Abstract
Inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene occurs in the majority of clear-cell renal cell carcinomas (RCC). It was previously shown that VHL decreased the abundance of integrin α2, α5 and β1 – consistent with VHL-associated changes in cell-cell and cell-extracellular matrix adhesions. We investigated the mechanism by which VHL down-regulates integrins. Although VHL can target hypoxia-inducible factor alpha (HIFα) subunits for degradation, VHL-dependent reduction of integrins was independent of O2 concentration and HIFα levels. VHL reduced the half-lives of integrins and this activity was blocked by proteasomal inhibition. Ectopic expression of Flag-VHL, while retaining activity for HIFα degradation, neither down-regulated integrins nor promoted adherens and tight intercellular junctions, in contrast to expression of wild-type VHL. Moreover, integrins co-immunoprecipitaed with wild type VHL, but not Flag-VHL. These data indicate that down-regulation of integrins by VHL is distinct from VHL’s regulation of HIF α subunits, and suggests that loss of this activity contributes to VHL-associated RCC development through disruption of adherens and tight junctions.
Keywords: VHL, integrin, adherens junctions, tight junctions, HIF
Introduction
Disruption of the VHL tumor suppressor gene causes a hereditary cancer syndrome characterized by clear-cell renal cell carcinomas (RCC), hemangioblastomas within the central nervous system and retina, and pheochromocytomas. Biallelic VHL inactivation also occurs in the majority of sporadic RCC (Kim and Kaelin, 2004; Kaelin, 2007). Moreover, ectopic expression of VHL in VHL-negative RCC cells inhibits their ability to form tumors in nude mice (Iliopoulos et al., 1995; Schoenfeld et al., 1998). Two native, biologically active VHL gene products have been previously identified as VHLp24(MPR) and VHLp18(MEA) (Schoenfeld et al., 1998).
At least one function of VHL is as a component of an E3 ubiquitin ligase complex that targets subunits of hypoxia-inducible factor α (HIFα) for ubiquitin-mediated proteasomal degradation under normoxic conditions (Maxwell et al., 1999; Kim and Kaelin, 2004). Nevertheless, genotype-phenotype correlations in VHL disease suggest that VHL has HIF-independent functions (Hoffman et al., 2001; Kim and Kaelin, 2004). Current studies indicate that both HIF-dependent and HIF-independent pathways are essential for VHL’s role as a tumor suppressor (Hoffman et al., 2001; Kondo et al., 2003; Kim and Kaelin, 2004; Stickle et al., 2004).
We previously demonstrated that VHL directs renal cells to form organized epithelial-like morphology through cell-cell and cell-extracellular matrix (ECM) signaling (Davidowitz et al., 2001). Cell-ECM signaling is primarily mediated by integrins (Giancotti and Ruoslahti, 1999). Integrins are cellular adhesion receptors that mediate cell binding to extracellular matrix and adjacent cells (Giancotti and Ruoslahti, 1999; Hynes, 2002). Each integrin is a heterodimeric molecule consisting of one α and one β subunit. Integrin signaling influences cell survival, proliferation, migration and differentiation (Giancotti and Ruoslahti, 1999; Hynes, 2002; Guo and Giancotti, 2004). In general, tumor cells frequently overexpress integrins (Guo and Giancotti, 2004) and specifically the clear cells of renal cysts and carcinomas have elevated integrin levels compared to normal proximal tubule cells (Paraf et al., 2000). Previous data showed that reintroduction of VHL into VHL-negative RCC cells resulted in reduced levels of integrins (Davidowitz et al., 2001).
Several research groups have demonstrated that integrin engagement disrupted intercellular adherens junctions (Kawano et al., 2001; Wang et al., 2006). In renal epithelial cells, adherens junctions and tight junctions are closely interrelated (Calzada et al., 2006). The formation and maintenance of tight junctions are generally dependent on the formation and maintenance of adherens junctions (Yamada et al., 2006). These junctions play key roles not only in epithelial cell adhesion and polarization, but also in their movement and proliferation (Yamada et al., 2006). The junctional complex is impaired in many cancer cells, and this alteration is associated with tumor initiation and progression (Cavallaro and Christofori, 2004; Calzada et al., 2006). VHL is required for proper assembly of adherens and tight intercellular junctions in renal cancer cells (Calzada et al., 2006).
In the current study, we investigated the mechanisms by which VHL down-regulates integrins. Our results show that VHL down-regulates integrins through an HIF-independent mechanism. Furthermore, down-regulation of integrins by VHL was associated with the restoration of adherens and tight junctions in renal cells.
Materials and Methods
Cell lines and culture
Parental 786-0 cells and cell lines stably expressing empty vector pCR3 (Invitrogen, Carlsbad, CA), VHLp24(MPR), VHLp18(MEA) and Flag-VHLp24(MPR) have been previous described (Schoenfeld et al., 1998; Schoenfeld et al., 2000). Flag-VHLp18(MEA) containing a FLAG epitope at the N-terminal of VHLp18 was produced as previously described (Schoenfeld et al., 2000) and used to generate stable expression in 786-0 and A498 cells. HIF2α knockdowns were created using retroviral supernatants containing HIF2α shRNA #2+#3 generously provided by Dr. William G. Kaelin, Jr., Harvard Medical School, Boston, MA (Kondo et al., 2003). Parental A498 cells and subclones stably expressing either empty vector (pLNCX2) or VHLp18(MEA) have been previously described (Lutz and Burk, 2006). The cell line RCC10 and its derived VHL expressing line RCC90 were generously provided by Dr. Miguel Esteban, Imperial College London, London, United Kingdom (Esteban et al., 2006a). All cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum.
