Abstract
Pancreatic ductal adenocarcinoma (PDAC), one of the deadliest malignancies, is resistant to current chemotherapies. We previously showed that triptolide inhibits PDAC cell growth in vitro and blocks metastatic spread in vivo. Triptolide downregulates heat shock protein 70 (HSP70), a molecular chaperone upregulated in several tumor types. This study investigates the mechanism by which triptolide inhibits HSP70. As microRNAs (miRNAs) are becoming increasingly recognized as negative regulators of gene expression, we tested whether triptolide regulates HSP70 via miRNAs. Here we show that triptolide, as well as quercetin but not gemcitabine, upregulated miR-142-3p in PDAC cells (MIA PaCa-2, Capan-1, and S2-013). Ectopic expression of miR-142-3p inhibited cell proliferation, measured by Electric Cell-substrate Impedance Sensing, and decreased HSP70 expression, measured by real-time PCR and immunoblotting, compared with controls. We demonstrated that miR-142-3p directly binds to the 3’UTR of HSP70, and that this interaction is important as HSP70 overexpression rescued miR-142-3p-induced cell death. We found that miR-142-3p regulates HSP70 independently of heat shock factor 1. Furthermore, Minnelide, a water soluble prodrug of triptolide, induced the expression of miR-142-3p in vivo. This is the first description of an miRNA-mediated mechanism of HSP70 regulation in cancer, making miR-142-3p an attractive target for PDAC therapeutic intervention.
Keywords: pancreatic cancer, triptolide, miR-142-3p, gene expression profiling, HSP70
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the United States (1). The 5-year survival rate for patients with localized disease after surgical resection is 20% and for those with metastatic disease, the survival rate is 5–6% (1). Over the past several decades these survival rates have not improved due in large part to aggressive growth, metastasis, and drug resistance of PDAC cells (1). Efforts are ongoing to understand the pathobiology of PDAC and to develop innovative and effective therapies. An important part of this process is to understand the mechanism of action of potential chemotherapeutic agents.
Triptolide, a diterpene triepoxide extract from the Chinese herb Tripterygium wilfordii, has been shown to inhibit PDAC cell viability in vitro (2, 3) and to block growth and metastatic spread in vivo (4). To date, in vivo studies have shown that triptolide inhibits the growth of cholangiocarcinoma cells in hamsters (5) and xenografts of human melanoma, breast cancer, bladder cancer, and gastric carcinoma in nude mice (6). Previous data from our laboratory have shown that triptolide inhibits the growth of neuroblastoma cells in vitro (7) and prevents tumor growth in vivo (8).
Because triptolide was identified in a small molecule screen to inhibit tumorigenic molecular chaperone heat shock protein gene transcription (9), our laboratory has continued to demonstrate that triptolide likewise inhibits cancer cell proliferation while concurrently inhibiting heat shock protein 70 (HSP70) expression in PDAC cells (2–4, 10) or neuroblastoma cells (11). Some studies suggest that triptolide inhibits the transcription factor heat shock factor 1 (HSF1) and in this way suppresses HSP70 transcription (9), but as microRNAs (miRNAs) become increasingly recognized as major negative regulators of gene expression, we asked whether triptolide may regulate HSP70 expression via miRNAs.
In cancer cells, the dysregulation of miRNAs expression serves as an efficient means to rewire the cell gene expression map and generate a cancer phenotype. In particular, the loss of tumor-suppressive miRNAs upregulates oncogenic targets (12). No previous reports have examined the effect of triptolide on the PDAC miRNAome nor evaluated miRNA-mediated regulation of HSP70 in PDAC cells. Consequently, the aims of this study are as follows: (a) examine the effect of triptolide on the miRNAome of PDAC cells in vitro and verify this in vivo, (b) evaluate whether miRNAs upregulated by triptolide play a tumor suppressive role in PDAC, and (c) validate that predicted miRNAs regulate HSP70 independent of the HSF1 pathway. We hypothesize that triptolide will upregulate tumor-suppressive miRNAs which directly regulate HSP70. This is the first evaluation of an miRNA-mediated mechanism of HSP70 regulation in cancer.
Materials and Methods
Cell culture and drug treatment
Cells from the MIA PaCa-2, Capan-1, and HEK-293 lines were obtained from ATCC (Manassus, VA) and cultured in DMEM (Life Technologies, Carlsbad, CA) containing 10% FBS (Life Technologies, Carlsbad, CA). S2-013 cells were kindly provided by Dr. Buchsbaum (University of Alabama at Birmingham) and cultured in RPMI medium (Life Technologies, Carlsbad, CA) containing 10% FBS. Multiple aliquots of cells were cryopreserved when initially grown. All the cell lines were used within 6 months of resuscitation. No authentication was done by the authors, but ATCC authenticates using Short Tandem Repeat Profiling. For all drug treatments, cells were seeded in 6-well plates (2.5 × 105 cells/well) and incubated for the periods indicated in the figure legends prior to RNA or protein analysis. Triptolide treatment (Calbiochem, San Diego, CA) was performed as previously described (2, 3). Quercetin (Sigma-Aldrich, St. Louis, MO) or gemcitabine (Eli Lilly Corporation, Indianapolis, IN) treatments were performed as previously reported (13, 14). Cells were maintained as previously described (2, 3).
MicroRNA expression profiling
MicroRNA was isolated using the mirVana RNA isolation kit (Ambion, Carlsbad, CA), and quantified using a Nanodrop spectrophotometer (Thermo Fisher, Rockford, IL). RNA quality (RNA index number of ≥5) was verified by an Agilent 6000 nanochip (Agilent, Santa Clara, CA), prior to miRNA analysis using miRNA BeadArrays (Illumina, San Diego, CA). Arrays were imaged using an Illumina BeadArray Reader, and the fluorescent intensity of miRNA probes were analyzed using BeadStudio version 3.3.3 (Illumina San Diego, CA). Quality control and statistical analyses of miRNA profiling were carried out as previously described (15). All gene array data were deposited into GEO (GSE46188).
Quantitative real-time PCR
Total RNA was reverse transcribed using the miScript II RT Kit (Qiagen, Valencia, CA). Real-time PCR was done using the QuantiTect or miScript SYBR green PCR kit (Qiagen, Valencia, CA) on an Applied Biosystems 7300 real-time PCR system. 18S was used as a control for HSP70, HSP27 or HSF1, and U6 was used as a control for miR-142-3p (miScript Primer Assay; Qiagen). To verify HSPA1B (HSP70) overexpression, HSP70 primers targeting the HSP70 ORF region (2) were used. Quantification was done using the ΔΔCt method.
