabioscienes.com/gncpro/gncpro.php.
Results
Carcinogenesis
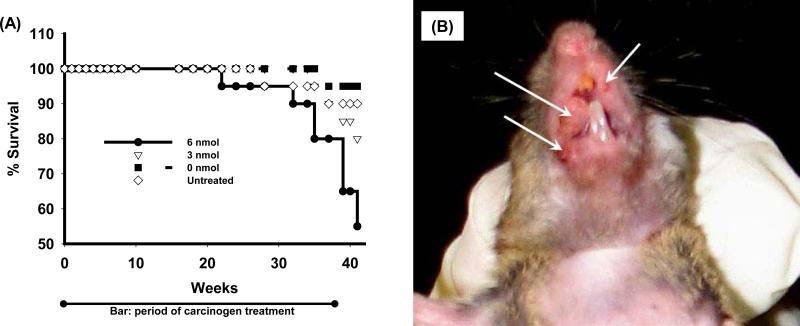
Figure 1A shows the cumulative mortality of mice in the carcinogenesis bioassay. Some mice were sacrificed prior to the termination because of weight loss or tumors over 2 cm in diameter. The probability of survival was analyzed using life-table analysis with death as an end point. The log-rank test was used to evaluate the difference between pairs of groups. Mice that received 3 or 6 nmol DB[a,l]PDE in DMSO had a shorter survival time than mice that were untreated or received only DMSO (P<0.001).The percent survival for the mice that received 6, 3, 0 nmol DB[a,l]PDE in DMSO and untreated were 55, 80, 95, and 90%, respectively.
Figure 1.
Cumulative mortality of mice, and tumors induced by DB[a,l]PDE. (A) Percentage survival of B6C3F1 mice treated by oral administration of DB[a,l]PDE at various doses three times a week for 38 weeks during the progress of the bioassay. (B) A representative picture showing the DB[a,l]PDE-induced SCC in oral cavity 42 weeks after the initiation of the treatment with DB[a,l]PDE.
Carcinogenesis induced by DB[a,l]PDE in the tongue and other oral tissues of mouse is summarized in Table 1. Figure 1B is a representative picture showing oral tumors induced by DB[a,l]PDE. DB[a,l]PDE is a highly potent carcinogen, and it induced OSCC in a dose-dependent manner in tongue and other oral tissues. Although we observed a low incidence (about 20%) of SCC in the facial skin surrounding the lip, no obvious tumors were observed in tissues distal to the head. Our results demonstrate that DB[a,l]PDE specifically induced tumors in the oral cavity; no other tumors were observed under the experimental conditions of our bioassay.. In mice receiving the higher dose of DB[a,l]PDE, tumor incidence was 74% in the tongue and 100% in other oral tissues; the corresponding values at the lower dose were 45 and 89%, respectively. In addition, hyperplasia, dysplasia, and papillomas were also observed in both tongue and other oral tissues. Table 1 reports mild and moderate dysplasia, and severe dysplasia are reported as carcinoma in situ (CIS), which is not found in DMSO-treated or untreated mice.
Table 1.
Mice with lesions in the oral cavity.
| DB[a,l]PDE Treatment | Number of mice | Effective no. of micea | Hyperplasia | Dysplasia | Neoplasia |
Othersb | |||
|---|---|---|---|---|---|---|---|---|---|
| CIS | SCC | total | |||||||
| Oral tissue | 6 nmol/L | 20 | 17 | 17 (100)c,d,f | 17 (100) | 12 (71)d,f | 13 (76)d,f | 17 (100)d,f | 5 (29)d,f |
| 3 nmol/L | 20 | 19 | 19 (100)d,f | 19 (100) | 14 (74)d,f | 14 (74)d,f | 17 (89)d,f | 3 (16)e,g | |
| DMSOh | 20 | 19 | 14 (74) | 18 (95) | 0(0) | 0(0) | 0 (0) | 0 (0) | |
| Untreated | 20 | 18 | 12 (67) | 17 (94) | 0(0) | 0(0) | 0 (0) | 0 (0) | |
| Tongue | 6 nmol/L | 20 | 19 | 19 (100)c,d,f | 18 (95)d,f | 10 (53)d,f | 6 (32)d,f | 14 (74)d,f | 5 (26)d,f |
| 3 nmol/L | 20 | 20 | 20 (100)d,f | 20 (100)d,f | 7 (35)d,f | 3 (15)d,f | 9 (45)d,f | 1 (5) | |
| DMSOh | 20 | 20 | 12 (60) | 11 (55) | 0 | 0 | 0 (0) | 0 (0) | |
| Untreated | 20 | 19 | 11 (58) | 10 (53) | 0 | 0 | 0 (0) | 0 (0) | |
Mice which died before the first tumor appeared in the study or did not reach histology due to cannibalism were not counted.
Others are primarily papillomas. In addition, we observed SCC in facial skin that can be considered HNSCC, but at lower incidence at about 20%.
Number in parentheses, percentage.
Significantly different from DMSO, p < 0.01.
Significantly different from DMSO, p < 0.05.