For experiments with cells cultured under hypoxic condition, cells were incubated for 16 h in BD BBL™ pouches (BD Biosciences, San Jose, CA) to achieve <2% oxygen or treated for 16 h with 250 µM Cobalt Chloride (CoCl2, Sigma, St. Louis, MO) to achieve chemical hypoxia (Bluyssen et al., 2004).
For determination of protein half-life, cells were treated with 50 µg/ml cycloheximide (CHX, Sigma) to inhibit new protein synthesis. To block proteasomal degradation, cells were incubated for 6 h with 10 µM MG132 (EMD Biosciences, Darmstadt, Germany) dissolved in DMSO.
Antibodies
Mouse anti-VHL monoclonal antibody 11E12 has been previous described (Schoenfeld et al., 1998). Rabbit anti-VHLp24(MPR) polyclonal antibody 888 was generated using a synthetic peptide corresponding to amino acids 1–14 of the VHL coding region. This antibody only recognizes VHLp24(MPR) and not VHLp18(MEA). Mouse antibodies recognizing integrin α2 (clone 2), integrin α5 (clone 1), integrin β1 (clone 18), fibronectin (clone 10), HIF-1α (clone 54), Cul-2 (clone 4) and ZO-1 (clone 1) were obtained from BD Biosciences. Goat anti-integrin α3 (C-18) and mouse anti-p21 (C-19) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-GLUT-1 (GT12-A) antibody was purchased from Alpha Diagnostic International (San Antonio, TX), rabbit anti-HIF-2α antibody from Novus Biologicals (Littleton, CO), and rabbit anti-β-catenin and mouse anti-β-tubulin (clone TUB 2.1) antibodies were from Sigma.
Western blots
Western blot experiments were performed as previously described (Davidowitz et al., 2001). Briefly, cells were lysed in RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA) (Upstate, Lake Placid, NY) supplemented with 200 µM phenylmethylsulfonyl fluoride (PMSF) and 1× complete mini protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) for 30 min at 4°C. Protein concentrations were determined by the Bradford assay (Bio-Rad, Hercules, CA). Lysates were then adjusted to load equal amounts per lane.
Co-immunoprecipitation
786-0 cells ectopically expressing VHLp24(MPR) were grown in 100-mm culture dishes and were lysed 9 days after reaching confluence in 0.5 ml of ELB buffer (50 mM Hepes, pH 7.6, 250 mM NaCl, 0.75% deoxycholic acid, 0.1% NP-40, 5 mM EDTA, 1 mM PMSF, 1 µg/ml each of aprotinin, pepstatin, bestatin, and leupeptin) for 30 min at 4°C. All subsequent steps were performed at 4°C. Lysates were clarified by centrifugation at 20,000 ×g for 15 min. A 400 µl aliquot of clarified lysate was incubated overnight with 4 µl of the rabbit anti-VHL(VHLp24) polyclonal antibody 888. Immune complexes were collected on protein G Plus-Agarose beads (Santa Cruz Biotechnology). Similarly, 786-0 cells ectopically expressing Flag-VHLp24(MPR) were lysed, clarified and then incubated overnight with anti-FLAG M2-agarose beads (Sigma). Immune complexes were washed four times with lysis buffer, boiled in SDS loading buffer, and separated by SDS-PAGE. VHL and coimmunoprecipitating proteins were visualized by Western blot analysis.
Immunofluorescence microscopy
Methods for immunofluorescence microscopy have been previously described (Lutz and Burk, 2006). Briefly, cells were plated on coverslips, fixed in 2% paraformaldehyde and extracted with 0.1% saponin (Sigma). Coverslips were incubated with primary antibodies: rabbit anti-β-catenin (1:800) and mouse anti-ZO-1 (1:50). After three washes in PBS (Sigma), secondary antibodies (Alexa Fluor 488 goat anti-mouse IgG and 568 goat anti-rabbit IgG; Molecular Probes, Carlsbad, CA) were used at 1:500. Samples were analyzed by wide-field, fluorescence microscopy using a Zeiss Axiovert 200M with Apotome for optical sectioning (Zeiss, Thornwood, NY) and a 63× 1.4 n.a. oil objective. Images were collected using a high-resolution AxioCam MRm digital camera and Zeiss Axiovision software.