Transfection of miR-142-3p mimic or inhibitor
Cells were seeded in 6-well (8 × 104 cells/well) or 24-well plates (1.5 × 104 cells/well) and incubated overnight prior to transfection. Transfection mastermixes were diluted in Opti-MEM (Life Technologies, Carlsbad, CA) containing HiPerFect (Qiagen, Valencia, CA), miR-142-3p mimic, miR-142-3p inhibitor or negative control (NC) miRIDIAN reagents (Thermo Scientific Dharmacon, Rockford, IL). Transfected cells were analyzed for Western blotting or cell viability as previously reported (2).
Cell proliferation using Electric Cell-substrate Impedance Sensing (ECIS)
Using the ECIS method, cells are grown on the surface of planar gold-film electrodes and the AC impedance of the cell-covered electrode is measured continuously at a frequency of 4000 Hz. Due to the insulating properties of cell membranes, the impedance increases with increasing coverage of the electrode. MIA PaCa-2 or Capan-1 cells (6 × 104 cells/well) and S2-013 (1 × 105 cells/well) were plated in 8-well, gold-film electrode coated 10E+ arrays (Applied Biophysics, Troy, NY). Proliferation rates were normalized to 6 h following transfection.
Dual-Luciferase reporter assay and 3’UTR binding site mutagenesis
HEK-293 cells were seeded in seeded in 24-well plates (6 × 104 cells/well). Mastermixes diluted in serum-free media (Life Technologies, Carlsbad, CA) containing Attractene (Qiagen, Valencia, CA), pGL4.73 control vector expressing firefly luciferase (Promega, Madison, WI), GoClone (HSPA1B) containing the wild-type HSPA1B 3’UTR expressing renilla luciferase (SwitchGear Genomics, Menlo Park, CA), miR-142-3p mimic or NC (Thermo Scientific, Rockford, IL). Mutagenesis was done using the QuickChange Site-Directed Mutagenesis Kit (Agilent Stratagene, Santa Clara, CA). The Dual-Luciferase Reporter Assay System (Promega, Madison, WI) was used on a Synergy2 luminometer (BioTek, Winooski, VT).
Transfection of HSPA1B (HSP70) or HSF1 ORF vector
Cells were seeded in 24-well plates (1.5 × 104 cells/well) and were incubated overnight prior to transfection. Transfection mastermixes diluted in Opti-MEM (Life Technologies, Carlsbad, CA) containing Attractene (Qiagen, Valencia, CA), HSP70 (HSPA1B isoform) ORF, HSF1 ORF, or negative control (GeneCopoeia, Rockville, MD), along with miRIDIAN reagents (Thermo Scientific Dharmacon, Rockford, IL). 400 ng of either plasmid was added to each well along with transfection of miRIDIAN reagents.
Transfection of HSF1 siRNA
Cells were seeded in seeded in 6-well plates (8.0 × 104 cells/well) and were incubated overnight prior to transfection. Transfection mastermixes diluted in Opti-MEM (Life Technologies, Carlsbad, CA) containing HiPerFect (Qiagen, Valencia, CA), HSF siRNA or non-silencing siRNA (Qiagen, Valencia, CA) at a final concentration of 25 nM.
Measurement of miR-142-3p and HSPA1B (HSP70) levels from human xenograft PDAC tumor model
Three de-identified human pancreatic tumors were implanted subcutaneously into female severe combined immunodeficient (SCID) mice (The Jackson Laboratory, Bar Harbor, ME). When tumor volumes reached 500 mm3, tumors were dissected, cut into 10 mm3 pieces, and propagated in additional SCID mice (one animal per tumor, n=3 animals). Strict animal care procedures from the University of Minnesota Institutional Animal Care and Use Committee were followed. Animals were randomized and tagged before daily intraperitoneal (i.p) injections of Minnelide (0.42 mg/kg) or saline for 7 days. Mice were sacrificed, and tumors were stored in RNAlater (Qiagen, Valencia, CA). Samples were homogenized in one ml of Trizol (Life Technologies, Carlsbad, CA). Gene levels were analyzed by real-time PCR.
Statistical Analysis
Values are expressed as the mean ± SEM. All experiments with cells were repeated at least thrice. The significance of the difference between the control and each experimental test condition was analyzed by unpaired Student’s t-test and a value of p<0.05 was considered statistically significant.
Results
Triptolide alters the microRNAome of PDAC cells, in particular inducing miR-142-3p expression
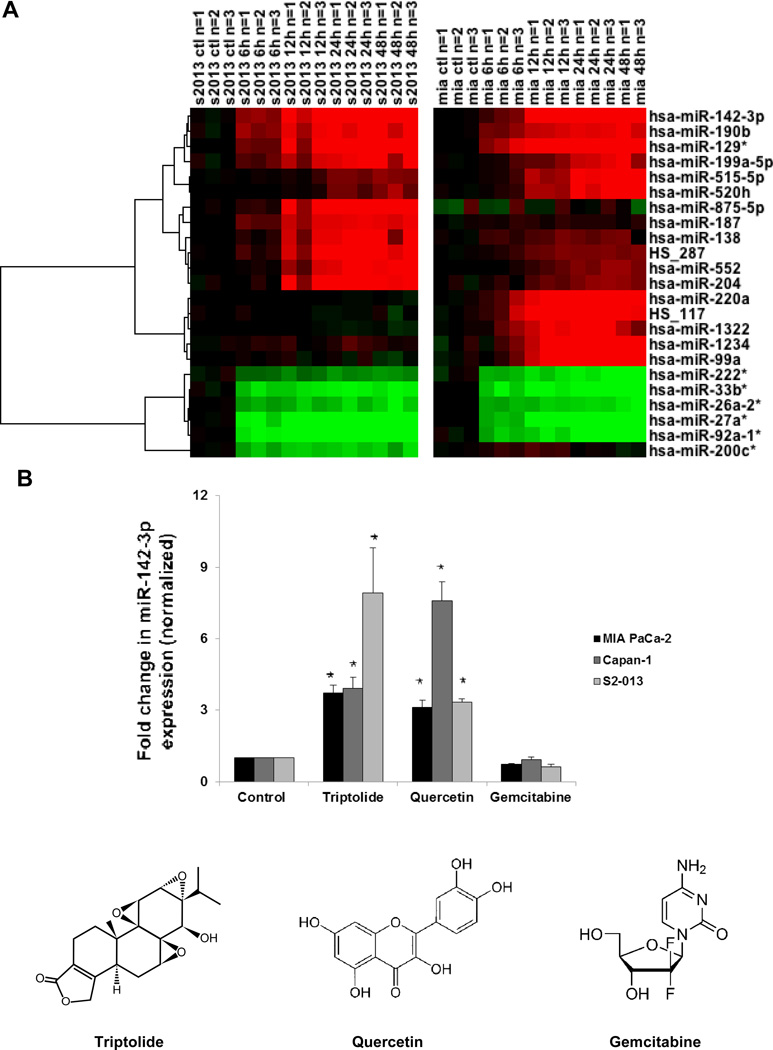
Previous data from our laboratory have shown that triptolide induces PDAC cell death in vitro and in vivo, while concurrently downregulating HSP70 expression (2–4, 10). Triptolide was identified as an HSP70 inhibitor by virtue of its ability to inhibit its main transcription factor, HSF1 (9). Given that miRNAs are becoming increasingly recognized as major negative regulators of gene expression (12), triptolide-induced upregulation of HSP70-targeting miRNAs was studied. We treated MIA PaCa-2 and S2-013 cells with triptolide and screened the collected miRNAs by microarray. These cell lines were chosen because triptolide induces different mechanisms of cell death in these primary and secondary (metastatic) tumor types, respectively (3). A 100 nM concentration of triptolide was chosen, for all studies in this manuscript, because this concentration has been shown to inhibit cell viability of both MIA PaCa-2 and S2-013 by 40% or 70% after 24 h or 48 h of treatment, respectively (3). Time points as early as 6 h following triptolide treatment were chosen so as to observe early microRNAome changes linked to