Abstract
Periodontitis is a prevalent oral inflammatory disease that leads to alveolar bone loss and may exert an adverse impact on systemic health. Experimental animal models are critical tools to investigate mechanisms of periodontal pathogenesis and test new therapeutic approaches. The ligature-induced periodontitis model has been used frequently in relatively large animals, including non-human primates, to assess the host response and its effects on the tooth-supporting tissues (gingiva and bone) under well-controlled conditions. Although mice constitute the most convenient and versatile model for mechanistic immunological research (plethora of genetically engineered strains and immunological reagents), the tiny size of the murine oral cavity has presented technical challenges for ligature placement. In this report, we present a straightforward method for ligating the second maxillary molar tooth, and, moreover, identified the most appropriate sites for evaluating inflammatory bone loss in a valid and reproducible manner. These optimizations are expected to facilitate the use of the mouse ligature-induced periodontitis model and consequently contribute to better understanding of the immunopathological mechanisms of periodontitis.
Keywords: Mouse model, oral infection, inflammation, periodontitis, bone loss, ligature
1. Introduction
Periodontitis is initiated by dysbiotic bacterial communities forming on subgingival tooth surfaces (Darveau, 2010; Hajishengallis and Lamont, 2012). This is a prevalent disease that affects >47% of US adults (Eke et al., 2012) and is characterized by inflammatory destruction of alveolar bone and connective tissues that surround and support the teeth (Pihlstrom et al., 2005). In its most severe form, which affects 8.5% of US adults (Eke et al., 2012), periodontitis can have an impact on systemic health, as it increases the risk for atherosclerosis, diabetes, and adverse pregnancy outcomes (Pihlstrom et al., 2005; Genco and Van Dyke, 2010; Jeffcoat et al., 2011; Lalla and Papapanou, 2011). Moreover, periodontitis is an attractive study model, as it is readily accessible for obtaining both microbial and host tissue samples to longitudinally investigate local inflammatory mechanisms and determine their effects on systemic diseases (Darveau, 2010).
Mouse models of periodontitis have been productively used to investigate periodontal disease mechanisms and to test the potential of novel therapeutic compounds (Gyurko et al., 2006; Li and Amar, 2007; Graves et al., 2008; Oz and Puleo, 2011; Abe et al., 2012). An important advantage of the so-called ligature-induced periodontitis model is that disease can be initiated at a known time with a predictable sequence of events culminating in alveolar bone loss within a few days (mice and rats) (Bezerra et al., 2000; Li and Amar, 2007; Graves et al., 2008; Abe et al., 2012) or several weeks (dogs and non-human primates) (Assuma et al., 1998; Martuscelli et al., 2000). Removal of the ligatures allows investigation of the resolution of inflammation and the healing response. The technical procedure involves placement of ligatures (usually made of silk or cotton) around posterior teeth (Assuma et al., 1998; Oz and Puleo, 2011). The ligatures are thought to facilitate local accumulation of bacteria and thereby enhance bacteria-mediated inflammation and bone loss (Graves et al., 2008).
Careful review of the literature indicates that the ligature-induced periodontitis model has been used quite infrequently in mice as compared to larger animals, such as rats, dogs, or non-human primates, despite the fact that mice represent the most convenient, inexpensive, and versatile model (Graves et al., 2008). In this regard, advantages of the mice as a model include the considerable background information on their immune system, a wide range of genetically engineered strains (e.g., gene knockouts for key immune receptors or signaling molecules) and availability of high-quality immunochemical and cellular reagents. It is possible that the relative paucity of the use of mice specifically in ligature-induced periodontitis (mice are widely used in oral infection models of periodontitis) arises from technical challenges having to do with the relatively small size of the mouse oral cavity. To facilitate the use of the mouse ligature-induced periodontitis model, we now provide an image-based description of the methodology, which has not been made publicly available before. We have moreover optimized the model by pinpointing the most appropriate sites for evaluation of bone loss in a valid and reproducible manner.