Results
VHL down-regulates integrins through a hypoxia-inducible factor-independent mechanism
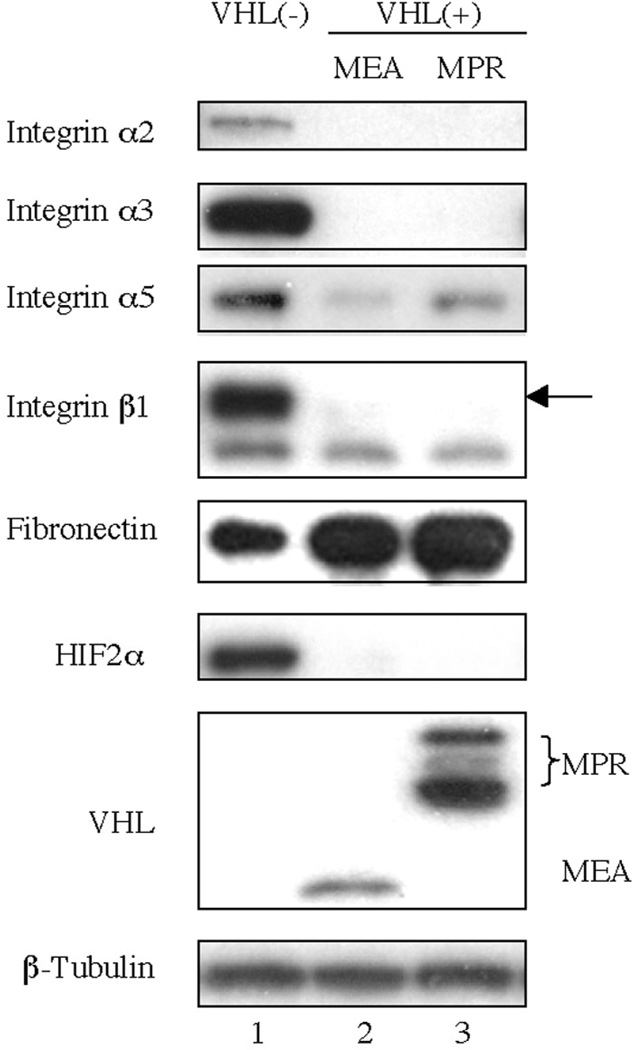
To study the mechanism by which VHL down-regulates integrins, we first determined integrin and fibronectin protein levels in 786-0 cells with and without VHL expression (Fig. 1). Increased fibronectin levels correlated with expression of VHL (MEA or MPR); whereas, levels of integrin α2, α3, α5 and β1 were reduced.
Fig. 1.
VHL decreases integrins and increase fibronectin levels. 786-0 cells stably expressing either empty vector pCR3, VHLp18(MEA) or VHLp24(MPR) were harvested 3 days after reaching confluence. Cell lysates were immunoblotted with the antibodies indicated to the left of the blots. VHL was detected using mAb 11E12. β1. β-tubulin was used to confirm equal protein loading.
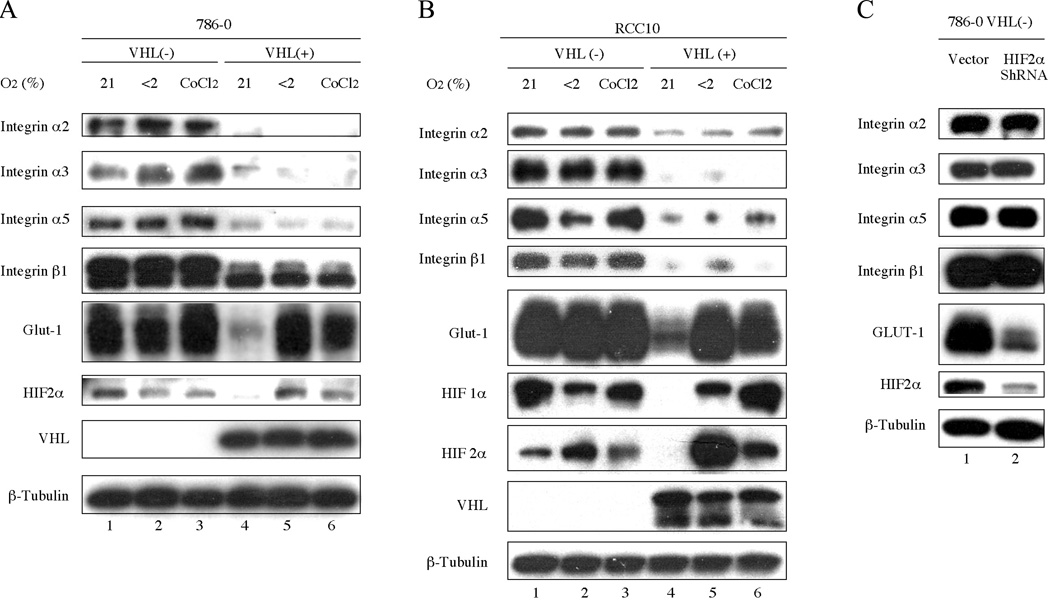
To evaluate the effect of hypoxia and HIFα levels on the expression of integrins, cells were cultured under normoxic or hypoxic conditions. As expected, HIF-2α levels were increased under hypoxic conditions in 786-0 (VHL+) cells (Fig. 2A, lanes 5 and 6) with a concomitant increase in levels of GLUT-1, an HIF target. However, 786-0 (VHL+) cells cultured under hypoxic conditions continued to have low levels of integrin α2, α3, α5 and β1 compared to the levels in 786-0 (VHL−) cells. In addition, observation by phase microscope indicated 786-0 (VHL+) cells maintained epithelial-like morphology under hypoxic conditions (data not shown).
Fig. 2.
Integrin expression is not influenced by hypoxia. (A) VHL-negative 786-0 cells stably expressing pCR3 and VHL-positive 786-0 cells stably expressing VHLp18(MEA). (B) VHL-negative RCC10 cells and VHL-positive RCC10 cells stably expressing VHL were grown in the presence of 21% O2, <2% O2 or 250 µM CoCl2 for 16 h. (C) 786-0 cells were infected with the empty parental retrovirus or retrovirus encoding HIF-2α shRNA. All cells were harvested 3 days after reaching confluence. Cell lysates were immunoblotted with the antibodies indicated to the left of the blots. β-tubulin was used to confirm equal protein loading.