proliferative pathways. Later time points were evaluated to verify that changes were sustained. In MIA PaCa-2 cells, 15 miRNAs significantly changed (10 upregulated, 5 downregulated) (p<0.05; Fig. 1A). In S2-013 cells, 14 miRNAs significantly changed (10 upregulated, 4 downregulated) (p<0.05; Fig. 1A). Principal component analysis of the resulting dataset shows that miRNA transcript levels changed linearly in both cell lines (Supplemental Fig. S1). Over the time-course of triptolide treatment, upregulation of miR-142-3p was one of the most significant changes in both cell lines (8–9 fold at 24 hours, Supplemental Table S1). This induction of miR-142-3p was validated in a third cell line, Capan-1 (Fig. 1B). Furthermore, independent validation of miRNA microarray was done by real-time PCR (Supplemental Fig. S2). In addition, the levels of those miRNAs known to play a role in PDAC, such as miR-155, miR-21 and miR-221 (16–18), were not observed to change in response to triptolide (Supplemental Fig. S3 and Supplemental Table S2). These results show that triptolide alters the microRNAome of PDAC cells, notably by increasing miR-142-3p.
Figure 1. Triptolide-regulated microRNAs in PDAC cells.
(A) Heatmap representation of differentially expressed human miRNAs after triptolide (100 nM) treatment (0, 6, 12, 24 or 48 h) in MIA PaCa-2 and S2-013 cells as measured by microarray. Only miRNAs with strong differential expression (t-test between 0 and 24 hour replicate time points, p<0.05, average fold change>5) in at least one of the two time-courses are shown. Log-transformed expression values are shown, compared with the untreated control, for each cell line independently. (B) Triptolide (100 nM) and quercetin (100 μM) treatments induced, whereas gemcitabine (1 μM) treatment had no effect on, miR-142-3p expression (as assessed by real-time PCR) in human MIA PaCa-2, Capan-1, and S2-013 cell lines following 24 h of treatment. Expression of miR-142-3p was normalized against U6. The bars represent mean ± SEM, n=3, *p<0.02 (t test).
Quercetin, but not gemcitabine, also induces miR-142-3p expression
To evaluate whether the induction of miR-142-3p was unique to HSP70 inhibiting compounds such as triptolide, we tested whether other chemotherapeutic agents upregulated miR-142-3p. As our laboratory has previously shown that quercetin (100 μM) inhibited HSP70 protein levels and decreased MIA PaCa-2 cell viability by 50% after 24 h (13), we tested whether quercetin likewise induced miR-142-3p. To address this, we treated PDAC cells with quercetin and assayed for miR-142-3p expression via real-time PCR. Expression of miR-142-3p was upregulated 3-fold by quercetin in MIA PaCa-2 and S2-013 cells and upregulated 8-fold in Capan-1 cells (Fig. 1B). To test whether the induction of miR-142-3p may be common among other chemotherapeutic agents, we measured miR-142-3p levels following gemcitabine treatment. We chose 1 μM gemcitabine treatment because it has been shown to inhibit MIA PaCa-2 (14, 19) viability by at least 50% after 72 h. This concentration allows us to evaluate early changes in the microRNAome directly linked proliferative pathways. Gemcitabine treatment did not alter miR-142-3p levels (Fig. 1B). These results show that miR-142-3p induction is common among HSP70 inhibitors triptolide and quercetin but is not present with the nucleoside analog gemcitabine.
Triptolide and ectopic expression of miR-142-3p inhibit PDAC cell proliferation
Because triptolide induced the expression of miR-142-3p, (Fig. 1) and inhibited cell viability in MIA PaCa-2, Capan-1, and S2-013 cells (3), we verified whether triptolide inhibits proliferation in these cell lines using Electric Cell-substrate Impedance Sensing (ECIS) assay, an established method to test cancer cell proliferation in real-time (20). Due to the insulating properties of cell membranes, the measured impedance increases with accumulating coverage of the electrode. We found that triptolide treatment inhibited proliferation of MIA PaCa-2, Capan-1, and S2-013 as early as 15 hours following treatment (Fig. 2A). To test whether ectopic expression of miR-142-3p was playing a tumor suppressive role in PDAC cells, we measured PDAC cell proliferation rates following miR-142-3p overexpression. Likewise, we found that overexpression of miR-142-3p inhibited proliferation of MIA PaCa-2, Capan-1, and S2-013 as early as 15 h following transfection (Fig. 2B). These data show that miR-142-3p and triptolide both suppress PDAC cell proliferation.
Figure 2. Effect of triptolide treatment or microRNA-142-3p mimic overexpression PDAC cell proliferation.
(A) Triptolide (100 nM) reduced the rate of cell proliferation as measured in real-time on the Electric Cell-substrate Impedance Sensing (ECIS) instrument in MIA PaCa-2, Capan-1, and S2-013 cell lines. (B) MicroRNA-142-3p mimic (5 nM) reduced the rate of proliferation as measured in all cell lines tested by ECIS. Proliferation rates are representative of n≥4 experiments and average of 4 replicates. Because proliferation rates are normalized to 6 h following transfection, the 0 h time point represents 6 h following transfection. SEM bars (not shown) overlap from 0–15 h, * represents point after which proliferation rates differ.