2. Materials and methods
2.1. Animals
Specific-pathogen-free female C57BL/6 mice were purchased from the Jackson Laboratory. All mouse experimental procedures described in this study have been reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee, in compliance with established federal and state policies. Mice were maintained in individually ventilated cages, and provided sterile food and water under specific-pathogen-free conditions. Mice were used for experiments at the age of 9–10 weeks and were divided into five groups of four mice each. One group was left untreated and four groups had their left maxillary second molar tooth ligated and then sacrificed at four different timepoints post-ligation (days 1, 3, 5 and 8).
2.2. Placement of ligatures
To induce bone loss, a 5–0 silk ligature (Roboz Surgical Instrument Co., MD, USA) was tied around the maxillary left second molar. Suture was applied and tied gently to prevent damage to the periodontal tissue. The contralateral molar tooth in each mouse was left unligated to serve as baseline control for bone height measurements. The ligatures remained in place in all mice throughout the experimental period. The tools required for the procedure are shown in Table 1 and the technical steps involved are depicted in Figure 1.
Table 1.
Tools required for ligature-induced periodontitis in mice
| Vendor (catalog number) | Tool name | Purpose |
|---|---|---|
| Roboz Surgical Instrument (SUT-15-1) | 5–0 silk suture | To be tied around second molar, to facilitate bacterial accumulation and inflammation |
| Fine Science Tools (11251–33) | Dumont Forceps | Passing suture through interdentium |
| Fine Science Tools (11251–35) | Dumont Forceps | Passing suture through interdentium |
| Fine Science Tools (00272–13) | Suture-tying forceps | Tying suture |
| Fine Science Tools (18025–10) | Suture-tying forceps | Tying suture |
| Fine Science Tools (15003–08) | Spring Scissors | Cutting suture |
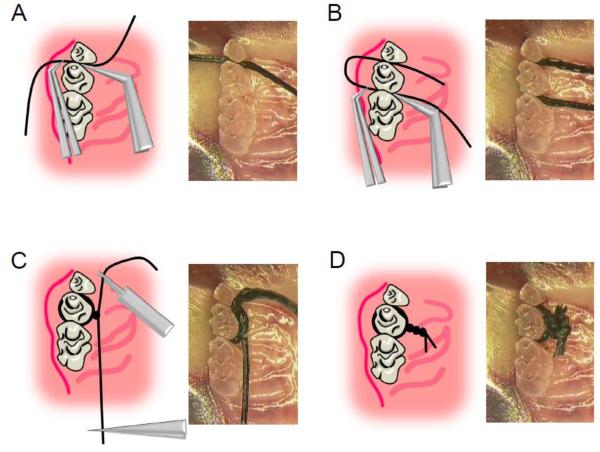
Figure 1. Technical procedures of ligation.
(A) 5–0 silk suture was passed through interdentium between second molar and third molar using Dumont forceps. (B) Suture was passed through interdentium between first molar and second molar using Dumont forceps. (C) Suture was looped around the second molar (taking care to remove the slack) using suture-tying forceps. (D) Suture was tied firmly using a triple-knot and excess suture was cut using spring scissors.
2.3. Determination of bacterial accumulation
The ligatures were recovered from euthanized mice and gently washed with PBS to remove food residue and other debris. Subsequently, the sutures were placed in Eppendorf tubes with 1 ml PBS and the bacteria were extracted by vortexing for 2 minutes at 3000 rpm. Serial dilutions of the bacterial suspensions were plated onto blood agar plates and CFU were enumerated following anaerobic growth at 37°C for 7 days. Results were normalized by dividing CFU by the length (mm) of the corresponding suture.
