Abstract
Context:
Mutations in the electron donor enzyme P450 oxidoreductase (POR) result in congenital adrenal hyperplasia with apparent combined 17α-hydroxylase/17,20 lyase and 21-hydroxylase deficiencies, also termed P450 oxidoreductase deficiency (PORD). Major clinical features present in PORD are disordered sex development in affected individuals of both sexes, glucocorticoid deficiency, and multiple skeletal malformations.
Objective:
The objective of the study was to establish a noninvasive approach to prenatal diagnosis of PORD including assessment of malformation severity to facilitate optimized prenatal diagnosis and timely treatment.
Design:
We analyzed 20 pregnancies with children homozygous or compound heterozygous for disease-causing POR mutations and 1 pregnancy with a child carrying a heterozygous POR mutation by recording clinical and biochemical presentations and fetal ultrasound findings. In 4 of the pregnancies (3 homozygous and 1 heterozygous for disease-causing POR mutations), prenatal analysis of steroid metabolite excretion in maternal urine was carried out by gas chromatography/mass spectrometry during gestational weeks 11–23.
Results:
Pregnancy complications in our cohort included maternal virilization (6 of 20) with onset in the second trimester. Seven pregnant women presented with low unconjugated estriol at prenatal screening (triple or quadruple antenatal screening test). Overt dysmorphic features were noted in 19 of the 20 babies at birth but observed in only 5 by prenatal ultrasound. These 5 had the most severe malformation phenotypes and poor outcome, whereas the other babies showed normal development. Steroid profiling of maternal urine revealed significantly increased steroids of fetal origin, namely the pregnenolone metabolite epiallopregnanediol and the androgen metabolite androsterone, with concomitant low values for estriol. Diagnostic steroid ratios conclusively indicated PORD as early as gestational week 12. In the heterozygous pregnancy, steroid ratios were only slightly elevated and estriol excretion was normal.
Conclusion:
Prenatal diagnosis in PORD is readily established via urinary steroid metabolite analysis of maternal urine. Visible malformations at prenatal ultrasound predict a severe malformation phenotype.
Congenital adrenal hyperplasia due to P450 oxidoreductase deficiency (PORD) is a condition with autosomal recessive inheritance, caused by mutations in the P450 oxidoreductase (POR) gene (1–3). PORD patients present with biochemical evidence of partial deficiencies in 21-hydroxylase (CYP21A2) and 17α-hydroxylase/17,20-lyase (CYP17A1) activities and manifest clinically in many but not all cases with disorder of sex development (DSD) in both sexes; glucocorticoid deficiency; and skeletal malformations including craniosynostosis, midface hypoplasia, radiohumeral synostosis, and phalangeal abnormalities (4–8). POR is the crucial electron donor to all microsomal cytochrome P450 (CYP) enzymes including the steroidogenic enzymes CYP21A2, CYP17A1, and P450 aromatase (CYP19A1), thus explaining the deficiencies in steroid synthesis observed in patients with PORD (2–3). POR also serves as the mandatory electron donor to squalene epoxidase and 14α-lanosterol demethylase (CYP51A1) catalyzing important steps in cholesterol synthesis. Sterols are involved in hedgehog-mediated regulation of fetal bone development. Hence, decreased 14α-lanosterol demethylase activity as documented ex vivo in a patient with PORD (9) may explain the observed skeletal malformation phenotype, which resembles that observed after the disruption of downstream processing of lanosterol by fluconazole (10–13). Distinct POR mutations can differentially affect the activities of electron-accepting CYP enzymes, a likely explanation for the observed phenotypic variability (5, 14–16).
The prognosis and outcome of PORD patients relies heavily on early diagnosis and timely treatment with optimal perinatal care including assessment of glucocorticoid replacement requirements as well as corrective treatment of skeletal malformations and DSD manifestations. Therefore, the aim of this study was to establish tools for prenatal diagnosis and estimation of disease severity.
Subjects and Methods
Patients
We analyzed pregnancy data from 20 patients (8 males, 12 females) with genetically confirmed PORD and 1 pregnancy with a fetus carrying a heterozygous mutation in the POR gene. Four of these pregnancies have been prospectively monitored (PORD A, B, C, and the heterozygous baby D) and are described in detail below.
PORD pregnancy A was the second pregnancy of healthy, nonconsanguineous parents. Their first child (46,XX) was affected by PORD, born with overt craniofacial malformations and severely virilized external genitalia (Prader IV); a cosyntropin test confirmed adrenal insufficiency. The pregnancy had been complicated by signs of maternal androgen excess (acne from gestational weeks 14 to 16 and maternal voice deepening noted from week 24). Genetic analysis detected homozygous POR p.A287P (g.29,556 G>C) mutations, with both parents being heterozygous carriers.
During the second pregnancy, the mother did not experience signs of androgen excess. Prenatal ultrasound examinations for detection of skeletal malformations were carried out every 4–6 weeks, showing normal fetal development without visible malformations throughout the pregnancy. Amniocentesis was carried out during the 16th week of gestation and direct sequencing of amniocyte DNA revealed the same homozygous p.A287P mutation as in the first child; the karyotype was confirmed as 46,XY. After an otherwise uneventful pregnancy, labor initiated spontaneously at gestational week 35. Birth weight was 2830 g [0.9 SD score (SDS)], length was 53 cm (3.2 SDS), and head circumference was 34 cm (1.4 SDS) (SDS according to British 1990 growth reference data). Initially the patient suffered from respiratory distress and had to be referred to the neonatal intensive care unit for continuous positive airway pressure ventilation therapy. The baby had midface hypoplasia, a pear-shaped nose, a soft palate cleft, and mild acrocephaly without overriding cranial sutures and a mild left elbow contracture. Other malformations included arachnodactyly, rocker-bottom feet, and a narrow chest with mild pectus excavatum. The penis was of normal size and the testes were bilaterally descended. He was started on hydrocortisone replacement therapy in the first week of life. At 6 weeks of age, surgery for bilateral inguinal and umbilical hernias was performed. He underwent surgery for coronal and metopic synostosis at age 6 months; the soft palate cleft was repaired at 1 year of age. Postnatal growth and development including cognitive function were normal.