Significantly different from untreated, p < 0.01.
Significantly different from untreated, p < 0.05.
Previously reported in Ref. 18.
Immunohistochemical Analysis
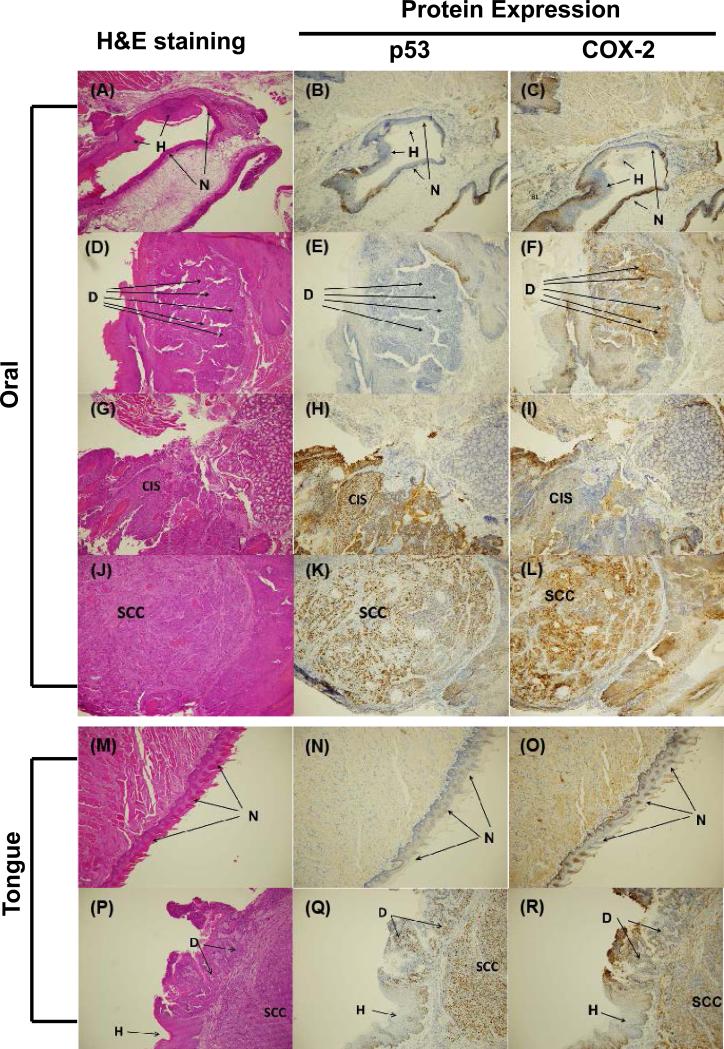
In tongue (Figure 2 M-R) and other oral tissues (Figure 2 A-L), p53 and COX-2 protein expression was elevated by DB[a,l]PDE treatment, which is consistent with results obtained with its parent DB[a,l]P. The induction of p53 and COX-2 proteins is in good agreement with the histological stages of disease progression. There was no p53 staining observed in normal and hyperplasia region, and the staining was weaker in dysplasia region than in the region of carcinoma. The COX-2 staining was similar in carcinoma and dysplasia regions, and no clear staining was observed in normal and hyperplasia region of both tongue and other oral tissues.
Figure 2.
Immunohistochemical analysis of p53 and COX-2 proteins expression in DB[a,l]PDE-treated oral (A-L) or tongue (M-R) tissues of B6C3F1 mouse (see “Materials and Methods”). (A), H&E staining of normal squamous epithelium and hyperplasia. (B), Expression of p53 protein in normal epithelium and hyperplasia. (C), Expression of COX-2 protein in normal epithelium and hyperplasia. (D), H&E staining of dysplasia. (E), Expression of p53 protein in dysplasia. (F), Expression of COX-2 protein in dysplasia. (G), H&E staining of CIS. (H), Expression of p53 protein in CIS. (I), Expression of COX-2 protein in CIS. (J), H&E staining of SCC. (K), Expression of p53 protein in SCC. (L), Expression of COX-2 protein in SCC. (M), H&E staining of normal squamous epithelium. (N), Expression of p53 protein in normal epithelium. (O), Expression of COX-2 protein in normal epithelium. (P), H&E staining of hyperplasia, dysplasia, and SCC. (Q), Expression of p53 protein in hyperplasia, dysplasia, and SCC. (R), Expression of COX-2 protein in hyperplasia, dysplasia, and SCC.
The cII Mutagenesis Assay
At the administered dose (3 nmol/application), DB[a,l]PDE induced a 6.7-fold increase in mutation fraction in tongue relative to control (17.5 ± 1.8 vs. 2.6 ± 0.68 × 10-5 plaque forming units; p < 0.001). The mutational profiles in tongues of mice treated with DB[a,l]PDE are shown in Table 2 along with those for untreated controls. The mouse tongues analyzed for the B[a]P mutational profiles were obtained from a previous study26 and the results are included for comparison (see discussion). Both DB[a,l]PDE and B[a]P induced more mutations at GC base pairs than AT base pairs, but DB[a,l]PDE induced a significant higher fraction of mutations at AT base pairs (31.3% vs. 4.2%, respectively) using Fisher's exact test adjusted for multiple comparisons (p < 0.001), and this result is consistent with mutations induced by DB[a,l]P vs. B[a]P 18.