To determine whether these results were generalizable to other VHL(−) renal carcinoma cell lines, A498 (data not shown) and RCC10 cells were analyzed (Fig. 2B). Similarly, hypoxia had no discernable affect on integrin levels. Since RCC10 cells express both HIF-1α and HIF-2α, this VHL activity is not limited to cells lacking HIF-1α, such as 786-0 and A498 cells.
To confirm the HIF-independent regulation of integrins by VHL, HIF-2α was knockdown in 786-0 cells using short hairpin RNAs (shRNA). As shown in Fig. 2C, HIF-2α shRNA knockdown decreased levels of HIF-2α and GLUT-1. Despite reduced levels of HIF-2α by shRNA, the levels of integrin α2, α3, α5 and β1 remained unchanged. Thus, VHL down-regulation of integrins operates through an HIF-independent mechanism in different cell lines and shown by use of hypoxia and HIF-2α shRNA knockdown.
Integrins undergo VHL-dependent proteasomal degradation
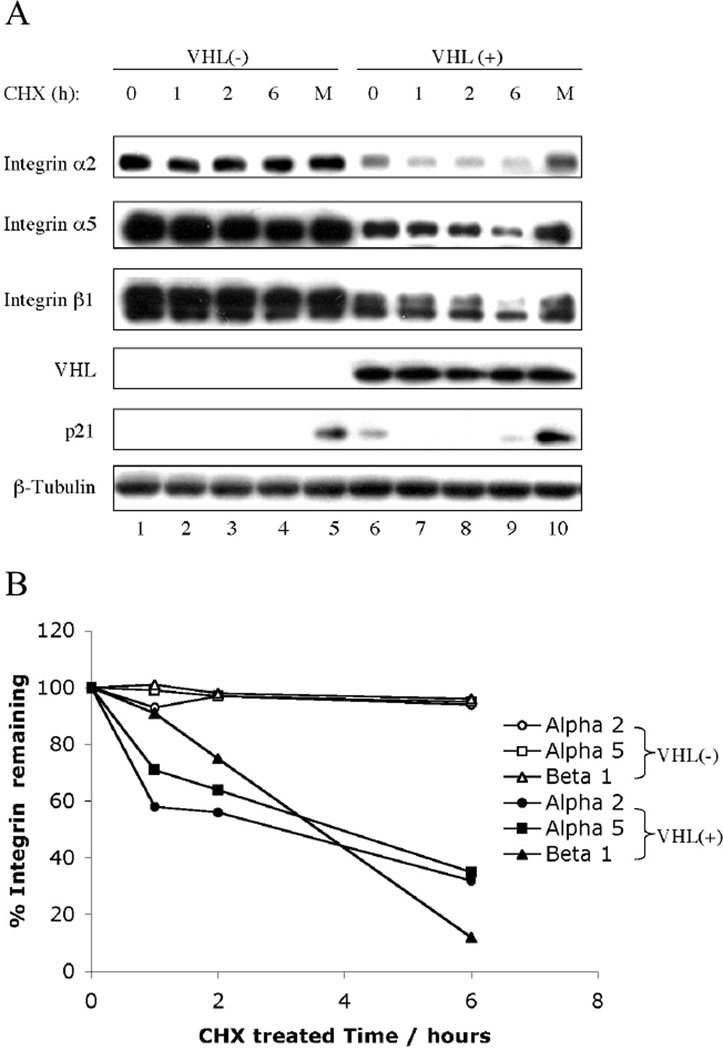
We next determined the effect of VHL on integrin stability. We examined integrin levels after treatment with cycloheximide (CHX). The integrin levels in 786-0 (VHL−) cells were stable over 6 hours and MG132 did not increase their levels (Fig. 3A, lanes 1–5). In contrast, integrin α2, α5 and β1 in 786-0 (VHL+) cells demonstrated a decreased half-life when protein synthesis was blocked by CHX treatment (Fig. 3A, lanes 6–9). MG132 treatment restored integrin protein levels to those present in untreated 786 (VHL+) cells (Fig. 3A, compare lane 10 to lane 6). Integrin α2, α5 and β1 levels were quantitated (Fig. 3B) and all had half-lives of 3–4 hours in the presence of VHL compared to >6 hours in VHL-negative lines. These data support the notion that integrins undergo VHL-dependent proteasomal degradation.
Fig. 3.
VHL leads to increased proteasome-dependent degradation of integrins. (A) Confluent VHL-negative 786-0 cells containing pCR3 and VHL-positive 786-0 cells ectopically expressing VHLp18(MEA) were treated with cycloheximide (CHX) for the times indicated about each blot or with both CHX and MG132 for 6 h. Cell lysates were separated in a 4~20% SDS-PAGE gel and immunoblotted with antibodies indicated to the left of the blots. Integrin α3 is undetectable in 786 (VHL+) cells (see Fig. 1). p21, a short-lived proteasomally degraded protein, was used as a positive control for CHX/MG132 treatment. (B) Integrin band intensities were quantified by IMAGEUANT 5.0 (Molecular Dynamics, Sunnyvale, CA) and are graphically represented.