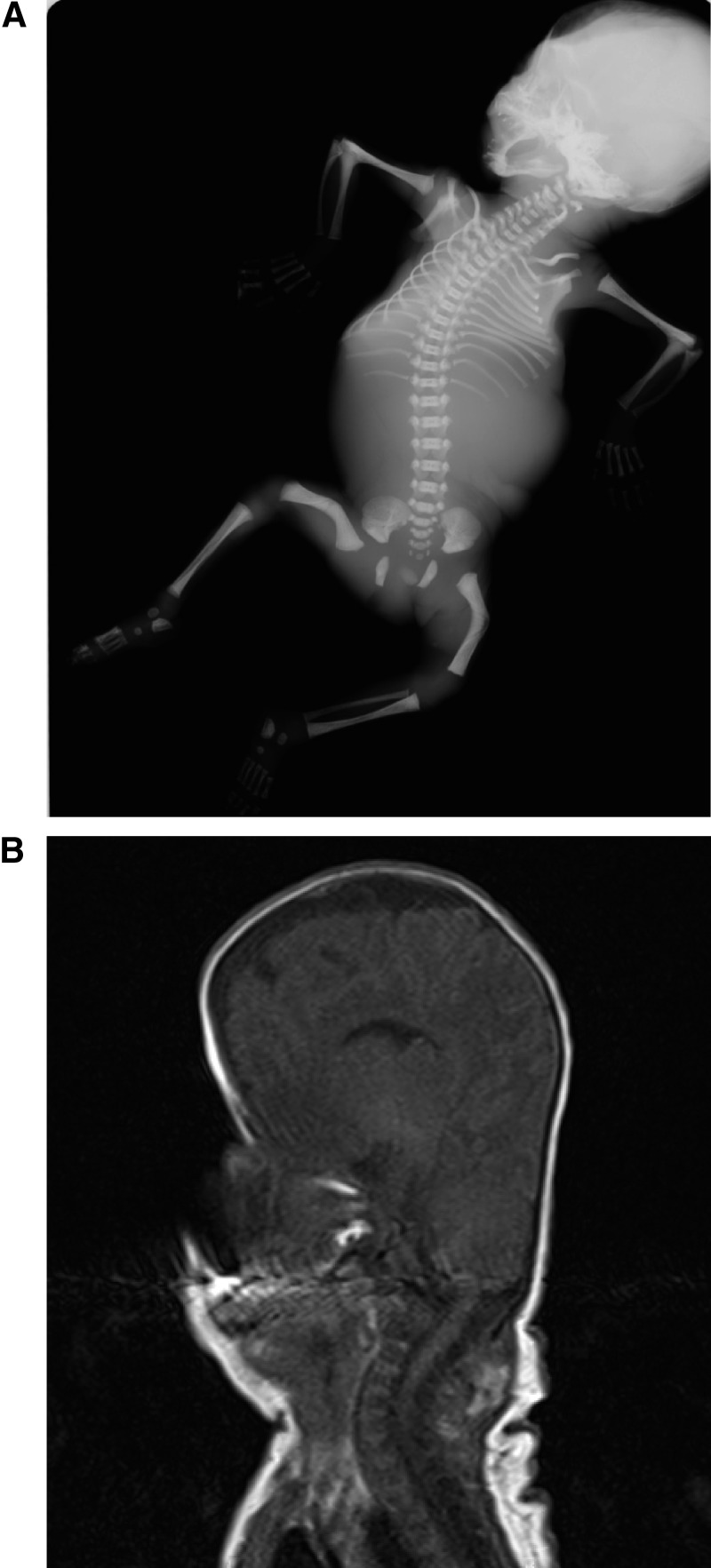
PORD pregnancy B was the first pregnancy of healthy, nonconsanguineous parents. Routine prenatal ultrasound at 16 weeks revealed an abnormally shaped skull, bowed femora, bilateral radioulnar synostosis, and ambiguous genitalia. Based on the severe malformation phenotype, the parents decided for termination of pregnancy (TOP), which was carried out in the 25th week of gestation. The postmortem on the fetus was consistent with a severe malformation phenotype including coronal synostosis, severe midface hypoplasia, bilateral radiohumeral synostosis, and bowed femora (all confirmed on posttermination x-ray, Figure 1A) as well as micropenis, absent scrotum, and undescended testes. The karyotype was 46,XY.
Figure 1.
A, An x-ray radiograph performed at postmortem after the termination of pregnancy PORD B at gestational week 25; note the abnormally shaped skull, thin ribs, ankylosis of both elbow joints (radioulnar synostosis), and bilateral bowed femora. B, Magnetic resonance imaging of the head of patient PORD B on postnatal day 2, illustrating the pronounced turricephaly consequent to PORD-associated craniosynostosis.
Sequencing analysis of fetal DNA commenced after termination revealed compound heterozygosity for p.A287P and a frameshift mutation I444fsX6 (g.31,146dupC).
PORD pregnancy C was the second pregnancy of healthy, nonconsanguineous parents (same father as in the first pregnancy). The first pregnancy had resulted in a healthy baby girl. The course of the second pregnancy was unremarkable, without signs of maternal virilization. At 19 weeks' gestation the triple test (human chorionic gonadotropin, α-fetoprotein, and unconjugated estriol) showed undetectable levels of estriol (<0.02 ng/mL). At 20 weeks' gestation, the following anomalies were detected by prenatal ultrasound: bowed femurs bilaterally, craniosynostosis, intrauterine growth retardation, and abnormal scissoring of the lower extremities (right foot positioned on left flank). A fetal magnetic resonance imaging did confirm the above. The amniocentesis performed at gestational week 20 revealed a female karyotype (46, XX). In gestational week 39, the baby was delivered by cesarean section without any complications. After birth the baby experienced respiratory distress due to a choanal stenosis [Apgar indices 8 at 1 minute, 9 at 5 minutes; birth weight 2610 g (−1.16 SDS), length 48 cm (−1.67 SDS), head circumference 31 cm (−2.89 SDS)] (SDS according to British 1990 growth reference data). Postnatally the following malformations were evident: craniosynostosis without hydrocephalus (Figure 1B), bilateral choanal stenosis, craniovertebral junction deformity, bilateral vesicoureteral reflux, bilateral hydronephrosis, contractures of the fingers and toes, arachnodactyly, humeroradial-ulnar synostosis of the upper extremities, shortened femurs, and humeri bilaterally. The external genitalia had normal female appearance. The baby was admitted to the intensive care unit and initially ventilated; concurrently hydrocortisone replacement was initiated. On day 69 she died due to respiratory insufficiency, most likely as a consequence of central apnea. Genetic analysis revealed compound heterozygous POR mutations (p.A287P/p.Q455Rfs166X).