2.4. Bone loss determination
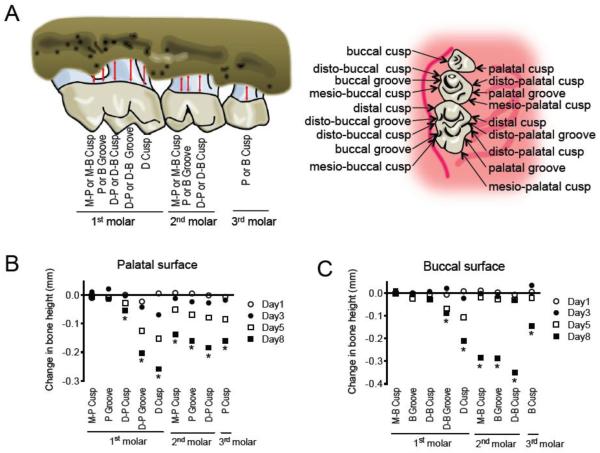
Freshly harvested skulls were boiled in water at 15 psi for 10 min. Following defleshing, the skulls were subjected to brushing and bleaching. The maxillae were stained with 0.5% eosin and 1% methylene blue (both from Ricca Chemical Company, TX, USA). Periodontal bone heights were assessed under a Nikon SMZ800 microscope (Nikon Instruments Inc., NY, USA) using a 40 × objective. Images of the maxillae were captured using a Nicon Digital Sight DS-U3 camera controller (Nikon Instruments Inc.) and bone heights were measured using NIS-Elements software (Nikon Instruments Inc.). Measurements were performed on the first molar (at five sites corresponding to mesio-palatal or mesio-buccal cusp, palatal or buccal groove, disto-palatal or disto-buccal cusp, disto-palatal or disto-buccal groove, and distal cusp), second molar (three sites corresponding to mesio-palatal or mesio-buccal cusp, palatal or buccal groove, and disto-palatal or disto-buccal cusp) and palatal or buccal cusp of the third molar, on the palatal and buccal surfaces of the maxillae (9 sites total for each of the palatal or buccal side; see Fig. 3A).
Figure 3. Identification of sites susceptible to bone loss.
(A) Schematics of sites subjected to measurements of CEJ-ABC distance. Key: M-P, mesio-palatal; M-B, mesio-buccal; P, palatal; B, buccal; D-P, disto-palatal; D-B, disto-buccal. (B) CEJ-ABC distance measurements at the palatal side, at the indicated days following placement of ligatures. (C) CEJ-ABC distance measurements at the buccal side, at the indicated days following placement of ligatures. *, P < 0.05 between day 8 and day 0.
Periodontal bone heights were measured as the distances from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC). After the identification of six sites that were susceptible to bone height changes following placement of ligature, the total bone loss in the ligated side relative to the contralateral unligated side was determined as follows: The 6-site total CEJ-ABC distance for the ligated side of each mouse was calculated and subtracted from the 6-site total CEJ-ABC distance of the contralateral unligated side of the same mouse. The results were presented in mm and negative values indicated bone loss relative to unligated controls.
2.5. Statistical analysis
For statistical analysis, data were evaluated by analysis of variance and the Dunnett multiple-comparison test with the InStat program (GraphPad Software, CA, USA). Where appropriate (comparison of two groups only), two-tailed t-tests were performed. P values < 0.05 were considered statistically significant.
3. Results
3.1. Evaluation of bacterial accumulation
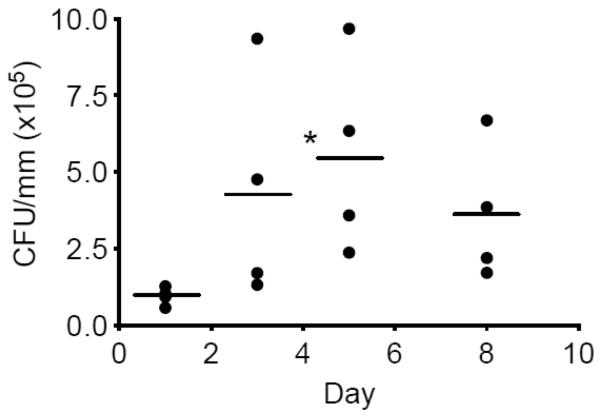
Dysbiotic microbial communities consisting predominantly of anaerobic bacteria are heavily involved in the initiation and progression of periodontitis (Marsh, 2003; Darveau, 2010; Hajishengallis et al., 2012). In fact, the transition from health to periodontitis in humans correlates with an increased microbial burden (Griffen et al., 2012; Abusleme et al., 2013). Although mice have many advantages as a model (see Introduction), it is technically very challenging to sample their periodontal microbiota for accurate quantitative determinations. However, in the ligature-induced periodontitis model, the cause of disease is intimately associated with the accumulation of the bacteria in specific regions, that is, in and around the ligature, which can be readily removed and analyzed. Therefore, to determine the local longitudinal accumulation of anaerobic bacteria in the ligature model, bacteria were extracted from sutures recovered 1, 3, 5, and 8 days post-ligation and subjected to anaerobic growth for CFU enumeration. The analysis showed a time-dependent accumulation of anaerobic bacteria which appeared to peak on day 5 (P < 0.05 compared with day 1). The importance of the bacteria in causing bone loss (technical details below) was confirmed by antibiotic treatment, which significantly inhibited bone loss (Supplementary fig. 1).