Down-regulation of integrins by VHL is associated with the restoration of renal cell adherens junctions and tight junctions
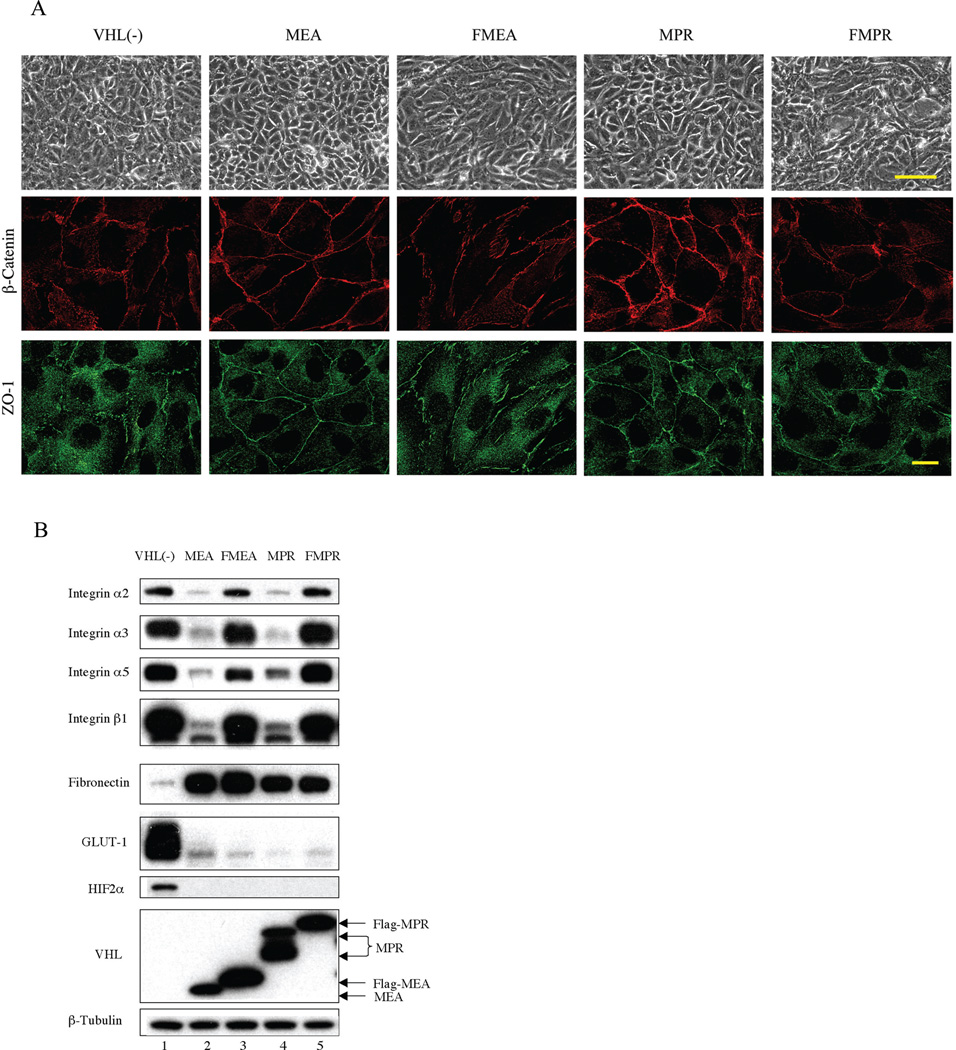
Ectopic expression of either VHLp24(MPR) or VHLp18(MEA) in 786-0 cells resulted in a monolayer of polygonal cells with characteristics of differentiated epithelia. However, ectopic expression of either Flag-MEA (FMEA) or Flag-MPR (FMPR) in 786-0 cells did not restore a differentiated epithelial morphology (Fig. 4A, top row). To determine whether adherens junctions and tight junctions were reestablished in cells containing wild type or epitope-tagged VHL, we examined β-catenin (adherens junctions marker) and ZO-1 (tight junctions marker) localization (Fig. 4A, middle and lower row). VHL-positive 786-0 (MEA) and 786-0 (MPR) cells showed intense staining of β-catenin at cell-cell contact boundaries. ZO-1 also displayed a preferential distribution along the cell periphery. In contrast, staining of β-catenin and ZO-1 in VHL-negative 786-0 (pCR3) cells was more randomly dispersed. 786-0 (FMEA) and 786-0 (FMPR) cells showed a more discontinuous and less focused staining pattern of β-catenin and ZO-1 compared with 786-0 (MEA) and 786-0 (MPR) cells. These results indicate that Flag-VHL failed to promote intercellular junctions in 786-0 cell.
Fig. 4.
Ectopic expression of VHL, but not Flag-VHL induces intercellular junctions and down-regulates integrin expression. (A) VHL-negative 786-0 cells containing pCR3 and 786-0 cells ectopically expressing VHLp18(MEA), Flag-VHLp18(MEA) (FMEA), VHLp24(MPR) or Flag- VHLp24(MPR) (FMPR) were grown on coverslips for 3 days post-confluence. Phase contrast images are shown in the top panels. Bar, 100 µm. Cells were then costained for β-catenin (middle row) and ZO-1 (lower row). Images were captured using a high-resolution AxioCam MRm digital camera on a Zeiss Axiovert 200M microscopy. Bar, 20 µm. (B) Western blot of cell extracts from cell lines shown in panel A. Cells were harvested 3 days post confluence and immunoblotted with antibodies indicated to the left of the blots. β-tubulin was used to confirm equal protein loading.
Since VHL epitope-tagged with Flag has been previously used for biochemical studies (Yu et al., 2001; Roe et al., 2006), we confirmed Flag-VHL activity by Western blotting (Fig. 4B). Both Flag-MEA and Flag-MPR containing cells led to degradation of HIF-2α, as well as decreased levels of GLUT-1. Flag-MEA and Flag-MPR also resulted in increased levels of fibronectin, similar to MEA and MPR. Strikingly, both Flag-MEA and Flag-MPR failed to down-regulate integrin levels. The ability of Flag-VHL to induce degradation of HIF-2α was not associated with the reappearance of adheres junctions and tight junctions.