Upregulation of miR-142-3p inhibits HSPA1B (HSP70) expression
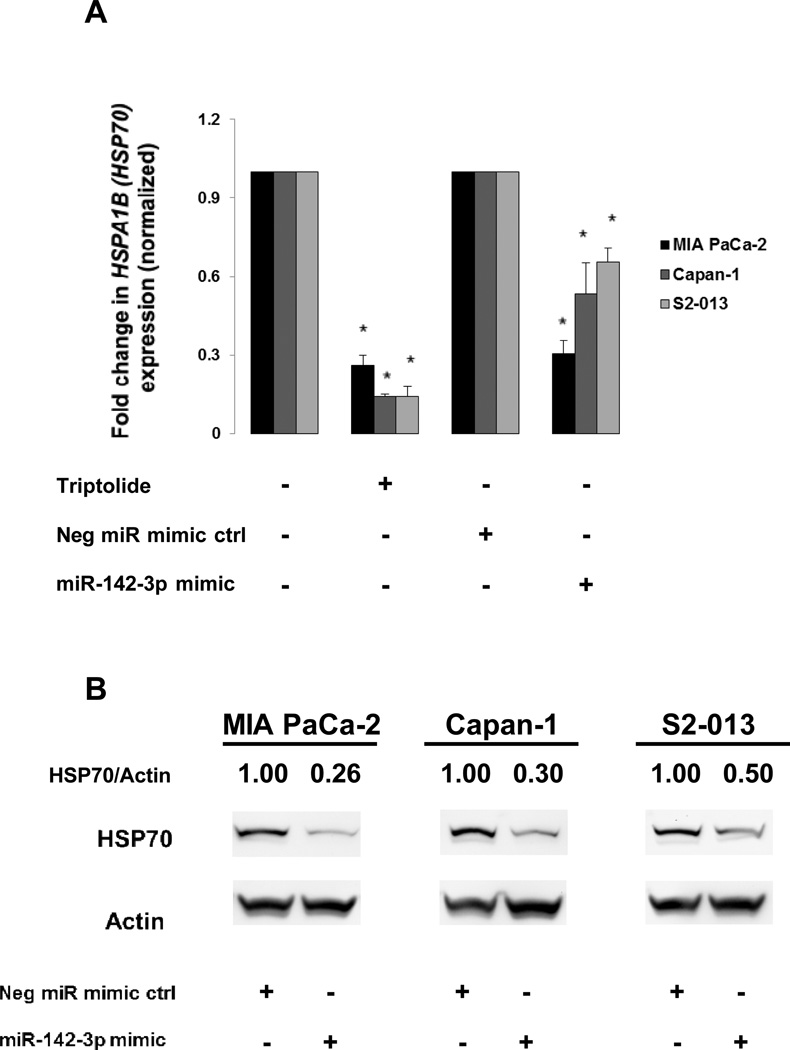
We tested whether ectopic expression of miR-142-3p inhibited HSPA1B (HSP70) expression for several reasons: first, our lab has previously shown that triptolide inhibits HSP70 expression (2–4, 10), second, triptolide inhibited HSPA1B (HSP70) mRNA expression by 74% or more (Fig. 3A), and third, miR-142-3p is predicted to target the HSPA1B isoform of HSP70 by three independent computational prediction programs (miRDB updated 4.2012, TargetScanHuman Release 6.2, MicroCosm Targets Version 5). Overexpression of the miR-142-3p mimic (5 nM) inhibited at least 31% of HSPA1B (HSP70) mRNA expression following 24 h of transfection (Fig. 3A). Similarly, overexpression of miR-142-3p inhibited total HSP70 (both HSPA1B and HSPA1A) protein expression by at least 50% following transfection for 72 h (Fig. 3B). These results show that ectopic expression of mIR-142-3p decreases HSPA1B (HSP70) mRNA and protein levels. Further, pancreatic cancer cells do not compensate for the loss in HSPA1B expression, induced by miR-142-3p, by upregulating the HSPA1A isoform of HSP70 (Supplemental Fig. S4).
Figure 3. Effect of triptolide treatment or microRNA-142-3p mimic overexpression on HSPA1B (HSP70) levels.
(A) Triptolide treatment (100 nM) and miR-142-3p (5 nM) ectopic expression reduced HSPA1B (HSP70) mRNA expression (as assessed by real-time PCR) in MIA PaCa-2, Capan-1, and S2-013 cell lines following 24 h of exposure. Expression of HSPA1B (HSP70) was normalized against 18S. The bars represent mean ± SEM, n=3, *p<0.02 (t test). (B) MicroRNA-142-3p mimic (5 nM for 48 h) reduced HSP70 protein expression in MIA PaCa-2, Capan-1, and S2-013 cell lines. Expression of HSP70 was normalized against actin.
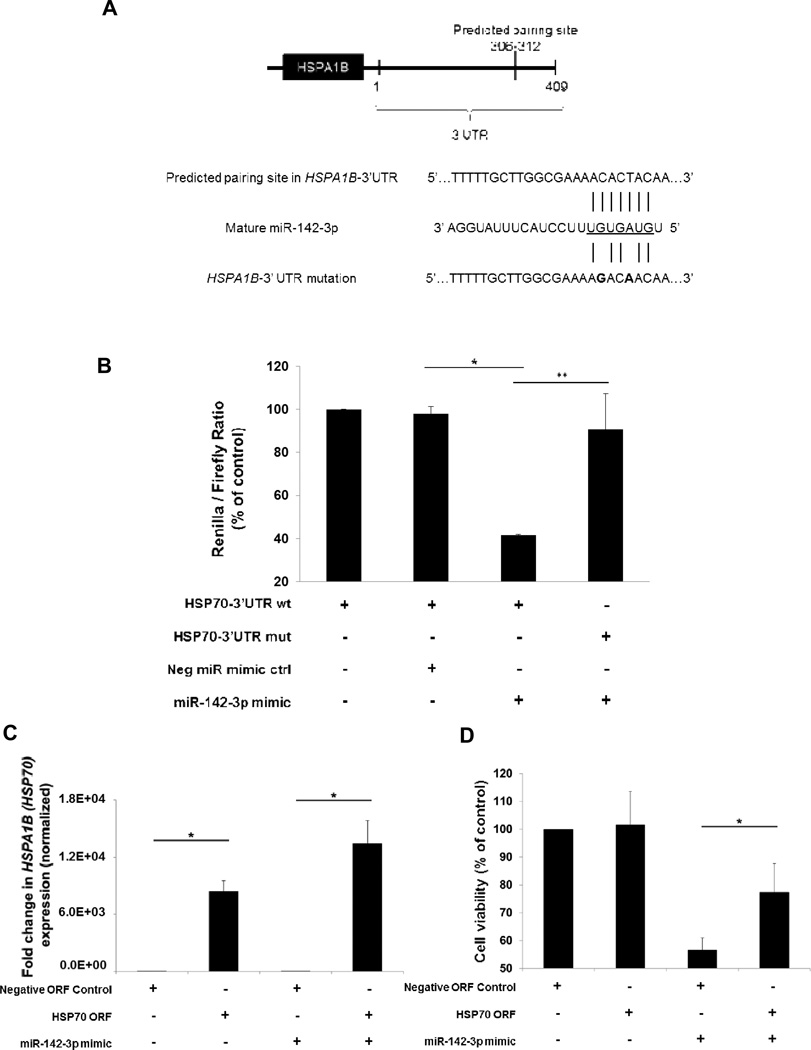
MicroRNA-142-3p directly binds to the 3’UTR of HSPA1B (HSP70)
Three independent programs (miRDB updated 4.2012, TargetScanHuman Release 6.2, MicroCosm Targets Version 5) predict that miR-142-3p binding site is located within the HSPA1B (HSP70) 3’UTR (Fig. 4A). To verify this interaction, a double point mutation (C to G and T to A) was inserted into the predicted binding site of HSPA1B (HSP70), preventing the miR-142-3p seed sequence from interacting with this region (Fig. 4A). Overexpression of miR-142-3p decreased the renilla-to-firefly ratio to 43% of control (Fig. 4B). Upon mutating the miR-142-3p binding site of the renilla-expressing construct, the renilla-to-firefly ratio was rescued to 92% of control (Fig. 4B). These results support the prediction that miR-142-3p regulates HSPA1B (HSP70) transcript levels by binding to its 3’UTR.