3.2. Morphometric analysis of alveolar bone loss
To assess bone loss, the CEJ-ABC distances were measured at 9 predetermined sites in each of the buccal or palatal surfaces (Fig. 3A) of the ligated side and of the corresponding contralateral unligated side (control). Changes in bone heights over time were calculated for each ligated site by subtracting its CEJ-ABC distance value from that of the corresponding unligated site. Negligible or very modest changes in bone heights were observed at three sites in each of the palatalal or buccal side of the first molar, namely, mesio-palatal cusp, palatal groove, disto-palatal cusp (Fig. 3B), mesio-buccal cusp, buccal groove, and disto-buccal cusp (Fig. 3C). In contrast, 6 sites ranging from the disto-palatal or disto-buccal groove of the first molar to the third molar palatal or buccal cusp displayed significantly increased bone heights during the course of the experiment in both palatal and buccal surfaces (Fig. 3, B and C, respectively). The affected sites corresponded to mesio-palatal or mesio-buccal cusp, palatal or buccal groove, and disto-palatal or disto-buccal cusp of the ligated second molar and immediately adjacent regions, that is, the sites corresponding to disto-palatal or disto-buccal groove and distal cusp of the first molar, and palatal or buccal cusp of the third molar. It is noted that the CEJ-ABC distance values of control sites (i.e., contralateral to the ligated side) were similar to the corresponding sites in mice that received no ligatures at all (data not shown), suggesting that the contralateral control sites are not significantly affected by the local accumulation of bacteria in the ligated sites.
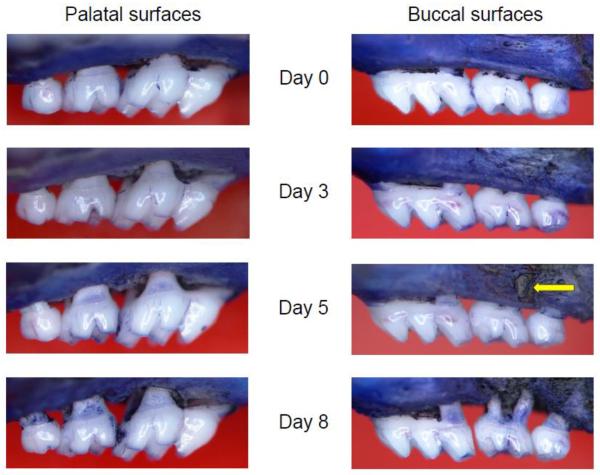
These six affected sites (i.e., which exhibited significant changes in bone heights; Fig. 3, B and C) were used to calculate the 6-site total bone loss. In the palatal side, the 6-site total bone loss displayed a relatively smooth progression over time (Fig. 4A). In contrast, the buccal side showed abrupt acceleration of bone loss from day 5 to day 8, despite little or no bone loss at earlier timepoints (Fig. 4B). To understand these differences, we carefully examined morphological changes in buccal and palatal bone throughout the experimental duration. At day 5 post-ligation, we observed abnormal fenestration on the buccal side that subsequently led to the collapse of overlying alveolar bone (Fig. 5), thereby giving the impression that bone loss was accelerated from day 5 to day 8 post-ligation.
Figure 4. Kinetics of total bone loss in palatal vs. buccal side.
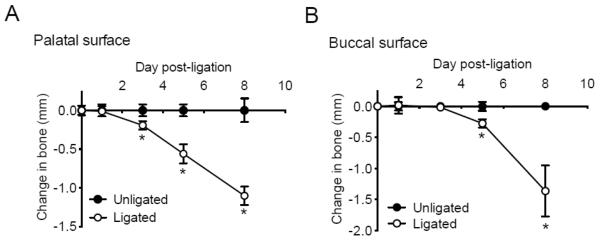
Groups of mice were euthanized 1, 3, 5, and 8 days after the placement of ligatures on the left maxillary molars. The 6-site total CEJ-ABC distance for the ligated side of each mouse was subtracted from the 6-site total CEJ-ABC distance of the contralateral unligated side of the same mouse. Panels A and B show measurements on the palatal and buccal surfaces, respectively. Data are means ± S.D. (n = 4) and negative values indicated bone loss relative to unligated baseline control. *, P < 0.05 compared to unligated baseline control.