Table 2.
Mutational profiles of (±)-anti-DB[a,l]PDE-and B[a]P-induced mutations in tongue and other oral tissues: numbers and percentages of mutants
| (±)-anti-DB[a,l]PDE | B[a]Pa | Controlb | ||||
|---|---|---|---|---|---|---|
| mutation class | number | % | number | % | % | |
| transitions | GC:AT | 7 | 11.8 | 15 | 31.3 | 63 |
| AT:GC | 3 | 7.8 | 0 | 0 | 3 | |
| transversions | GC:TA | 19 | 37.3 | 19 | 39.6 | 14 |
| GC:CG | 8 | 15.7 | 5 | 10.4 | 3 | |
| AT:CG | 2 | 3.9 | 0 | 0 | 3 | |
| AT:TA | 10 | 19.6 | 2 | 4.2 | 6 | |
| in/delc | 2 | 3.9 | 7 | 14.6 | 9 | |
| total | 51 | 100 | 48 | 100 | 100 | |
Tongue and other oral tissues for B[a]P were obtained from a previous study (26). The MF in the cII gene for the tongues from the B[a]P treated mice was 15.7 ± 2.9 × 10-5 vs. 2.1 ± 1.1 for control.
Percentage taken from ref. 18.
Insertions and deletions.
P53 mutations
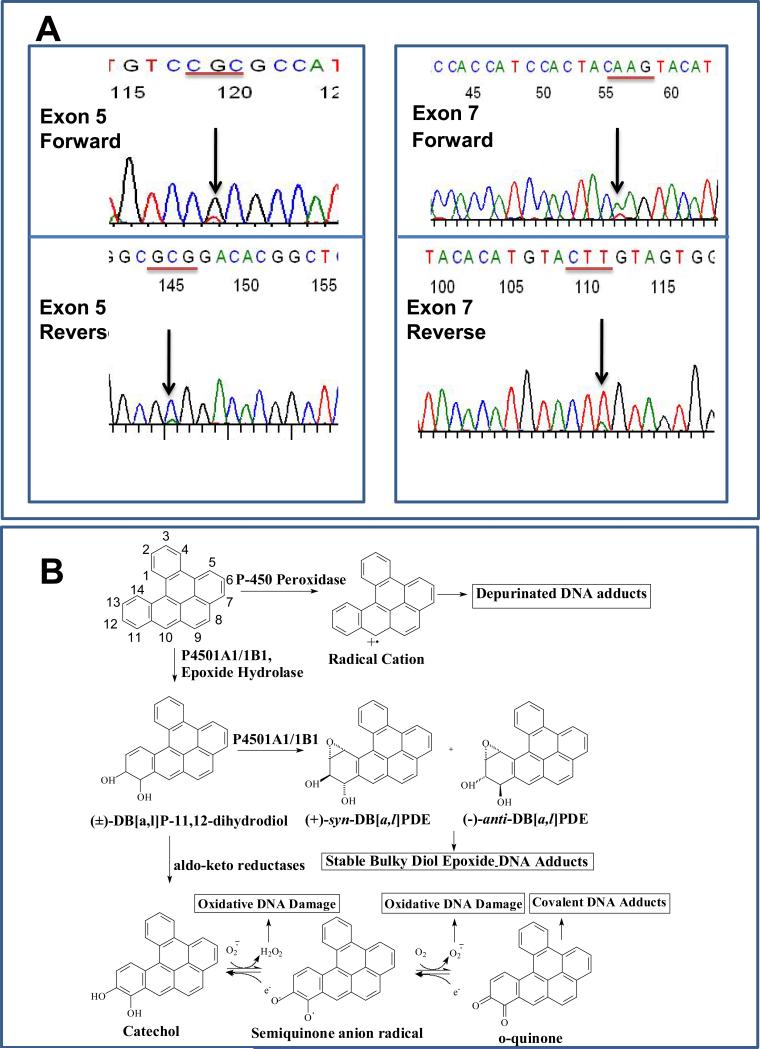
P53 is frequently mutated or lost in OSCC in humans 27. In an attempt to determine whether overexpression of p53 protein observed in this study is associated with mutations induced by DB[a,l]PDE, we subsequently isolated DNA from frozen tumor tissues obtained from our bioassay. Mutations in exons 5 and 7 were observed from 2 tumor tissues out of 5 (Figure 3A). A point mutation in exon 5 was classified as missense (codon 155, a CGC to CTC substitution mutation resulting in an amino acid change of Arg to Leu), and the other in exon 7 was a nonsense mutation (codon 232, an AAG to TAG substitution resulting in an amino acid change of Lys to a stop codon). Subsequently, the presence of p53 mutations was verified using SSCP (data not shown).
Figure 3.