Figure 4. MicroRNA-142-3p modulates HSPA1B (HSP70) expression by binding to its 3’UTR.
(A) Schematic of HSPA1B (HSP70) mRNA showing predicted miR-142-3p interaction site (upper). Seven-nucleotide interaction sequence between wildtype (wt) HSPA1B (HSP70)-3’UTR and miR-142-3p and mutant HSPA1B (HSP70)-3’UTR construct shown (lower). (B) Luciferase reporter assay using HEK-293 cells to demonstrate the direct interaction of miR-142-3p and the 3’UTR of HSPA1B (HSP70). After 24 h, miR-142-3p mimic (10 nM) reduced the ratio of renilla-to-firefly expression but not when the 3’UTR bears two point mutations in the miR-142-3p binding site. (C) HSPA1B (HSP70) ORF (lacking the 3’UTR containing the miR-142-3p binding site) transfection causes overexpression of HSPA1B (HSP70) expression (as assessed by real-time PCR) in MIA PaCa-2. (D) HSPA1B (HSP70) ORF overexpression rescued loss in cell viability caused by miR-142-3p (5 nM) overexpression for 48 h in MIA PaCa-2 cells. The bars represent mean ± SEM, n=3, *or **p<0.05 (t test).
HSPA1B (HSP70) is a functional target of miR-142-3p in PDAC cells
Because one miRNA may control the expression of many targets within the cell (12), we evaluated whether miR-142-3p was targeting HSPA1B (HSP70) as a means to control PDAC cell viability. To test this, we measured whether the loss in cell viability induced by miR-142-3p could be rescued by HSPA1B (HSP70) ORF overexpression. This construct was used because it lacks the miR-142-3p binding site. Following 24 h of transfection, HSPA1B (HSP70) was upregulated 13 × 103-fold in MIA PaCa-2 cells overexpressing miR-142-3p and upregulated 8 × 103-fold upregulation in the same cells but in the control group (Fig. 4C). Following 48 h of transfection, miR-142-3p caused cell viability to decrease to 56% of control, but cotransfection of HSPA1B (HSP70) ORF rescues this level to 77% of control. Because a significant, but not complete, rescue was observed, miR-142-3p may be targeting other predicted downstream targets (Supplemental Table S3). These results show, however, that miR-142-3p is targeting HSPA1B (HSP70) in PDAC cells and that this interaction is important in regulating cell viability.
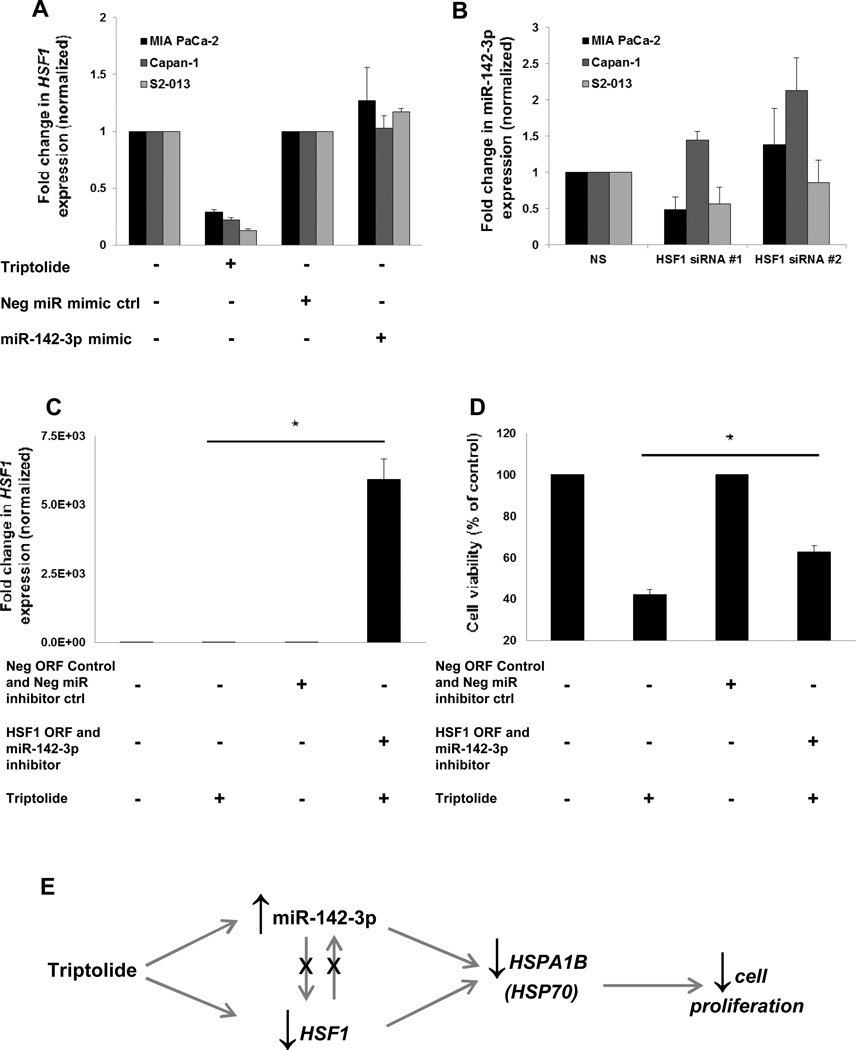
HSF1 and miR-142-3p independently regulate HSPA1B (HSP70)
We tested whether miR-142-3p regulation of HSPA1B (HSP70) was independent of HSF1 because our lab has shown that HSF1 inhibition decreases HSP70 expression (21). This was evaluated by measuring HSF1 levels by real-time PCR in PDAC cells following ectopic expression of miR-142-3p. While triptolide inhibited HSF1 mRNA expression (Fig. 5A), as well as downstream transcriptional targets HSPA1B (HSP70) (Fig. 3A) and HSP27 (Supplemental Fig. 5A), ectopic expression of miR-142-3p had no effect on either HSF1 or HSP27 levels (Fig. 5A and Supplemental Fig. 5A). These data show that miR-142-3p does not regulate HSF1, but these results do not address whether HSF1 controls miR-142-3P levels. To test this, we silenced HSF1 and measured miR-142-3p levels. Both HSF1 siRNA sequences independently inhibited HSF1 mRNA expression (Supplemental Fig. 5B). Levels of miR-142-3p did not change, compared with the control cells, following 24 h of transfection (Fig. 5B).