To determine whether this phenomenon was generalizable to other renal-derived cells, we also examined VHL construct expression in A498 cells and RCC10 cells. Flag-VHL, while restoring the regulation of HIF-α and fibronectin, neither down-regulated integrin levels nor restored adherens and tight junctions (data not shown). These results indicate that down-regulation of integrins by wild type VHL is associated with the restoration of adherens and tight junctions in renal cells.
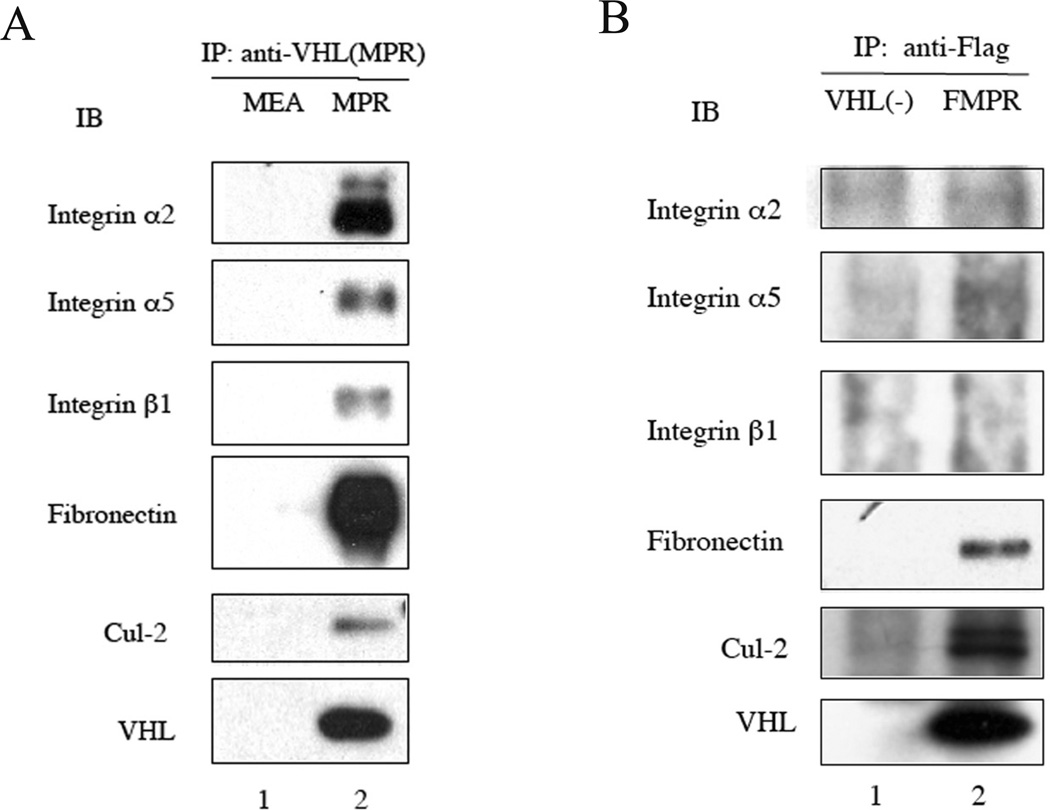
Integrins co-immunoprecipitate with wild type VHL
Since integrins undergo VHL-dependent proteasomal degradation, whereas Flag-VHL lacks this activity, we hypothesized that integrins would associate with VHL but not Flag-VHL. To test this hypothesis, 786-0(MPR) cells were lysed and immunoprecipitated with rabbit anti-VHLp24(MPR) polyclonal Ab888. Ab888 only recognizes the VHLp24(MPR) form. Immunoprecipitation of VHLp24(MPR) pulled down known VHL binding partners: Cul-2 and fibronectin, as well as integrin α2, α5 and β1 (Fig. 5A, lane 2). In contrast, no integrins were detected in immunoprecipitates from 786-0 (MEA) cells, since Ab888 does not recognize VHLp18(MEA). Integrin α2, α5 and β1 also co-immunoprecipitated with native VHL in the renal-derived 293T cells (data not shown), indicating the interaction between integrins and VHL was not related to heterologous expression.
Fig. 5.
Integrins co-immunoprecipitate with VHL but not Flag-VHL. (A) 786-0 cells stably expressing VHLp18(MEA) or VHLp24(MPR) cells were harvested after reaching confluence and cell lysates were immunoprecipitated with rabbit anti-VHLp24(MPR) polyclonal antibody 888. Immunoprecipitates were collected on protein G plus-Agarose beads. (B) VHL-negative 786-0 cells containing pCR3 and 786-0 cells ectopically expressing Flag-VHLp24(MPR) (FMPR) were harvested after reaching confluence and cell lysates were immunoprecipitated with anti-FLAG M2-Agarose. Immunoprecipitates were immunoblotted with antibodies corresponding to the proteins shown to the left of the blots. VHL was detected using mAb 11E12.
Cellular extracts of 786-0 (Flag-MPR) cells were then immunoprecipitated with anti-flag M2 agarose beads followed by immunoblotting. Flag-MPR immunoprecipitated VHL binding partners: Cul-2 and fibronectin, but not integrin α2, α5 and β1 (Fig. 5B, lane 2).