Figure 5. Representative images of maxillae exhibiting time-dependent bone loss.
The palatal side is contrasted with the buccal side in terms of time-dependent bone loss in ligature-induced periodontitis. Note the fenestration (arrow) forming on the buccal side of the second molar at day 5.
4. Discussion
Our analysis has identified appropriate sites for morphometric evaluation of bone loss in ligature-induced periodontitis in mice. Although the buccal side has been used for measurements of ligature-induced bone loss, our analysis has shown that the buccal side may not be as reliable as the palatal side. At the buccal side, the thickness of the bone in the apical region is thinner than in the marginal region, and the apical region may be destroyed (see fenestration in Fig. 5) earlier than the marginal region. Consequently, the bone overlying a fenestration may abruptly collapse giving the false impression that intensive and uniform resorption of bone has occurred. In contrast, at the palatal side, the bone at the apical region is thicker than in the marginal region and bone loss proceeds quite smoothly. In addition to the presented results in this study (Fig. 4B), we have consistently noticed a relatively high standard deviation in buccal bone loss at day 8 (unpublished data from groups of 5–6 mice); this is probably because the collapse of the bone overlying fenestrations does not happen simultaneously in all mice within the group. On the other hand, the standard deviation of the palatal bone loss is consistently smaller (Fig. 4A) (Abe et al., 2012; Eskan et al., 2012).
We further identified six sites that are quite sensitive to bacterially-induced inflammatory bone loss following ligation of the second maxillary molar (Fig. 3 B and C). At the palatal side, the identified sites (disto-palatal groove and distal cusp of the first molar; mesio-palatal cusp, palatal groove, and disto-palatal cusp of the ligated second molar; and palatal cusp of the third molar) can reliably be monitored to assess the effects of experimental therapeutic treatments. Indeed, using the 6-site total bone loss, as determined and justified in this study, we have recently determined the protective effects of complement inhibitors (Abe et al., 2012) and other anti-inflammatory compounds (Eskan et al., 2012). In other models of mouse periodontitis, such as the oral gavage model (e.g., oral inoculation with a human periodontal bacterium) (Hart et al., 2004), it is often challenging to reliably assess the host inflammatory response using dissected gingival tissue. This is because the dissected tissue from the inoculated mice may contain both inflamed and healthy tissue, which would thus tend to blunt differences with sham-infected controls. On the other hand, it is easy to locate and dissect the inflamed gingival tissue in ligature-induced periodontitis (the tissue around the ligated second molar) in order to assess the inflammatory response (Abe et al., 2012; Eskan et al., 2012) (see also Supplementary file for relevant methodology). Moreover, intravital microscopy of gingival vessels can be used to assess leukocyte adhesion, rolling, and transmigration to the inflamed periodontium (Gyurko et al., 2006).
The placement of ligatures does not involve mechanical trauma in large animals such as non-human primates (Brecx et al., 1985) and the ligatures do not cause inflammation or bone loss by themselves in germ-free rats (Rovin et al., 1966; Graves et al., 2008). However, in small animals such as mice, we cannot exclude the possibility for mechanical trauma by the ligatures, which could thus contribute to bone loss. Regardless of this possibility, the bacteria do constitute a major factor in the induction of bone loss in this model, since treatment of mice with antibiotics significantly inhibited bone loss (Supplementary fig. 1).