Figure 5. HSF1 and miR-142-3p independently regulate HSPA1B (HSP70) and mediate triptolide-induced suppression of PDAC proliferation.
(A) Triptolide (100 nM) treatment for 24 h decreases HSF1 levels, but ectopic expression of miR-142-3p mimic (5 nM for 24 h) has no effect (as assessed by real-time PCR) in MIA PaCa-2, Capan-1, and S2-013 cell lines. Expression of HSF1 was normalized against 18S. (B) Real-time PCR measurement of miR-142-3p following HSF1 silencing (25 nM for 24 h). Expression of miR-142-3p was normalized against U6. (C) HSF1 ORF transfection causes overexpression of HSF1 expression (as assessed by real-time PCR) in MIA PaCa-2. (D) HSF1 ORF overexpression rescues loss in cell viability caused by triptolide (100 nM for 48 h) overexpression in MIA PaCa-2 cells. The bars represent mean ± SEM, n=3, *p<0.05 (t test). (E) Model of triptolide action in PDAC cells.
HSF1 and miR-142-3p mediate triptolide-induced suppression of PDAC proliferation
HSF1 and miR-142-3p have each been shown to independently regulate HSP70 and to be inhibited or induced by triptolide, respectively. For this reason, we tested whether HSF1 and miR-142-3p were important in mediating triptolide-induced suppression of PDAC proliferation via HSP70 modulation. We found that simultaneous overexpression of HSF1 and inhibition of miR-142-3p significantly rescued from triptolide-induced cell death. The HSF1 ORF vector yielded sufficient overexpression (Fig. 5C). Triptolide deceases cell viability to 42% of control, but overexpression of HSF1 and inhibition of miR-142-3p rescues this to 63% of control (Fig. 5D). We also tested whether these conditions could rescue from triptolide-induced loss of HSPA1B (HSP70) expression. Triptolide suppresses HSPA1B (HSP70) levels to 26% of control, but while concurrently overexpressing of HSF1 and inhibiting miR-142-3p, this is increased to 35% of control. Although this was expected, the difference was not statistically significant (Supplemental Fig. S5C). These data corroborate our results showing that HSPA1B (HSP70) is an important, although not the only, target of miR-142-3p (Figs. 3 and 4). Likewise, HSPA1B (HSP70) is one of several transcriptional targets of HSF1 (22). Both HSF1 and miR-142-3p play an important role in mediating triptolide-induced suppression of cell proliferation, each independently regulate HSPA1B (HSP70) expression but also regulate other targets (Fig. 5E).
Minnelide induces miR-142-3p expression in a mouse model of PDAC
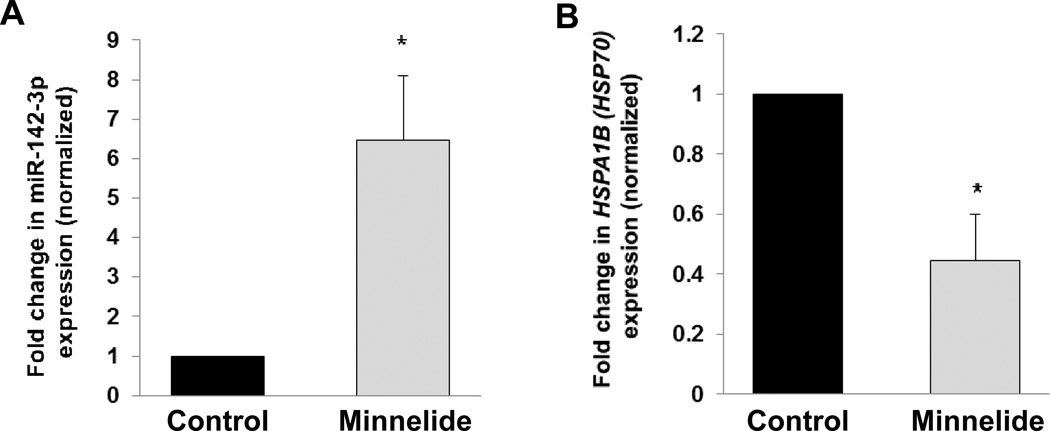
Our laboratory has shown that Minnelide, a prodrug of triptolide, is effective in decreasing tumor burden in vivo (4). To verify that the induction of miR-142-3p observed in vitro would also take place in vivo, we tested whether Minnelide (0.42 mg/kg for 7 days i.p.) upregulated miR-142-3p levels in three independent human PDAC tumor tissues propagated in SCID mice. Minnelide treatment, as compared to saline treatment, led to a 6-fold upregulation of miR-142-3p levels in SCID mice bearing human PDAC tumors (Fig. 6A). In these same samples, Minnelide led to a 55% inhibition of HSPA1B (HSP70) mRNA. These results show that Minnelide is concurrently inducing miR-142-3p and inhibiting HSPA1B (HSP70) in vivo.
Figure 6. Effect of Minnelide on miR-142-3p or HSPA1B (HSP70) expression in vivo.
(A) Minnelide (0.42 mg/kg daily for seven days i.p.) induces miR-142-3p expression (as assessed by real-time PCR) in three independent human tumor xenografts grown in vivo. Expression of miR-142-3p was normalized against U6. (B) Minnelide reduces HSPA1B (HSP70) mRNA expression (as assessed by real-time PCR) of these same samples in vivo. Expression of HSPA1B (HSP70) was normalized against 18S. The bars represent mean ± SEM, n=3, *p<0.05 (t test).