(A) Direct sequencing of p53 in exon 5 and exon 7 in both forward and reverse directions. In exon 5, nucleotide G was changed to T (upper panel), and nucleotide C was changed to A (lower panel). In exon 7, nucleotide A was changed to T (upper panel), and nucleotide T was changed to A (lower panel). (B) A schematic representation of the metabolic activation and subsequent events of DB[a,l]P.
Effect of DB[a,l]P on gene expression
To further investigate the mechanisms by which DB[a,l]P and presumably its diol epoxides may be involved in oral carcinogenesis, the effects of DB[a,l]P on gene expression in mouse oral cavity was explored using a focused real-time cancer pathway PCR array. The dose and duration of treatment with DB[a,l]P in this experiment were those previously shown to form DB[a,l]P-DNA adducts in the oral tissues of mouse 23. Among the 84 representative genes included in the array, 22 of them were upregulated 2 or more folds and only one gene was downregulated (Table 3). As mutations in p53 were observed, genes affecting or modulated by p53 were of interest. We found that 12 of the genes modulated by DB[a,l]P have been reported to affect expression of, or be modulated by p53 (S100A4, CHEK2, CDKN2A, E2F1, CDK4, TNFRSF10B, BAX, SERPINE1, TERT, MTA2, MUC1 and CCNE1), and all of them were upregulated.
Table 3.
Modulation of gene expression by DB[a,l]P in mouse oral tissues.
| symbol | fold | description |
|---|---|---|
| 1. Cell Cycle Control and DNA Damage Repair | ||
| Ccne1 | 2.56 | Cyclin E1 |
| Cdk4 | 1.96 | Cyclin-dependent kinase 4 |
| Cdkn2a | 2.76 | Cyclin-dependent kinase inhibitor 2A |
| Chek2 | 3.10 | CHK2 checkpoint homolog (S. pombe) |
| E2f1 | 2.68 | E2F transcription factor 1 |
| 2. Apoptosis and Cell Senescence | ||
| Bax | 2.02 | Bcl2-associated X protein |
| Tert | 4.27 | Telomerase reverse transcriptase |
| Tnfrsf10b | 2.22 | Tumor necrosis factor receptor superfamily, 10b |
| 3. Signal Transduction Molecular and Transcription Factors | ||
| Fos | 4.77 | Interferon beta 1, fibroblast |
| Pik3r1 | 2.47 | Phosphatidylinositol 3-kinase, regulatory |
| 4. Adhesion | ||
| Itga2 | 3.01 | Integrin alpha 2 |
| Itgb3 | 2.36 | Integrin beta 3 |
| 5. Angiongenesis | ||
| Angpt1 | 3.02 | Angiopoietin 1 |
| Ifnb1 | 4.77 | FBJ osteosarcoma oncogene |
| Pdgfa | 2.55 | Platelet derived growth factor, alpha |
| 6. Invasion and Metastasis | ||
| S100A4 | 2.41 | S100 calcium binding protein A4 |
| Mta2 | 1.97 | Metastasis-associated gene family, member 2 |
| Mcam | 3.22 | Melanoma cell adhesion molecule |
| Serpine1 | 3.99 | Serine (or cysteine) peptidase inhibitor, clade E, member 1 |
| Timp1 | 2.18 | Tissue inhibitor of metalloproteinase 1 |
| Kiss1 | 2.03 | KiSS-1 metastasis-suppressor |
| Muc1 | 2.25 | Mucin 1, transmembrane |
| Serpinb2 | -2.53 | Serine (or cysteine) peptidase inhibitor, clade B, member 2 |
Discussion
While the carcinogenicity of DB[a,l]P in the oral tissues of mice has been reported 16, the mechanisms and pathways leading to this tumor formation have not been thoroughly investigated. Therefore, in the present study we tested the hypothesis that initiation of DB[a,l]P-induced oral carcinogenesis is primarily due to the formation of its fjord region diol epoxide. In figure 3B, a schematic representation shows the three different pathways which have been proposed to be involved in the activation of DB[a,l]P: (1) the radical cation pathway (mediated by P450 peroxidases and other peroxidases) to yield depurinating adducts; (2) the diol epoxides pathway (mediated by cytochromes P4501A1/1A2 and 1B1) to yield stable bulky diol epoxide-DNA adducts; and (3) the PAH ortho-quinone pathway (mediated by aldo-keto reductases) which results in bulky stable DNA adducts, depurinating adducts, and oxidatively modified DNA lesions 22. Previously, we demonstrated that both DB[a,l]PDE and its parent DB[a,l]P resulted in the formation of DB[a,l]PDE-dA adducts in mouse oral tissue using a sensitive LC-MS/MS method 23. Although the detection of DB[a,l]PDE-dA adducts provides some evidence that DB[a,l]P can be metabolized to its diol epoxides and that it is stable enough to react with DNA in the oral tissues of mouse, the formation of this adduct cannot fully explain the mutagenicity and carcinogenicity 18. In this report, we show for the first time that oral application of DB[a,l]PDE induces tumors exclusively in tongue and other oral tissues of B6C3F1 mice. DB[a,l]PDE induced more tumors in the oral tissues and at lower doses (6 and 3 nmol) than did DB[a,l]P at a higher dose (24 nmol) 18. The tumors induced by DB[a,l]PDE closely resemble those induced by DB[a,l]P. Both DB[a,l]P and DB[a,l]PDE similarly affect protein expression of COX-2 and p53, and mutation profiles (Fig. 2), 18. Taken together, we conclude that DB[a,l]P exerts its oral mutagenicity and tumorigenicity mainly through the formation of its diol epoxide, supporting our hypothesis.