Heterozygous PORD pregnancy D was the second pregnancy of a consanguineous Pakistani couple. The first baby (46, XY) had presented at delivery with a dysmorphic face including frontal bossing, hypotelorism, proptosis, low nasal bridge, short columella, pronounced philtrum, and small, low-set ears. Other malformations at birth included bilateral radiohumeral synostosis and arachnodactyly. During the first trimester of pregnancy, the mother had started to develop hirsutism, resolving after delivery. POR sequencing in the index child revealed a homozygous p.R498P mutation.
Because of this history, the second pregnancy was closely monitored by ultrasound, but no malformations could be detected. Chorionic villous sampling during gestational week 11 revealed a 46,XX karyotype. The baby was well and healthy at delivery, with no sign of malformations; genetic analysis after birth revealed heterozygosity for p.R498P.
PORD malformation score
The clinical malformation phenotype was assessed after birth using a scoring algorithm we recently developed (8). In brief, this score assesses the absence or presence of 6 different types of malformations: midface hypoplasia, craniosynostosis, phalangeal malformations, large joint synostoses, femoral bowing, and additional malformations. The severity of each malformation is graded on a scale ranging from 0 (not present), 1 (mild), 2 (severe), to 3 (severe with additional complications). The minimum of the total PORD malformation score is 0 (no malformations), and the maximum score is 16 (most severe malformation phenotype).
Urinary steroid profiling
Urinary steroid metabolite excretion was analyzed by gas chromatography/mass spectrometry (GC/MS). Characterization and quantification of all excreted steroids has been previously established in a longitudinal study of a pregnancy with a PORD fetus (17). This allowed designation of key analytes for diagnosis of the condition by the development of an abbreviated selected ion monitoring quantitative method for their analysis. In the 4 prospective PORD pregnancies followed up, maternal urine for steroid metabolite assessment was collected during gestational weeks 12, 22, and 28 (PORD A), 23 (PORD B), 20 (PORD C), and 11 (heterozygous PORD pregnancy D), respectively, and results were compared with the urinary steroid profiles from 60 apparently normal pregnancies of gestational weeks 15–23.
Results
Prenatal maternal and fetal findings
Overall, 7 of 20 pregnancies with a baby affected by PORD were complicated by signs of maternal virilization, which usually manifested during the second trimester (Table 1). Clinical signs and symptoms included acne, hirsutism, and deepening of the voice, all resolving within a few weeks after delivery. The occurrence of prenatal maternal virilization did not show any conclusive correlation to genotype or sex of the affected child. In 7 pregnancies a low unconjugated estriol were found at routine prenatal screening (triple or quadruple antenatal screening test) during gestational weeks 14–19 (cases 2, 3, 15, 17, PORD A, PORD B, and PORD C).
Table 1.
Prenatal and Postnatal Presentation in 19 Patients Affected by PORD
| Patient Number | Genotype | Karyotype | Malformations Detected by Prenatal Ultrasound | Total Malformation Score at Birtha | Maternal Androgen Excess | DSD Detected by Prenatal Ultrasound | DSD Evident at Birth | Outcome | Follow-Up Period |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IVS7 + 2dupT/p.Q455RfsX90 | XY | Y | 15 | N | N | Y | Death at 19 mo | 19 mo |
| 2 | p.A287P/ IVS6-2A>T | XY | Y | 15 | Y | Y | Y | Developmental delay, death at age 2 y | 2 y |
| 3 | p.A287P/ IVS6-2A>T | XX | Y | 15 | Y | N | N | Delayed motor and speech development | 23 mo |
| PORD B | p.A287P/p.I444HfsX6 | XY | Y | >10b | n.d. | Y | Y | TOP gestational wk 25 | TOP gestational wk 25c |
| PORD C | p.A287P/p.Q455Rfs166X | XX | Y | 12 | N | N | N | Death at d 69 | 69 d |
| 4 | p.A287P/p.V472AfsX102 | XY | N | 10 | N | N | Y | Conductive hearing loss, normal intelligence | 11 y |
| 5 | p.A287P/IVS8 + 1G>A | XX | N | 9 | N | N | Y | Normalc | 11 y |
| 6 | p.A287P/p.G188V191dup | XY | N | 9 | Y | N | Y | Delayed motor and speech development | 23 mo |
| 7 | p.A287P/− | XX | N | 9 | Y | N | Y | Normal | 9 y |
| PORD A | p.A287P/p.A287P | XY | N | 8 | N | N | N | Normal | 4 y |
| 8 | p.R498P/p.R498P | XX | N | 7 | Y | N | N | Normal | 1.5 y |
| 9 | p.A287P/p.A287P | XX | N | 7 | Y | N | Y | Normal | 6 y |
| 10 | p.A287P/p.H628P | XX | N | 7 | N | N | Y | Conductive hearing loss at age 4 y, slightly delayed development | 9 y |
| 11 | p.A287P/p.A287P | XX | N | 5 | N | N | Y | Normal | 14 y |
| 12 | p.Y376 LfsX74/p.T142A | XX | N | 5 | N | N | N | Normal | 20 y |
| 13 | p.R457H/p.A287P | XX | N | 4 | N | N | Y | Normal | 16 y |
| 14 | p.A287P/Del exU1-1 | XX | N | 4 | N | N | N | Normal | 3.5 y |
| 15 | p.C569Y/p.Y181D | XX | N | 4 | N | N | Y | Normal | 4 y |
| 16 | p.A287P/p.A287P | XY | N | 4 | N | N | N | Normal | 7 y |
| 17 | p.C569Y/p.Y181D | XY | N | 0 | Y | N | N | Normal | 2 y |
Abbreviations: E3, estriol; −, no mutation detected; N, no; n.d., not determined; Y, yes. Bold indicates cases with visible malformations on prenatal ultrasound and a total malformation score above 10.