Discussion
In this report, we investigated the mechanism by which VHL down-regulates integrins in RCC cells. We show that HIFα levels were neither associated with VHL-mediated integrin levels nor intercellular junctions. VHL-mediated reduced steady state levels of integrins was due to decreased half-life that was blocked by proteasome inhibition and mechanistically correlated with the co-immunoprecipitation between VHL and integrins. Ectopic expression of Flag-MPR or Flag-MEA in multiple VHL-negative RCC cell lines restored HIFα regulation, yet neither down-regulated integrins nor promoted assembly of intercellular junctions. This infers that Flag disrupts the structural requirements of VHL for some activities, but not the ubiquitin ligase activity for HIFα.
The best-documented function of VHL is targeting subunits of hypoxia-inducible factor α (HIFα) for proteasomal degradation (Maxwell et al., 1999; Kim and Kaelin, 2004). VHL’s activities that are independent of HIF regulation include assembly of extracellular fibronectin and collagen IV matrix, assembly of intercellular adherens junctions and tight junctions, promotion of cell differentiation, and cilia production (Hoffman et al., 2001; Bluyssen et al., 2004; Calzada et al., 2006; Lutz and Burk, 2006; Grosfeld et al., 2007). Gene expression studies also indicated that VHL influences abundant HIF-independent target genes (Wykoff et al., 2000; Bluyssen et al., 2004; Abdulrahman et al., 2007). Additionally, VHL is involved in several cellular processes for which a link to HIFα is unclear, such as regulation of cytoskeletal stability (Hergovich et al., 2003) and cell-cycle arrest (Davidowitz et al., 2001).
VHL directs renal cells to form organized epithelial-like morphology, adherens junctions and tight junctions. This event is independent of HIFα alteration, cell cycle effects and HGF signaling (Davidowitz et al., 2001; Stickle et al., 2004; Calzada et al., 2006). Blocking fibronectin matrix assembly in 786-0 cells ectopically expressing VHL had no influence on cell monolayer organization, tight junction assembly and paracellular permeability (Davidowitz et al., 2001; Calzada et al., 2006). A role for E-cadherin has been proposed for assembly of adherens junctions; however, no E-cadherin protein was detected in 786-0 cells ectopically expressing VHL. Nevertheless, ectopic expression of VHL in 786-0 cells promotes the assembly of intercellular junctions (Davidowitz et al., 2001; Calzada et al., 2006). E-cadherin is also unnecessary for the formation of tight junctions in MDCK cells (Yamada et al., 2006). Furthermore, restoration of E-cadherin expression by VHL in two other VHL-defective cell lines (RCC4 and RCC10) was recently found to be mediated via HIF (Esteban et al., 2006b). Thus, VHL regulation of E-cadherin and fibronectin matrix is unlikely to be responsible for the assembly of adherens and tight intercellular junctions in renal cells.
Several research groups have demonstrated that integrin engagement induced the disruption of intercellular adherens junctions (Kawano et al., 2001; Wang et al., 2006). Here we showed that wild type VHL down-regulated integrins and promoted adherens junctions and tight junctions; whereas, Flag-VHL neither down-regulated integrins nor induced adherens junctions or tight junctions. These results indicate VHL-mediated assembly of adherens junctions and tight junctions is associated with down-regulation of integrins.
VHL mRNA is abundantly expressed within the epithelial lining of proximal tubules in the kidney during embryogenesis, consistent with a role for VHL in renal proximal tubular differentiation (Kessler et al., 1995). A neddylation-defective VHL mutant, while restoring the regulation of HIF, was reported to fail to promote cell morphologic differentiation and was insufficient to suppress tumor formation in nude mice (Stickle et al., 2004).
VHL induces renal cell differentiation through cell-cell and cell-extracellular matrix (ECM) signaling (Davidowitz et al., 2001). Cell-ECM signaling is primarily mediated by integrins (Giancotti and Ruoslahti, 1999). Since integrin signaling influences cell survival, proliferation, migration and differentiation (Giancotti and Ruoslahti, 1999; Hynes, 2002; Guo and Giancotti, 2004), we reasoned that integrins may contribute to VHL-mediated renal cell differentiation. This notion is supported by the observation that clear cells of renal cysts and carcinomas have elevated integrin levels compared to normal proximal tubule cells (Paraf et al., 2000). Moreover, poorly differentiated RCC tumors have increased expression levels of integrins when compared to normal kidney tissues or differentiated RCC tumors (Anastassiou et al., 1995).
No differences in mRNA levels of integrin α2, α3, α5 and β1 were observed in previous gene expression studies comparing VHL-negative and positive 786-0 cells (Wykoff et al., 2000; Bluyssen et al., 2004; Abdulrahman et al., 2007). Moreover, of 113 genes identified as having differential gene expression in at least three of six independent microarray studies comparing RCC tumors and normal kidney tissue, no changes in integrin expression was reported (Lenburg et al., 2003). In addition, recent gene expression data showed 786-0 cells ectopically expressing MPR or Flag-MPR have similar mRNA levels of integrin α2, α3, α5 and β1 (Q. Ji and R.D. Burk, unpublished data). These observations support the results presented in this report that VHL regulates integrins at a post-transcriptional level. Furthermore, we showed that integrins in 786-0 cells ectopically expressing VHL had markedly reduced half-lives after CHX treatment that was restored with proteasome inhibition. This suggests a mechanism whereby VHL might interact with one or more of the integrins, targeting them for proteasomal degradation. In support of this, integrin α2, α5 and β1 specifically co-immunoprecipitated with wild type VHL but not Flag-VHL.