Previous reports suggested that exposure to specific environmental pollutants give rise to specific types of molecular alterations in HNSCC; i.e. different etiological factors are reflected in different molecular characteristics and clinical outcome of these tumors 28, 29. The up-regulation of p53 protein expression in oral lesions appears to correlate well with oral malignancy, and this result is consistent with the histological progression of OSCC 30, 31.
It has been suggested that p53 mutations may be responsible for COX-2 up-regulation frequently observed in HNSCCs 32, 33. Although it was also reported that there was no significant association between COX-2 and p53 expression in patients with oral and pharyngeal squamous cell carcinoma 34, it is noted that, unlike mutant p53, wild-type p53 has been shown to suppress COX-2 transcription 33. As mentioned above, p53 is frequently mutated or lost in OSCC. The inactivation or mutation of p53 and elevations in COX-2 protein represent common events in human malignant transformation, and their expression/alteration could therefore be important for the evaluation of chemotherapeutic or chemopreventive agents in animal models.
The mutational spectra of p53 have been suggested to reflect the exposure to specific exogenous and endogenous carcinogenic agents and processes 35. It has been reported that the most frequent types of p53 mutations identified in HNSCC are G:C > A:T transitions and G:C > T:A transversions 29, 32. G:C > T:A transversions are characteristic of exogenous DNA-damaging agents derived from bulky carcinogens including tobacco smoke carcinogens and certain other agents 29, 35. Additionally these mutations can be result from the reactive-oxygen-species DNA adduct, 8-oxodeoxyguanosine 36.
Among the carcinogenic polycyclic aromatic hydrocarbons in tobacco, B[a]P is often considered the most relevant to human cancer induction 37, but the results of the present and our recent report on carcinogenesis induced by DB[a,l]P 18 suggest that the latter may also be an important contributor in the development of this disease . Although DB[a,l]P has been detected in cigarette smoke, but not quantified. However, in soil and sediment samples, the levels of DB[a,l]P contents were determined to be in the range of tens to hundreds ppb for samples with different level of pollution, and they are roughly more than 2 orders of magnitude lower than those of B[a]P, the gap is reduced by a 40-fold difference in the case of strong contamination 16, 22. DB[a,l]P, DB[a,l]PDE and B[a]P all induce oral tissue mutations at GC base-pairs at about 60% of the total mutations (Table 2 and ref. 21). However, mutations at AT base pairs accounted for about 30% of the DB[a,l]PDE-induced mutations also consistent with DB[a,l]P-induced mutations 18 and much higher than the 4% observed in the oral cavity of mice treated with B[a]P 18. In tumors of the oral cavity in humans the fraction of AT base pair mutations in the p53 gene in tumors of the oral cavity is about 24% 35, similar to the percentage observed here and much higher than that induced by B[a]P. Although speculative these results suggest that our DB[a,l]P / DB[a,l]PDE model of oral cancer is relevant to tobacco-smoke induced cancer of the oral cavity.
B[a]P has been implicated in p53 mutations in smoking-related cancers based partially on its ability to preferentially induce GC > TA mutations, and its ability to target specific p53 “hotspots” 37, 38. However, it has been pointed out that certain hotspots (e.g. codon 179, position 2) not targeted by B[a]P, contain AT > GC mutations 37. AT > GC mutations were induced in mouse oral tissues by DB[a,l]P and DB[a,l]PDE, but not B[a]P (Table 2 and ref. 21). We further demonstrated that DB[a,l]PDE induced p53 mutations in a limited number of tumor tissues obtained from our carcinogenesis bioassay. The mutational characteristics observed in our model (cII and p53 genes) appear to be in line with those reported for human oral squamous cell carcinomas.
The fact that p53 abnormalities resulting from DB[a,l]P and its diol epoxide treatments may be actively involved in oral carcinogenesis induced by DB[a,l]P is further supported using a cancer pathway-specific PCR expression array. The effect of DB[a,l]P on gene expression in mouse oral cavity was explored as an initial attempt to provide some insights into the mechanisms of oral carcinogenesis in this animal model. Clearly, our results demonstrate that exposure of mice to DB[a,l]P resulted in altered expression of a number of genes modulated by, or affecting p53 expression. Although future studies are required to explore in detail, the relevance of select gene expression to oral carcinogenesis, our results demonstrate, in general (Table 3), that DB[a,l]P influences the expression of several genes related to cell cycle, DNA damage and repair and apoptosis among other cellular events that are critical in the carcinogenic process. The expression of S100A4, CHEK2, CDKN2A, and E2F1 has been reported to result in the stabilization, activation or overexpression of p53 protein 39-42. Overexpression of CDKN2A (p21) is a p53- mediated response to DNA damage which slows progression through the cell cycle and allows more time for DNA repair 43.