For a detailed description of the PORD malformation score, please see published reference (8).
The malformation score for case PORD B is likely to be underestimated as scoring based on postmortem report rather than clinical examination.
Normal, normal postnatal development in terms of growth, speech, motor, and cognitive function.
Prenatal ultrasound detected skeletal malformations in 5 of the 20 PORD pregnancies (Table 1), in all cases describing clearly visible bowed femora. These 5 included the above-described PORD pregnancies B and D and cases 1–3 (Table 1). In case 1, bilaterally angulated femora were detected for the first time at gestational week 21 and a prominence of the forehead in addition to short and bent femora at gestational week 25. In case 2, ultrasound showed bowed femora and flexed distal limb joints of the extremities at gestational week 16. In case 3, ultrasound in gestational week 17 revealed a flattened nasal bridge, low set ears, abnormal hand posturing, ambiguous genitalia, and bowed humeri, femora and tibiae.
However, despite this low detection rate by prenatal ultrasound, at birth all 20 PORD cases presented with overt skeletal malformations; the median PORD malformation score of the cohort was 7.5, ranging from 4 to 15. Of note, the median malformation score in the 15 patients without evidence of malformations on prenatal ultrasound was 6 (range 0–10), ie, spanning from absent to mild and moderate malformations. By contrast, the 5 patients with prenatally detected malformations presented with the most severe skeletal malformation phenotypes (all scores >10, median score 15) (Table 1). Of these 5 pregnancies, 1 ended after the termination of pregnancy because of the severity of the observed malformations. Three patients died, due to recurrent respiratory infections (case 1), accidental displacement of the tracheostomy tube (case 2), and respiratory failure due to central apnea (PORD C). The fifth patient survived but suffered from delayed motor and cognitive development. By contrast, all 15 patients with a mild to intermediate malformation phenotype survived with a normal functional outcome with regard to growth, motor, speech, and cognitive development (Table 1). Characteristic malformations documented by prenatal ultrasound in the severely affected group were a bowed femora and an abnormally shaped skull, which was documented in all 5 patients. By contrast, only 2 of the 15 patients in the milder malformation group (cases 6 and 8, Table 1) had bowed femora and only 1 of 15 (case 6) had a midface malformation phenotype of similar severity to the severe malformation group; in both cases these had not been detected prenatally, and their total malformation scores were lower than in the 5 patients with the most severe malformation phenotype (Table 1).
Urinary steroid profiling during pregnancy
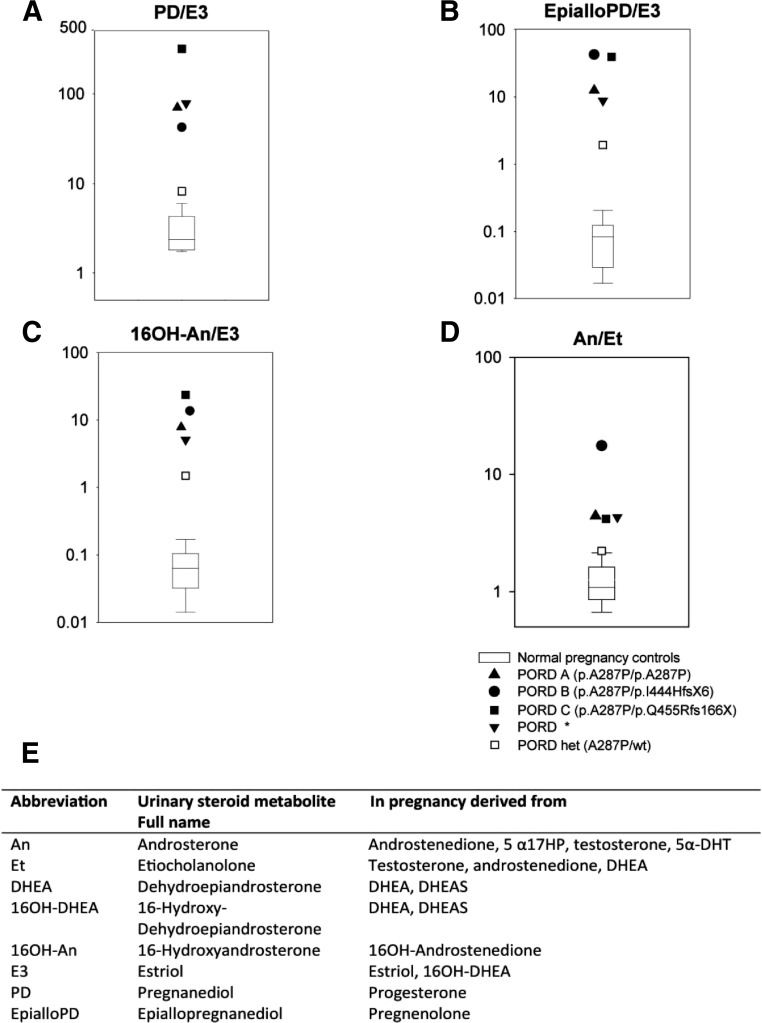
Four pregnancies, PORD pregnancies A, B, and C and the heterozygous pregnancy D, were monitored prospectively with GC/MS analysis of maternal urine collected between gestational weeks 11 and 23. This revealed significant reduction in the excretion of estriol in the PORD pregnancies, which in a normal pregnancy represents the second most important end product of fetoplacental steroid synthesis, after the progesterone metabolite pregnanediol (PD). The very high PD to estriol ratio in the 3 PORD pregnancies (Figure 2A) illustrates the pronounced estriol deficiency. All 3 mothers showed markedly increased excretion of the pregnenolone metabolite epiallopregnanediol (EpialloPD), which is usually found in only trace amounts in normal pregnancies. Consequently, the EpialloPD to estriol ratios in the 3 affected pregnancies were extremely elevated as compared with normal pregnancies (Figure 2B). The possible attenuated aromatization of 16α-hydroxyandrostenedione in the estriol biosynthetic pathway would be represented by an increased ratio of its metabolite 16α-hydroxyandrosterone to estriol, and this was the case in the 3 PORD pregnancies studied (Figure 2C).