Our quantitative analysis of the ligature-associated microbiota has shown a non-significant drop in bacterial counts after the peak at day 5 (Fig. 2). Obviously, a greater number of observations is required to determine whether this is a real trend, which in such case might reflect a successful host response to control the infection. On the other hand, it can be concluded that five days are adequate to assess inflammation and bone loss and, quite likely, represent a period of disease progression. This short period greatly facilitates the investigation of novel anti-inflammatory compounds, which– in the oral gavage model– need to be administered over several weeks; this is not always feasible if the experimental compound is available in limited quantities or involves prohibitive costs. Despite its multifactorial etiology, human periodontitis fundamentally represents disruption of periodontal tissue homeostasis (Darveau, 2010). In this regard, the placement of ligatures in effect disrupts periodontal homeostasis (by facilitating heavy local accumulation of bacteria). On the other hand, the removal of the ligatures (e.g., at day 5) can cause a transition into the resolution phase, which could be studied in an appropriate context (e.g., the ligatures could be removed from previously ligated normal or diabetic mice, to investigate the impact of diabetes on the resolution of periodontal infection and inflammation).
Figure 2. Quantification of anaerobic bacteria.
Bacteria were extracted from recovered sutures (at the indicated days following placement of ligatures) and serial dilutions of bacterial suspensions were plated onto blood agar plates for anaerobic growth and CFU enumeration. Each symbol represents an individual mouse; small horizontal lines indicate the mean. *, P < 0.05, as compared with day 1.
In conclusion, we have identified a number of factors that may help standardize and optimize the use of the murine ligature-periodontitis model, which we have described in image-based technical detail. This work may facilitate further use of this useful model to investigate host-microbe interactions and inflammation in periodontitis.
Supplementary Material
Highlights
Purpose: To standardize the ligature-induced periodontitis model in mice
An image-based description of the methodology was provided
The most appropriate sites for valid evaluation of bone loss were identified
Acknowledgments
This research was supported by grants from the NIH/NIDCR (DE017138 and DE021685 to GH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
T.A. performed research and data analysis and wrote the paper; G.H. conceived of, designed and supervised the research and edited the paper.
References
- Abe T, Hosur KB, Hajishengallis E, Reis ES, Ricklin D, Lambris JD, Hajishengallis G. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J Epub ahead of print. 2013 doi: 10.1038/ismej.2012.174. doi:10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403. [PubMed] [Google Scholar]
- Bezerra MM, de Lima V, Alencar VB, Vieira IB, Brito GA, Ribeiro RA, Rocha FA. Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. Journal of periodontology. 2000;71:1009. doi: 10.1902/jop.2000.71.6.1009. [DOI] [PubMed] [Google Scholar]
- Brecx MC, Nalbandian J, Ooya K, Kornman KS, Robertson PB. Morphological studies on periodontal disease in the cynomolgus monkey. II. Light microscopic observations on ligature-induced periodontitis. J Periodontal Res. 1985;20:165. doi: 10.1111/j.1600-0765.1985.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol. 2010;7:479. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, Van Dyke TE. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol. 2006;177:7250. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: The Polymicrobial Synergy and Dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GT, Shaffer DJ, Akilesh S, Brown AC, Moran L, Roopenian DC, Baker PJ. Quantitative gene expression profiling implicates genes for susceptibility and resistance to alveolar bone loss. Infect Immun. 2004;72:4471. doi: 10.1128/IAI.72.8.4471-4479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoat M, Parry S, Sammel M, Clothier B, Catlin A, Macones G. Periodontal infection and preterm birth: successful periodontal therapy reduces the risk of preterm birth. BJOG. 2011;118:250. doi: 10.1111/j.1471-0528.2010.02713.x. [DOI] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- Li CH, Amar S. Morphometric, histomorphometric, and microcomputed tomographic analysis of periodontal inflammatory lesions in a murine model. J Periodontol. 2007;78:1120. doi: 10.1902/jop.2007.060320. [DOI] [PubMed] [Google Scholar]
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- Martuscelli G, Fiorellini JP, Crohin CC, Howell TH. The effect of interleukin-11 on the progression of ligature-induced periodontal disease in the beagle dog. J Periodontol. 2000;71:573. doi: 10.1902/jop.2000.71.4.573. [DOI] [PubMed] [Google Scholar]
- Oz HS, Puleo DA. Animal models for periodontal disease. J Biomed Biotech. 2011;2011:754857. doi: 10.1155/2011/754857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Rovin S, Costich ER, Gordon HA. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J Periodontal Res. 1966;1:193. doi: 10.1111/j.1600-0765.1966.tb01860.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.