Tnfrsf10b, Bax and SERPINE1 were found to be activated or up-regulated by p53 44-46. High TERT immunostaining had been reported to be associated with p53 mutation in human breast tumors 47. It has also been shown that the amplification of CCNE1 was associated with p53 mutations and smoking in a series of non-small-cell lung carcinomas 48. Therefore, the overexpression of TERT and CCNE1 could result from mutated p53. The MUC1 oncogene is overexpressed in human carcinomas and suppresses p53-dependent apoptotic response to DNA damage 49. The expression of MAT strongly represses p53-dependent transcriptional activation, and modulates p53-mediated cell growth arrest and apoptosis 50. We propose that the induction of MUC1 and MAT might inhibit p53-dependent processes.
In conclusion, we have developed for the first time a non-surgical whole animal model demonstrating the potent carcinogenicity and high organ-specificity of a metabolite derived from an environmental and tobacco carcinogen in the oral cavity; this model is an appropriate platform to further explore the genetic and epigenetic alterations that can account for the development of OSCC in humans. This model will be especially valuable to test the chemopreventive efficacy of agents which can modulate p53, COX-2 or additional molecular targets that are critical in oral carcinogenesis.
Novelty & Impact Statements.
We have developed a novel animal model to demonstrate the potent carcinogenicity of (±)-anti-DB[a,l]PDE, a metabolite of the tobacco smoke constituent and environmental carcinogen dibenzo[a,l]pyrene in the oral cavity of mice. This model is an appropriate platform to explore genetic and epigenetic alterations that can account for the development of OSCC and to evaluate the chemopreventive efficacy of agents which can modulate p53, COX-2 or additional molecular targets that are critical in oral carcinogenesis.
Acknowledgement
Grant Support: by NCI grant R01-CA173465 and Penn State Cancer Institute Seed Funds.
References
- 1.Fuller CD, Wang SJ, Thomas CR, Jr., Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973-1998. Cancer. 2007;109:1331–43. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Boone CW, Kelloff GJ, Steel V. Histopathology of the premalignant process. Cancer Bull. 1991;43:481–4. [Google Scholar]
- 5.Kramer IR, Lucas RB, Pindborg JJ, Sobin LH. Definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46:518–39. [PubMed] [Google Scholar]
- 6.Franco EL, Kowalski LP, Kanda JL. Risk factors for second cancers of the upper respiratory and digestive systems: a case-control study. J Clin Epidemiol. 1991;44:615–25. doi: 10.1016/0895-4356(91)90021-z. [DOI] [PubMed] [Google Scholar]
- 7.Licciardello JT, Spitz MR, Hong WK. Multiple primary cancer in patients with cancer of the head and neck: second cancer of the head and neck, esophagus, and lung. Int J Radiat Oncol Biol Phys. 1989;17:467–76. doi: 10.1016/0360-3016(89)90096-5. [DOI] [PubMed] [Google Scholar]
- 8.Lippman SM, Hong WK. Second malignant tumors in head and neck squamous cell carcinoma: the overshadowing threat for patients with early-stage disease. Int J Radiat Oncol Biol Phys. 1989;17:691–4. doi: 10.1016/0360-3016(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 9.Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer. 1992;70:14–9. doi: 10.1002/1097-0142(19920701)70:1<14::aid-cncr2820700103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Jovanovic A, van der Tol IG, Schulten EA, Kostense PJ, de Vries N, Snow GB, van der Waal I. Risk of multiple primary tumors following oral squamous-cell carcinoma. International journal of cancer. 1994;56:320–3. doi: 10.1002/ijc.2910560304. [DOI] [PubMed] [Google Scholar]
- 11.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 12.Wong KK. Oral-specific chemical carcinogenesis in mice: an exciting model for cancer prevention and therapy. Cancer Prev Res (Phila Pa) 2009;2:10–3. doi: 10.1158/1940-6207.CAPR-08-0234. [DOI] [PubMed] [Google Scholar]
- 13.Vitale-Cross L, Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Chemical carcinogenesis models for evaluating molecular-targeted prevention and treatment of oral cancer. Cancer Prev Res (Phila Pa) 2009;2:419–22. doi: 10.1158/1940-6207.CAPR-09-0058. [DOI] [PubMed] [Google Scholar]