Figure 2.
Proposed urine steroid metabolite ratios for a prenatal diagnosis of PORD. Illustrated are normative values from gestational week 15 to week 23 (n = 60) and 4 pregnancies with affected PORD babies and 1 pregnancy with a heterozygous carrier. Panel A shows reduced estriol relative to the excretion of the progesterone metabolite PD. Panel B shows increased value of the EpialloPD to estriol ratio resulting from pregnenolone accumulation caused by attenuated 17-hydroxylase/17,20-lyase and decreased estriol production. Panel C shows increased 16-hydroxyandrosterone excretion in relation to decreased estriol, indicative of the effect of mutant POR on CYP19A1 aromatase activity. Panel D shows increased androsterone excretion compared with etiocholanolone, indicative of alternative pathway androgen synthesis activity (2). Panel E gives the abbreviation and systematic name of the urinary steroid metabolites and from which they derive. The ratios denoted by an asterisk were obtained from a previously published report on a patient (17).
A further hallmark of the pregnancies affected by PORD was the overproduction of 5α-reduced androgens, reflected by highly increased excretion of the 5α-reduced androgen metabolite androsterone. This excess is most easily represented by relating its excretion to that of its 5β-epimer etiocholanolone, ie, the androsterone to etiocholanolone ratio. These ratio values were very high in the 3 PORD pregnancies as compared with normal pregnancies (Figure 2D).
The typical features of PORD were identified in all urine samples collected in the 3 affected pregnancies during gestational weeks 12–28. All maternal steroid metabolites in urine normalized after delivery (data not shown), consistent with a fetal source of abnormality during pregnancy.
In the heterozygous pregnancy D, the above-mentioned pathogenic urinary metabolite ratios for PORD were only slightly elevated, and the estriol concentration was completely normal.
Discussion
Analysis of the course of 20 pregnancies with fetuses affected by PORD and 1 pregnancy heterozygous for a POR mutation revealed 3 cardinal features indicative of the presence of the disorder that may be evident prenatally: maternal virilization, severe bone malformations detected by fetal ultrasound, and, invariably in the prospectively monitored cases, a distinctly altered steroid metabolite excretion in maternal urine indicative of PORD from gestational week 12 onward. Remarkably, these changes were uniformly present in all affected pregnancies and did not vary as a function of genotype or phenotype, similar to our previous experience with urine GC/MS analysis in affected patients (8).
The characteristically low maternal urinary estriol excretion during the affected pregnancies was mirrored by the finding of low serum estriol in the antenatal multiple marker screening test carried out in 7 of the patients. These findings were similar to those in the 2 previously published prenatal cases, describing low urinary and serum estriol, respectively (17, 18). The maternal blood screening test is usually performed between the 16th and 18th week of pregnancy. The triple test comprises measurement of serum α-fetoprotein, human chorionic gonadotropin, and estriol, but a quad test is gaining popularity and also includes inhibin A. The primary object of the screening test is for detection of neural tube defects and Down's syndrome (19, 20).
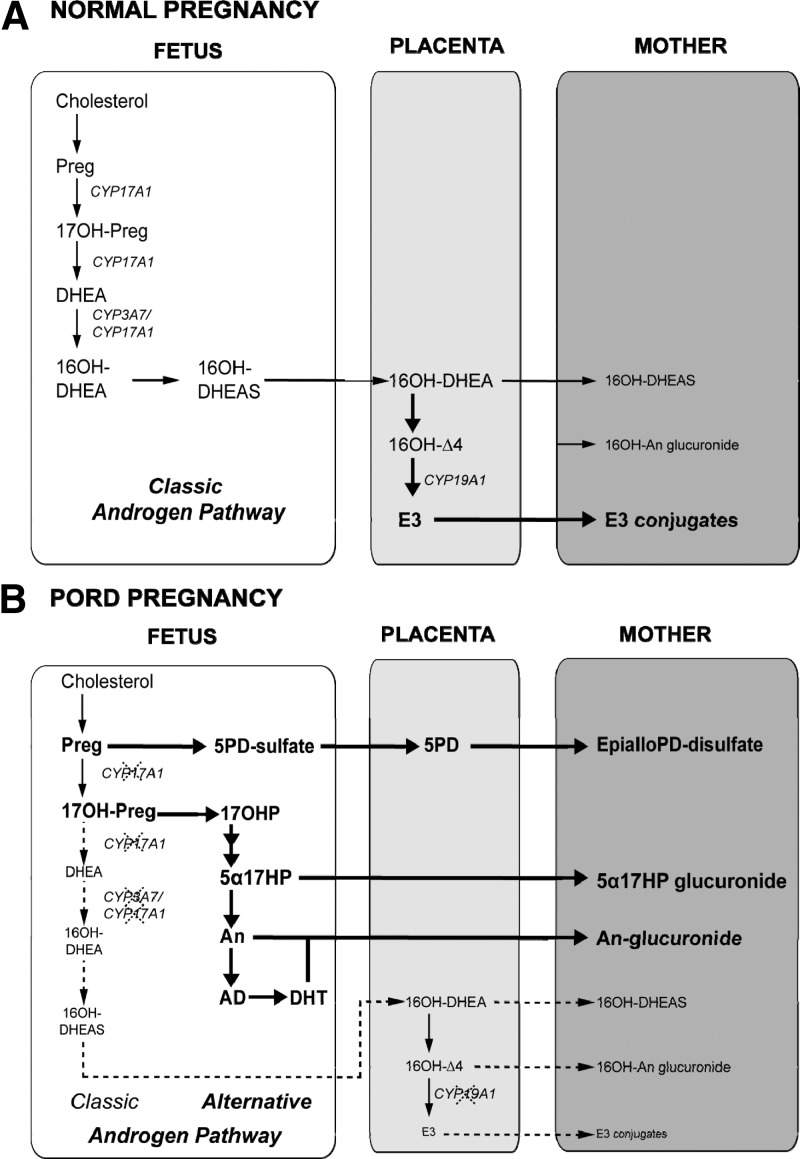
In a physiological pregnancy, estriol is generated in the placenta from fetal dehydroepiandrosterone (DHEA), the major product of fetal adrenal steroid synthesis (21). In PORD, 3 enzymatic reactions that are involved in the pathway leading to estriol synthesis are dependent on electron transfer by POR and thus may be compromised. First, mutant POR results in impaired 17-hydroxylase/17,20-lyase activity of CYP17A1. This consequently diminishes the production of DHEA and downstream androgens, thereby decreasing the amount of androgenic substrate available for conversion to estriol by aromatase (Figure 3). Second, steroid 16α-hydroxylase is required for the production of 16α-hydroxy-DHEA, but there is conflicting evidence as to whether it has diminished activity due to PORD. Finally, CYP19A1 aromatase activity itself could be impaired by mutant POR, further contributing to low estriol production, which again could show some variability in PORD with distinct mutants having been shown to have a differential impact on aromatase activity in vitro (15). Impaired aromatase activity would predictably result in an accumulation of its immediate precursor 16α-hydroxyandrostenedione and reflected in increased excretion of its metabolite 16α-hydroxyandrosterone. These markers were modestly raised in the pregnancies described here; however, similar to our previously reported case (17), these increases were certainly not in the range that would be expected in cases of aromatase deficiency due to CYP19A1 mutations. Thus, it appears that the major determinant of low estriol in PORD is reduced synthesis of DHEA due to impairment of 17,20-lyase activity.