Discussion
These studies show that triptolide induces the expression of miR-142-3p: a negative regulator of HSP70 and a tumor suppressor in PDAC cells. To date, there have only been three studies evaluating the effect of triptolide on miRNAs. One study found that triptolide inhibited proliferation of lymphocytic leukemic cells via downregulating nuclear factor κB (NF-κB) and miR-16-1* (23). Another study reported that triptolide enhanced the sensitivity of multiple myeloma cells to dexamethasone via the downregulation of miR-142-5p and miR-181a (24). Most recently, it has been shown that triptolide may sensitize leukemic cells to adriamycin via inhibiting miR-21 (25). This is in contrast to what we observe, given that a reduction in miR-21 levels was not observed in this study (Supplemental Fig. S3 and Supplemental Table S2). None of the studies addressed whether triptolide affected miR-142-3p expression.
Among more than 200 reports evaluating the role of miRNAs in PDAC, only two evaluate the role of the mir-142 precursor, and none study miR-142-3p. In one study, SUIT-2, the cell line from which S2-013 was derived (26), and Capan-1 were studied to find miRNAs which were altered upon developing gemcitabine resistance. This report showed that gemcitabine-treated patients with high miR-142-5p and miR-204 expression had longer survival times than those with low expression (27). Interestingly, triptolide induces miR-204 expression, though not to the degree that it induces the expression of miR-142-3p (Fig. 1A). Another real-time PCR profiling study found that 100 miRNA precursors were aberrantly expressed in PDAC, including miRNAs previously reported as upregulated in other human cancers such as miR-155, miR-21 and miR-221 (16–18). The mir-142 precursor was found to be 15-fold downregulated in PDAC compared with normal pancreas (28). These findings support our hypothesis that miR-142-3p plays a tumor suppressive role in PDAC cells. Although studying the biological role of miRNAs dysregulated in PDAC has proven informative, our findings support the importance of evaluating miRNA changes in PDAC following chemotherapy treatment to better understand key miRNAs which regulate proliferation.
While this study is the first to evaluate the role of miR-142-3p in PDAC, over 20 studies have evaluated the tumor suppressive role of miR-142-3p in other cancer types. In miRNA profiling studies of acute myeloid leukemia patients, miR-142-3p was downregulated (29) and shown to regulate tumorigenic targets: CCNT2 and TAB2 (30). In hepatocellular carcinoma, miR-142-3p was downregulated compared with normal liver and shown to target RAC1, a GTPase involved in cell growth, migration, and the activation of protein kinases (31). Because HSPA1B (HSP70) significantly, though not completely, rescued from miR-142-3p-induced cell death (Fig. 4D), and the miR-142-3p inhibitor somewhat, although not significantly, rescued from triptolide-induced suppression of HSPA1B (HSP70) (Supplemental Fig. 5C), miR-142-3p may bind to additional targets in PDAC cells. There are a nine other targets predicted by multiple algorithms (Supplemental Table S3). Testing whether mir-142-3p targets RLF rearranged L-myc fusion protein merits further investigation because it has been established as an oncogene in small cell lung carcinoma (32). CCNT2, TAB2, RAC1 or RLT may be important miR-142-3p targets in PDAC, and this is worthy of future study. Our data support the hypothesis that miR-142-3p plays a tumor suppressive role by regulating HSPA1B (HSP70) in PDAC (Fig. 3 & 4).
Although there are several cardiovascular and pulmonary studies evaluating miRNA regulation of HSP70, none have focused on HSPA1B isoform of (HSP70). Hsp70.3 (HSP2A isoform) has been shown to possess general cytoprotective properties in preventing ischemic damage. One study has shown that the Hsp70.3 (HSP2A) gene product is subject to miRNA regulation via miR-378* and miR-711 (33). In lung tissue, it has been shown that HSP70 is regulated by miR-146a and miR-146b-5b. These miRNAs were found to increase greatly, and inversely correlate with HSP70 levels, following treatment with gefitinib; this may contribute to pulmonary fibrosis (34). The results obtained in this study support an miRNA-mediated mechanism of HSP70 regulation independent of HSF1 (Fig. 5). Though HSF1 regulation of HSP70 has been well-documented (9, 21, 22), miRNA regulation of HSP70 merits further investigation.
Because miR-142-3p negatively regulates protumorigenic genes, it holds promise as a target for future PDAC therapeutic development. As with many miRNAs, miR-142-3p may play an opposite role in different cancer types. For example, miR-142-3p has been reported to be oncogenic and upregulated in human T-cell acute lymphoblastic leukemia (35). Although the majority of studies evaluating miR-142-3p in cancer demonstrate its tumor suppressive role (29–31), those who further develop miR-142-3p as a therapy in PDAC will need to verify its tumor suppressive role in prospective patients. Restoring repressed miRNA levels in patients holds promise because it has been shown to be feasible via systemic delivery of lipid nanoparticles carrying miR-34a, miR-143, and miR-145 in treating orthotopic PDAC tumors in vivo (36). This study was especially important as blood flow to the pancreas is thought to be low (37). Understanding an miRNA-mediated mechanism of triptolide action, especially the induction of miR-142-3p, will be useful as Minnelide moves into clinical trials. Moreover, miR-142-3p can be a target for future PDAC therapeutic development.
Supplementary Material
Acknowledgements
The authors would like to thank Michael Raleigh for editing this manuscript.
Grant Support: NIH grants T32 DA07097 (to T.N. MacKenzie) and R01 CA124723 and R01 CA170946 (to A.K. Saluja), grants from the Hirshberg Foundation for Pancreatic Cancer Research and Robert and Katherine Goodale Foundation (to A.K. Saluja), and by intramural funding from the University of Minnesota’s Surgery Department.