- 14.Raimondi AR, Molinolo A, Gutkind JS. Rapamycin prevents early onset of tumorigenesis in an oral-specific K-ras and p53 two-hit carcinogenesis model. Cancer Res. 2009;69:4159–66. doi: 10.1158/0008-5472.CAN-08-4645. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–90. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 16.Luch A. On the impact of the molecule structure in chemical carcinogenesis. Exs. 2009;99:151–79. doi: 10.1007/978-3-7643-8336-7_6. [DOI] [PubMed] [Google Scholar]
- 17.Leavitt SA, George MH, Moore T, Ross JA. Mutations induced by benzo[a]pyrene and dibenzo[a,l]pyrene in lacI transgenic B6C3F1 mouse lung result from stable DNA adducts. Mutagenesis. 2008;23:445–50. doi: 10.1093/mutage/gen033. [DOI] [PubMed] [Google Scholar]
- 18.Guttenplan JB, Kosinska W, Zhao ZL, Chen KM, Aliaga C, Deltondo J, Cooper T, Sun YW, Zhang SM, Jiang K, Bruggeman R, Sharma AK, et al. Mutagenesis and carcinogenesis induced by dibenzo[a,l]pyrene in the mouse oral cavity: a potential new model for oral cancer. International journal of cancer. 2012;130:2783–90. doi: 10.1002/ijc.26344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krzeminski J, Lin JM, Amin S, Hecht SS. Synthesis of Fjord region diol epoxides as potential ultimate carcinogens of dibenzo[a,l]pyrene. Chem Res Toxicol. 1994;7:125–9. doi: 10.1021/tx00038a002. [DOI] [PubMed] [Google Scholar]
- 20.Leininger JR, Jokinen MP, et al. Oral Cavity, Esophagus, and Stomached. Cache River Press; St. Louis, MO: 1999. [Google Scholar]
- 21.Boyiri T, Guttenplan J, Khmelnitsky M, Kosinska W, Lin JM, Desai D, Amin S, Pittman B, El-Bayoumy K. Mammary carcinogenesis and molecular analysis of in vivo cII gene mutations in the mammary tissue of female transgenic rats treated with the environmental pollutant 6-nitrochrysene. Carcinogen. 2004;25:637–43. doi: 10.1093/carcin/bgh040. [DOI] [PubMed] [Google Scholar]
- 22.Chen KM, Zhang SM, Aliaga C, Sun YW, Cooper T, Gowdahalli K, Zhu J, Amin S, El-Bayoumy K. Induction of ovarian cancer and DNA adducts by Dibenzo[a,l]pyrene in the mouse. Chem Res Toxicol. 2012;25:374–80. doi: 10.1021/tx2004322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang SM, Chen KM, Aliaga C, Sun YW, Lin JM, Sharma AK, Amin S, El-Bayoumy K. Identification and quantification of DNA adducts in the oral tissues of mice treated with the environmental carcinogen dibenzo[a,l]pyrene by HPLC-MS/MS. Chem Res Toxicol. 2011;24:1297–303. doi: 10.1021/tx200188j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tam AS, Foley JF, Devereux TR, Maronpot RR, Massey TE. High frequency and heterogeneous distribution of p53 mutations in aflatoxin B1-induced mouse lung tumors. Cancer Res. 1999;59:3634–40. [PubMed] [Google Scholar]
- 25.Trukhanova LS, Hong HH, Sills RC, Bowser AD, Gaul B, Boorman GA, Turusov VS, Devereux TR, Dixon D. Predominant p53 G-->A transition mutation and enhanced cell proliferation in uterine sarcomas of CBA mice treated with 1,2-dimethylhydrazine. Toxicologic pathology. 1998;26:367–74. doi: 10.1177/019262339802600310. [DOI] [PubMed] [Google Scholar]
- 26.Guttenplan JB, Spratt TE, Khmelnitsky M, Kosinska W, Desai D, El-Bayoumy K. Effects of 3H-1,2-dithiole-3-thione, 1,4-phenylenebis(methylene)selenocyanate, and selenium-enriched yeast individually and in combination on benzo[a]pyrene-induced mutagenesis in oral tissue and esophagus in lacZ mice. Mutat Res. 2004;559:199–210. doi: 10.1016/j.mrgentox.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Bradley G, Irish J, MacMillan C, Mancer K, Witterick I, Hartwick W, Gullane P, Kamel-Reid S, Benchimol S. Abnormalities of the ARF-p53 pathway in oral squamous cell carcinoma. Oncogene. 2001;20:654–8. doi: 10.1038/sj.onc.1204131. [DOI] [PubMed] [Google Scholar]
- 28.Marsit CJ, Christensen BC, Houseman EA, Karagas MR, Wrensch MR, Yeh RF, Nelson HH, Wiemels JL, Zheng S, Posner MR, McClean MD, Wiencke JK, et al. Epigenetic profiling reveals etiologically distinct patterns of DNA methylation in head and neck squamous cell carcinoma. Carcinogenesis. 2009;30:416–22. doi: 10.1093/carcin/bgp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hjortsberg L, Rubio-Nevado JM, Hamroun D, Claustres M, Beroud C, Soussi T. The p53 Handbook. (ed. 2) 2008 Available from: http://p53.free.fr/Database/p53_database.html.