Figure 3.
Simplified representation of steroid synthesis and metabolism in normal (A) and PORD pregnancies (B). The precise locations of certain transformations, for example 5PD to EpialloPD are unconfirmed. Chol, cholesterol; Preg, pregnenolone; 5PD, pregnenediol (5-pregnene-3β,20α-diol); Prog, progesterone; 16OH-DHEA-sulfate, 16-hydroxydehydroepiandrosterone sulfate; 16OH-Δ4, 16-hydroxyandrostenedione; 5α17HP, EpialloPD 17-hydroxyallopregnanolone (5α-pregnane-3α,17α-diol-20-one); An, androsterone; AD, androstanediol (5α-androstane-3α,17β-diol); DHT, 5α-dihydrotestosterone; 17OH-Preg, 17-hydroxypregnenolone; DHEA, dehydroepiandrosterone; 17OHP, 17-hydroxyprogesterone.
Low estriol on its own, however, is a nonspecific marker that can be associated with a number of conditions, including several single gene disorders underlying sterol and steroid synthesis defects such as Smith-Lemli-Opitz syndrome, steroid sulfatase deficiency, aromatase deficiency, and severe neonatal adrenal insufficiency due to mutations in NR0B1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1), NR5A1 (steroidogenic factor 1), CYP11A1 (P450 side chain cleavage enzyme), and TBX19 (TPIT, corticotroph specific T-box transcription factor) (22–35). However, here a distinct combination of low estriol and other fetal steroid markers enabled us to make the specific diagnosis of PORD based on the analysis of maternal urine at midpregnancy. Interestingly, normal estriol in the pregnancy with the heterozygous baby clearly distinguished this pregnancy from the PORD pregnancies. In this case the PORD specific ratios showed only very mild aberrations from the control cohort due to a normal estriol, suggesting that the baby was not affected by PORD. In addition to low estriol, a characteristic finding in the PORD pregnancies was the highly increased excretion of EpialloPD, a metabolite of fetal pregnenolone, which accumulates in PORD due to the concurrent impairment of CYP17A1 and CYP21A2 activities. Thus, in PORD EpialloPD essentially replaces estriol as the major terminal product of fetal adrenal steroid synthesis and metabolism (17). Consequently, the altered fetal synthesis and metabolism in PORD pregnancy is most effectively documented by measuring the urinary ratio of the abnormal end-product (EpialloPD) to the normal end-product (estriol). Importantly, we could identify all 4 prospectively monitored PORD cases by the above-described urinary steroid ratios despite an appreciable degree of biochemical variability observed in PORD. The identified ratios in our view reflect the key biochemical changes detectable in prenatal PORD cases. However, further studies in larger cohorts will be needed to further validate our findings. We should add that 2 other rare disorders, 17-hydroxylase deficiency and cytochrome b5 deficiency, are also associated with attenuated 17-hydroxylase/17,20 lyase and pregnenolone build-up and may therefore give similar results regarding the increased excretion of EpialloPD and low excretion of estriol. However, in both conditions the maternal urine androsterone to etiocholanolone ratio would be normal and not elevated as was observed in PORD.
In normal pregnancies the excretion of the 2 epimeric major androgen metabolites androsterone (5α-reduced) and etiocholanolone (5β-reduced) is approximately equivalent. Increased excretion of 5α-reduced androgens was demonstrated by the significantly elevated androsterone to etiocholanolone ratios in the PORD pregnancies reported here and also in the previously reported case (17), providing a clue to the cause of maternal and female fetal virilization in PORD. We have previously proposed that this is caused by increased activity of an alternative androgen biosynthetic pathway during fetal and early neonatal life (2), resulting in the increased production of androsterone and 5α-dihydrotestosterone. PORD results in an accumulation of 17α-hydroxypregnenolone and 17α-hydroxyprogesterone that can enter the alternative pathway (Figure 3). This pathway is accessed through a 5α-reduced 17-hydroxypregnanolone intermediate (3α,17α-dihydroxy-5α-pregnan-20-one) that is a substrate with higher affinity for the 17,20 lyase activity of CYP17A1 than the other 17-hydroxysteroids, 17α-hydroxypregnenolone and 17α-hydroxyprogesterone (36). In PORD, 5α-reduced 17-hydroxypregnanolone intermediate conversion by 17,20 lyase via the alternative pathway results in increased fetal production of androsterone, which in turn can be converted to active androgen in the fetoplacental unit, even while the conventional (classic) androgen synthesis pathway is largely blocked. It is important to note that the presence of maternal virilization was independent of genotype and other phenotypic characteristics (Table 1) and, for example, was observed in 1 affected child but not in its sibling with the same genotype (PORD pregnancy A). Future research will have to be conducted to reveal the underlying mechanisms explaining this disparity.