Abbreviations
- siRNA
Short interfering RNA
- miR
microRNA
Footnotes
Conflict of interest: University of Minnesota has filed a patent for Minnelide, which has been licensed to Minneamrita Therapeutics LLC. Inventors on this patent include S.V. and A.K.S. S.V. and A.K.S. have financial interests in this company. The other authors declare that they have no competing interests. Minnelide synthesis has been filed under patent WO/2010/129918.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock. Cancer research. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 3.Mujumdar N, Mackenzie TN, Dudeja V, Chugh R, Antonoff MB, Borja-Cacho D, et al. Triptolide induces cell death in pancreatic cancer cells by apoptotic and. Gastroenterology. 2010;139:598–608. doi: 10.1053/j.gastro.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3004334. 156ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tengchaisri T, Chawengkirttikul R, Rachaphaew N, Reutrakul V, Sangsuwan R, Sirisinha S. Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and. Cancer letters. 1998;133:169–175. doi: 10.1016/s0304-3835(98)00222-5. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, et al. Triptolide inhibits the growth and metastasis of solid tumors. Molecular cancer therapeutics. 2003;2:65–72. [PubMed] [Google Scholar]
- 7.Krosch TC, Sangwan V, Banerjee S, Mujumdar N, Dudeja V, Saluja AK, et al. Triptolide-mediated cell death in neuroblastoma occurs by both apoptosis and autophagy pathways and results in inhibition of nuclear factor-kappa B activity. American journal of surgery. 2013;205:387–396. doi: 10.1016/j.amjsurg.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, et al. Triptolide therapy for neuroblastoma decreases cell viability in vitro and. Surgery. 2009;146:282–290. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances. The Journal of biological chemistry. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 10.Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, et al. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and. Gastroenterology. 2009;136:1772–1782. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonoff MB, Chugh R, Skube SJ, Dudeja V, Borja-Cacho D, Clawson KA, et al. Role of Hsp-70 in triptolide-mediated cell death of neuroblastoma. The Journal of surgical research. 2010;163:172–178. doi: 10.1016/j.jss.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in. Nature reviews Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in. Cancer research. 2007;67:616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 14.Awasthi N, Zhang C, Ruan W, Schwarz MA, Schwarz RE. BMS-754807, a Small Molecule Inhibitor of Insulin-like Growth Factor-1 Receptor. Molecular cancer therapeutics. 2012 doi: 10.1158/1535-7163.MCT-12-0447. [DOI] [PubMed] [Google Scholar]
- 15.Sarver AL. Toward understanding the informatics and statistical aspects of micro-RNA. Journal of cardiovascular translational research. 2010;3:204–211. doi: 10.1007/s12265-010-9180-z. [DOI] [PubMed] [Google Scholar]
- 16.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. England. 2010:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan Q, Wang X, Gong W, Ni L, Chen C, He X, et al. ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PloS one. United States. 2012:e31518. doi: 10.1371/journal.pone.0031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. United States. 2007:16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreutzer JN, Ruzzene M, Guerra B. Enhancing chemosensitivity to gemcitabine via RNA interference targeting the. BMC cancer. 2010;10:440. doi: 10.1186/1471-2407-10-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan T, Skaftnesmo KO, Leiss L, Sleire L, Wang J, Li X, et al. Neuronal markers are expressed in human gliomas and NSE knockdown sensitizes. BMC cancer. 2011;11:524. doi: 10.1186/1471-2407-11-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudeja V, Chugh RK, Sangwan V, Skube SJ, Mujumdar NR, Antonoff MB, et al. Prosurvival role of heat shock factor 1 in the pathogenesis of pancreatobiliary. American journal of physiology Gastrointestinal and liver physiology. 2011;300:G948–G955. doi: 10.1152/ajpgi.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. England. 2004:2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 23.Meng HT, Zhu L, Ni WM, You LS, Jin J, Qian WB. Triptolide inhibits the proliferation of cells from lymphocytic leukemic cell. Acta pharmacologica Sinica. 2011;32:503–511. doi: 10.1038/aps.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Yang M, Jin J. Triptolide enhances the sensitivity of multiple myeloma cells to dexamethasone. Leukemia & lymphoma. 2012;53:1188–1195. doi: 10.3109/10428194.2011.638069. [DOI] [PubMed] [Google Scholar]
- 25.Matthaios D, Zarogoulidis P, Balgouranidou I, Chatzaki E, Kakolyris S. Oncology. Switzerland: Basel; 2011. Molecular pathogenesis of pancreatic cancer and clinical perspectives; pp. 259–272. [DOI] [PubMed] [Google Scholar]
- 26.Iwamura T, Caffrey TC, Kitamura N, Yamanari H, Setoguchi T, Hollingsworth MA. P-selectin expression in a metastatic pancreatic tumor cell line (SUIT-2) Cancer research. 1997;57:1206–1212. [PubMed] [Google Scholar]
- 27.Ohuchida K, Mizumoto K, Kayashima T, Fujita H, Moriyama T, Ohtsuka T, et al. MicroRNA expression as a predictive marker for gemcitabine response after. Annals of surgical oncology. 2011;18:2381–2387. doi: 10.1245/s10434-011-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, et al. Expression profiling identifies microRNA signature in pancreatic cancer. International journal of cancer Journal international du cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Wang XS, Yang GH, Zhai PF, Xiao Z, Xia LY, et al. miR-29a and miR-142-3p downregulation and diagnostic implication in human acute. Molecular biology reports. 2012;39:2713–2722. doi: 10.1007/s11033-011-1026-5. [DOI] [PubMed] [Google Scholar]
- 30.Wang XS, Gong JN, Yu J, Wang F, Zhang XH, Yin XL, et al. MicroRNA-29a and microRNA-142-3p are regulators of myeloid differentiation and. Blood. 2012;119:4992–5004. doi: 10.1182/blood-2011-10-385716. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Cai C, Wang X, Liu M, Li X, Tang H. MicroRNA-142-3p, a new regulator of RAC1, suppresses the migration and invasion. FEBS letters. 2011;585:1322–1330. doi: 10.1016/j.febslet.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Hui L, Xu W, Shen H, Chen Q, Long L, et al. Triptolide modulates the sensitivity of K562/A02 cells to adriamycin by regulating miR-21 expression. Pharmaceutical biology. 2012;50:1233–1240. doi: 10.3109/13880209.2012.665931. [DOI] [PubMed] [Google Scholar]
- 33.Tranter M, Helsley RN, Paulding WR, McGuinness M, Brokamp C, Haar L, et al. Coordinated post-transcriptional regulation of Hsp70.3 gene expression by. The Journal of biological chemistry. 2011;286:29828–29837. doi: 10.1074/jbc.M111.221796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namba T, Tanaka K, Hoshino T, Azuma A, Mizushima T. Suppression of expression of heat shock protein 70 by gefitinib and its. PloS one. 2011;6:e27296. doi: 10.1371/journal.pone.0027296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv M, Zhang X, Jia H, Li D, Zhang B, Zhang H, et al. An oncogenic role of miR-142-3p in human T-cell acute lymphoblastic leukemia. Leukemia: official journal of the Leukemia Society of America, Leukemia Research. 2012;26:769–777. doi: 10.1038/leu.2011.273. [DOI] [PubMed] [Google Scholar]
- 36.Goloudina AR, Demidov ON, Garrido C. Inhibition of HSP70: a challenging anticancer strategy. Cancer letters. Ireland 2012 Elsevier Ireland Ltd. 2012:117–124. doi: 10.1016/j.canlet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. England. 2010:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.