- 30.Ogden GR, Kiddie RA, Lunny DP, Lane DP. Assessment of p53 protein expression in normal, benign, and malignant oral mucosa. J Pathol. 1992;166:389–94. doi: 10.1002/path.1711660411. [DOI] [PubMed] [Google Scholar]
- 31.Field JK, Spandidos DA, Malliri A, Gosney JR, Yiagnisis M, Stell PM. Elevated P53 expression correlates with a history of heavy smoking in squamous cell carcinoma of the head and neck. British journal of cancer. 1991;64:573–7. doi: 10.1038/bjc.1991.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallo O, Schiavone N, Papucci L, Sardi I, Magnelli L, Franchi A, Masini E, Capaccioli S. Down-regulation of nitric oxide synthase-2 and cyclooxygenase-2 pathways by p53 in squamous cell carcinoma. Am J Pathol. 2003;163:723–32. doi: 10.1016/S0002-9440(10)63699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendes RA, Carvalho JF, Waal I. An overview on the expression of cyclooxygenase-2 in tumors of the head and neck. Oral Oncol. 2009;45:e124–8. doi: 10.1016/j.oraloncology.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Atula T, Hedstrom J, Ristimaki A, Finne P, Leivo I, Markkanen-Leppanen M, Haglund C. Cyclooxygenase-2 expression in squamous cell carcinoma of the oral cavity and pharynx: association to p53 and clinical outcome. Oncol Rep. 2006;16:485–90. [PubMed] [Google Scholar]
- 35.Olshan AF, Weissler MC, Pei H, Conway K. p53 mutations in head and neck cancer: new data and evaluation of mutational spectra. Cancer Epidemiol Biomarkers Prev. 1997;6:499–504. [PubMed] [Google Scholar]
- 36.Le Page F, Margot A, Grollman AP, Sarasin A, Gentil A. Mutagenicity of a unique 8-oxoguanine in a human Ha-ras sequence in mammalian cells. Carcinogenesis. 1995;16:2779–84. doi: 10.1093/carcin/16.11.2779. [DOI] [PubMed] [Google Scholar]
- 37.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274:430–2. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 38.Ruggeri B, DiRado M, Zhang SY, Bauer B, Goodrow T, Klein-Szanto AJ. Benzo[a]pyrene-induced murine skin tumors exhibit frequent and characteristic G to T mutations in the p53 gene. Proc Natl Acad Sci U S A. 1993;90:1013–7. doi: 10.1073/pnas.90.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klingelhofer J, Senolt L, Baslund B, Nielsen GH, Skibshoj I, Pavelka K, Neidhart M, Gay S, Ambartsumian N, Hansen BS, Petersen J, Lukanidin E, et al. Up-regulation of metastasis-promoting S100A4 (Mts-1) in rheumatoid arthritis: putative involvement in the pathogenesis of rheumatoid arthritis. Arthritis and rheumatism. 2007;56:779–89. doi: 10.1002/art.22398. [DOI] [PubMed] [Google Scholar]
- 40.Bruno T, De Nicola F, Iezzi S, Lecis D, D'Angelo C, Di Padova M, Corbi N, Dimiziani L, Zannini L, Jekimovs C, Scarsella M, Porrello A, et al. Che-1 phosphorylation by ATM/ATR and Chk2 kinases activates p53 transcription and the G2/M checkpoint. Cancer Cell. 2006;10:473–86. doi: 10.1016/j.ccr.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH, Peters G. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. The EMBO journal. 1998;17:5001–14. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong S, Pusapati RV, Powers JT, Johnson DG. Oncogenes and the DNA damage response: Myc and E2F1 engage the ATM signaling pathway to activate p53 and induce apoptosis. Cell cycle (Georgetown, Tex. 2006;5:801–3. doi: 10.4161/cc.5.8.2638. [DOI] [PubMed] [Google Scholar]
- 43.Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–90. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 44.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 45.Takimoto R, Wang W, Dicker DT, Rastinejad F, Lyssikatos J, el-Deiry WS. The mutant p53-conformation modifying drug, CP-31398, can induce apoptosis of human cancer cells and can stabilize wild-type p53 protein. Cancer biology & therapy. 2002;1:47–55. doi: 10.4161/cbt.1.1.41. [DOI] [PubMed] [Google Scholar]
- 46.Shetty S, Shetty P, Idell S, Velusamy T, Bhandary YP, Shetty RS. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein p53. The Journal of biological chemistry. 2008;283:19570–80. doi: 10.1074/jbc.M710268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bodvarsdottir SK, Steinarsdottir M, Hilmarsdottir H, Jonasson JG, Eyfjord JE. MYC amplification and TERT expression in breast tumor progression. Cancer genetics and cytogenetics. 2007;176:93–9. doi: 10.1016/j.cancergencyto.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Blons H, Pallier K, Le Corre D, Danel C, Tremblay-Gravel M, Houdayer C, Fabre-Guillevin E, Riquet M, Dessen P, Laurent-Puig P. Genome wide SNP comparative analysis between EGFR and KRAS mutated NSCLC and characterization of two models of oncogenic cooperation in non-small cell lung carcinoma. BMC medical genomics. 2008;1:25. doi: 10.1186/1755-8794-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–78. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408:377–81. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]