Most of the 20 patients we reported here underwent only routine prenatal ultrasound because no malformations were suspected prior to birth. Anatomic ultrasound performed in prenatal specialist centers in the second trimester has a reported detection rate of approximately 70%–90% for fetal congenital abnormalities (37, 38). Thus, one could assume that specialized malformation ultrasound may have detected skeletal abnormalities in the milder cases. However, this assumption contrasts with the longitudinally documented findings in a PORD pregnancy A in which the pregnancy was very closely monitored by specialist ultrasound after the birth of an index patient with multiple skeletal malformations of intermediate severity. However, prenatal ultrasound failed to detect the malformations in the second child, which were clearly visible after birth. Of note, in our cohort, prenatal ultrasound seemed to indicate disease severity and long-term outcome in PORD. All 5 patients with the highest malformation scores based on postnatal clinical assessment were found to have skeletal malformations during routine prenatal ultrasound examinations, whereas no malformations were detected in the 15 patients with clinically intermediate or mild malformation phenotypes. This would suggest that only the most severe PORD-related malformation phenotypes can be detected reliably by routine prenatal ultrasound, and according to our data, these are invariably associated with a poor prognosis, whereas children with intermediate, mild, or absent malformation phenotypes had a good clinical outcome.
The option of prenatal diagnosis always comes with ethical challenges and when deciding on the pros and cons of termination of pregnancy, it is important to consider the normal cognitive capacity and normal intellectual development in affected children irrespective of presence and severity of malformations. The parents of the child described as PORD pregnancy A asked for a prenatal diagnosis to ascertain whether they should temporarily move from a very rural area closer to a tertiary care hospital offering peri- and postpartal fetal medicine and pediatric specialist care. Fetal sexing using fetal cells circulating in maternal blood will become available in the foreseeable future. The parents of PORD pregnancy A considered prenatal dexamethasone treatment to avoid the virilization observed in their firstborn daughter, the index child. This girl carried homozygous A287P mutations associated with 46,XX DSD but normal genital phenotype in affected 46,XY individuals (8). In PORD it is highly likely that the major part of virilization is driven by the alternative androgen synthesis pathway (2). Thus, irrespective of other concerns regarding prenatal dexamethasone treatment, in a male fetus such a treatment is highly likely to significantly reduce the substrate flow to the alternative pathway and consequently result in undervirilization.
In conclusion, our data demonstrate that diagnosis of PORD can be established prenatally via GC/MS steroid analysis of maternal urine, with further data needed on diagnostic sensitivity and on the differentiation of fetuses with homozygous POR mutations from heterozygous carriers. Prenatal ultrasound is helpful in estimating the severity of the PORD-associated skeletal malformation phenotype and appears to correlate with long-term outcome. These 2 noninvasive diagnostic tools are of significant promise for the prenatal diagnosis of PORD in pregnancies affected by maternal virilization or fetal malformations and those occurring after the birth of an index child with PORD. This diagnostic approach will hopefully facilitate improved perinatal care and the timely involvement of a multidisciplinary specialist team including endocrine expertise. Although on an individual patient basis, urinary steroid profiling of pregnant women with low estriol in the triple or quadruple screening test is readily performed and a powerful tool for the early diagnosis of PORD, more routine and widespread use of the technique for identifying steroid or sterol synthesis disorders such as PORD, steroid sulfatase deficiency, and Smith-Lemli-Opitz syndrome in all low-estriol pregnancies is unlikely to occur because of the costs involved.
Acknowledgments
This work was supported by the Medical Research Council UK Program Grant 0900567, (to W.A.) and Research Fellowship G1001964 (to J.I.); the European Community's Seventh Framework Program (Marie Curie Intra-European Fellowship PIEF-GA-2008-221058 and Marie Curie European Reintegration Grant PERG-GA-2010-268270, to N.R.; Collaborative Research Project Grant EuroDSD FP7-GA-2008-201444 (to W.A.); and the Wellcome Trust (Clinician Scientist Fellowship GR079865MA, to N.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CYP
- cytochrome P450
- CYP17A1
- 17α-hydroxylase/17,20-lyase
- CYP19A1
- P450 aromatase
- CYP21A2
- 21-hydroxylase
- DHEA
- dehydroepiandrosterone
- DSD
- disorder of sex development
- EpialloPD
- epiallopregnanediol
- GC/MS
- gas chromatography/mass spectrometry
- PD
- pregnanediol
- POR
- P450 oxidoreductase
- PORD
- POR deficiency
- SDS
- SD score
- TOP
- termination of pregnancy.
References
- 1. Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arlt W, Walker EA, Draper N, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363:2128–2135 [DOI] [PubMed] [Google Scholar]
- 3. Fluck CE, Tajima T, Pandey AV, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36:228–230 [DOI] [PubMed] [Google Scholar]
- 4. Krone N, Dhir V, Ivison HE, Arlt W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol (Oxf). 2007;66:162–172 [DOI] [PubMed] [Google Scholar]
- 5. Huang N, Pandey AV, Agrawal V, et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76:729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukami M, Nishimura G, Homma K, et al. Cytochrome P450 oxidoreductase deficiency: identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. J Clin Endocrinol Metab. 2009;94:1723–1731 [DOI] [PubMed] [Google Scholar]
- 7. Idkowiak J, O'Riordan S, Reisch N, et al. Pubertal presentation in seven patients with congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2011;96:E453–E462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krone N, Reisch N, Idkowiak J, et al. Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2012;97:E257–E267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelley RI, Kratz LE, Glaser RL, Netzloff ML, Wolf LM, Jabs EW. Abnormal sterol metabolism in a patient with Antley-Bixler syndrome and ambiguous genitalia. Am J Med Genet. 2002;110:95–102 [DOI] [PubMed] [Google Scholar]
- 10. Andersson HC, Kratz L, Kelley R. Desmosterolosis presenting with multiple congenital anomalies and profound developmental delay. Am J Med Genet. 2002;113:315–319 [DOI] [PubMed] [Google Scholar]
- 11. Pursley TJ, Blomquist IK, Abraham J, Andersen HF, Bartley JA. Fluconazole-induced congenital anomalies in three infants. Clin Infect Dis. 1996;22:336–340 [DOI] [PubMed] [Google Scholar]
- 12. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253–256 [PubMed] [Google Scholar]
- 13. Lee BE, Feinberg M, Abraham JJ, Murthy AR. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062–1064 [PubMed] [Google Scholar]
- 14. Dhir V, Ivison HE, Krone N, et al. Differential inhibition of CYP17A1 and CYP21A2 activities by the P450 oxidoreductase mutant A287P. Mol Endocrinol. 2007;21:1958–1968 [DOI] [PubMed] [Google Scholar]
- 15. Pandey AV, Kempna P, Hofer G, Mullis PE, Fluck CE. Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase. Mol Endocrinol. 2007;21:2579–2595 [DOI] [PubMed] [Google Scholar]
- 16. Idkowiak J, Malunowicz EM, Dhir V, et al. Concomitant mutations in the P450 oxidoreductase and androgen receptor genes presenting with 46,XY disordered sex development and androgenization at adrenarche. J Clin Endocrinol Metab. 2010;95:3418–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shackleton C, Marcos J, Arlt W, Hauffa BP. Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): a disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley-Bixler syndrome phenotype. Am J Med Genet A. 2004;129A:105–112 [DOI] [PubMed] [Google Scholar]
- 18. Cragun DL, Trumpy SK, Shackleton CH, et al. Undetectable maternal serum uE3 and postnatal abnormal sterol and steroid metabolism in Antley-Bixler syndrome. Am J Med Genet A. 2004;129A:1–7 [DOI] [PubMed] [Google Scholar]
- 19. Lao MR, Calhoun BC, Bracero LA, et al. The ability of the quadruple test to predict adverse perinatal outcomes in a high-risk obstetric population. J Med Screen. 2009;16:55–59 [DOI] [PubMed] [Google Scholar]
- 20. Dugoff L, Hobbins JC, Malone FD, et al. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260–267 [DOI] [PubMed] [Google Scholar]
- 21. Auchus RJ, Rainey WE. Adrenarche—physiology, biochemistry and human disease. Clin Endocrinol (Oxf). 2004;60:288–296 [DOI] [PubMed] [Google Scholar]
- 22. Marcos J, Craig WY, Palomaki GE, et al. Maternal urine and serum steroid measurements to identify steroid sulfatase deficiency (STSD) in second trimester pregnancies. Prenat Diagn. 2009;29:771–780 [DOI] [PubMed] [Google Scholar]
- 23. Shackleton CH, Marcos J, Palomaki GE, et al. Dehydrosteroid measurements in maternal urine or serum for the prenatal diagnosis of Smith-Lemli-Opitz syndrome (SLOS). Am J Med Genet A. 2007;143A:2129–2136 [DOI] [PubMed] [Google Scholar]
- 24. Glass IA, Lam RC, Chang T, Roitman E, Shapiro LJ, Shackleton CH. Steroid sulphatase deficiency is the major cause of extremely low oestriol production at mid-pregnancy: a urinary steroid assay for the discrimination of steroid sulphatase deficiency from other causes. Prenat Diagn. 1998;18:789–800 [PubMed] [Google Scholar]
- 25. Wald N, Densem J, Kennard A. Antenatal screening for Down's syndrome. BMJ. 1992;305:1017–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wald N, Kennard A. The value of performing a dating scan on all women in antenatal screening for Down's syndrome and neural tube defects. Ultrasound Obstet Gynecol. 1993;3:3–5 [DOI] [PubMed] [Google Scholar]
- 27. Fang YM, Benn P, Campbell W, Bolnick J, Prabulos AM, Egan JF. Down syndrome screening in the United States in 2001 and 2007: a survey of maternal-fetal medicine specialists. Am J Obstet Gynecol 2009;201:97.e1–e5 [DOI] [PubMed] [Google Scholar]
- 28. Kim CJ, Lin L, Huang N, et al. Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab. 2008;93:696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malpuech G, Vanlieferinghen P, Dechelotte P, Gaulme J, Labbe A, Guiot F. Isolated familial adrenocorticotropin deficiency: prenatal diagnosis by maternal plasma estriol assay. Am J Med Genet. 1988;29:125–130 [DOI] [PubMed] [Google Scholar]
- 30. Lin L, Philibert P, Ferraz-de-Souza B, et al. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab. 2007;92:991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weintrob N, Drouin J, Vallette-Kasic S, et al. Low estriol levels in the maternal triple-marker screen as a predictor of isolated adrenocorticotropic hormone deficiency caused by a new mutation in the TPIT gene. Pediatrics. 2006;117:e322–e327 [DOI] [PubMed] [Google Scholar]
- 32. Marshall I, Ugrasbul F, Manginello F, et al. Congenital hypopituitarism as a cause of undetectable estriol levels in the maternal triple-marker screen. J Clin Endocrinol Metab. 2003;88:4144–4148 [DOI] [PubMed] [Google Scholar]
- 33. Pelissier P, Merlin E, Prieur F, et al. [Adrenal hypoplasia congenita: four new cases in children]. Arch Pediatr. 2005;12:380–384 [DOI] [PubMed] [Google Scholar]
- 34. Peter M, Partsch CJ, Dorr HG, Sippell WG. Prenatal diagnosis of congenital adrenal hypoplasia. Horm Res. 1996;46:41–45 [DOI] [PubMed] [Google Scholar]
- 35. Mullis PE, Yoshimura N, Kuhlmann B, Lippuner K, Jaeger P, Harada H. Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J Clin Endocrinol Metab. 1997;82:1739–1745 [DOI] [PubMed] [Google Scholar]
- 36. Gupta MK, Guryev OL, Auchus RJ. 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003;418:151–160 [DOI] [PubMed] [Google Scholar]
- 37. Nyberg DA, Souter VL. Sonographic markers of fetal trisomies: second trimester. J Ultrasound Med. 2001;20:655–674 [DOI] [PubMed] [Google Scholar]
- 38. Krakow D, Lachman RS, Rimoin DL. Guidelines for the prenatal diagnosis of fetal skeletal dysplasias. Genet Med. 2009;11:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]