How does VHL physically associate with integrins? First, VHL may directly bind to integrin α2, α5 or β1. Since VHL physically interacts with fibronectin and collagen IV, the ligands of integrins (Ohh et al., 1998; Grosfeld et al., 2007), the interaction between VHL and integrins may be indirect. Although, Flag-VHL immunoprecipitated fibronectin, but not integrins.
In conclusion, this report shows that VHL down-regulates integrins through an HIF-independent mechanism and this event is associated with restoration of adherens and tight junctions. These data support the notion that at least two distinct and independent pathways (degradation of HIF and down-regulation of integrins) contribute to VHL’s role as a tumor suppressor.
Acknowledgements
This work was supported by grant CA85412 from National Cancer Institute. We thank Dr. Mallory S. Lutz for assistance with microscopy techniques and critical review of this manuscript, Andrew Prior for assistance with co-immunoprecipitation.
Contributor Information
Qingzhou Ji, Department of Microbiology & Immunology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, New York, 10461, USA.
Robert D. Burk, Department of Pediatrics, Microbiology & Immunology, Epidemiology & Population Health, Obstetrics, Gynecology & Women’s Health, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, New York, 10461, USA.
References
- Abdulrahman M, Maina EN, Morris MR, Zatyka M, Raval RR, Banks RE, Wiesener MS, Richards FM, Johnson CM, Latif F, Maher ER. Identification of novel VHL targets that are associated with the development of renal cell carcinoma. Oncogene. 2007;26:1661–1672. doi: 10.1038/sj.onc.1209932. [DOI] [PubMed] [Google Scholar]
- Anastassiou G, Duensing S, Steinhoff G, Kirchner H, Ganser A, Atzpodien J. In vivo distribution of integrins in renal cell carcinoma: integrin-phenotype alteration in different degrees of tumor differentiation and VLA-2 involvement in tumor metastasis. Cancer Biother. 1995;10:287–292. doi: 10.1089/cbr.1995.10.287. [DOI] [PubMed] [Google Scholar]
- Bluyssen HA, Lolkema MP, van Beest M, Boone M, Snijckers CM, Los M, Gebbink MF, Braam B, Holstege FC, Giles RH, Voest EE. Fibronectin is a hypoxia-independent target of the tumor suppressor VHL. FEBS Lett. 2004;556:137–142. doi: 10.1016/s0014-5793(03)01392-9. [DOI] [PubMed] [Google Scholar]
- Calzada MJ, Esteban MA, Feijoo-Cuaresma M, Castellanos MC, Naranjo-Suarez S, Temes E, Mendez F, Yanez-Mo M, Ohh M, Landazuri MO. von Hippel-Lindau tumor suppressor protein regulates the assembly of intercellular junctions in renal cancer cells through hypoxia-inducible factor-independent mechanisms. Cancer Res. 2006;66:1553–1560. doi: 10.1158/0008-5472.CAN-05-3236. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Davidowitz EJ, Schoenfeld AR, Burk RD. VHL induces renal cell differentiation and growth arrest through integration of cell-cell and cell-extracellular matrix signaling. Mol. Cell. Biol. 2001;21:865–874. doi: 10.1128/MCB.21.3.865-874.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban MA, Harten SK, Tran MG, Maxwell PH. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J. Am. Soc. Nephrol. 2006a;17:1801–1806. doi: 10.1681/ASN.2006020181. [DOI] [PubMed] [Google Scholar]
- Esteban MA, Tran MG, Harten SK, Hill P, Castellanos MC, Chandra A, Raval R, O'Brien TS, Maxwell PH. Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res. 2006b;66:3567–3575. doi: 10.1158/0008-5472.CAN-05-2670. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Grosfeld A, Stolze IP, Cockman ME, Pugh CW, Edelmann M, Kessler B, Bullock AN, Ratcliffe PJ, Masson N. Interaction of hydroxylated collagen IV with the von hippel-lindau tumor suppressor. J. Biol. Chem. 2007;282:13264–13269. doi: 10.1074/jbc.M611648200. [DOI] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumor progression. Nat. Rev. Mol. Cell. Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumor suppressor protein pVHL. Nat. Cell. Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG., Jr von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum. Mol. Genet. 2001;10:1019–1027. doi: 10.1093/hmg/10.10.1019. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O, Kibel A, Gray S, Kaelin WG., Jr Tumor suppression by the human von Hippel-Lindau gene product. Nat. Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res. 2007;13:680s–684s. doi: 10.1158/1078-0432.CCR-06-1865. [DOI] [PubMed] [Google Scholar]
- Kawano K, Kantak SS, Murai M, Yao CC, Kramer RH. Integrin alpha3beta1 engagement disrupts intercellular adhesion. Exp. Cell. Res. 2001;262:180–196. doi: 10.1006/excr.2000.5083. [DOI] [PubMed] [Google Scholar]
- Kessler PM, Vasavada SP, Rackley RR, Stackhouse T, Duh FM, Latif F, Lerman MI, Zbar B, Williams BR. Expression of the Von Hippel-Lindau tumor suppressor gene, VHL, in human fetal kidney and during mouse embryogenesis. Mol. Med. 1995;1:457–466. [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J. Clin. Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenburg ME, Liou LS, Gerry NP, Frampton GM, Cohen HT, Christman MF. Previously unidentified changes in renal cell carcinoma gene expression identified by parametric analysis of microarray data. BMC Cancer. 2003;3:31. doi: 10.1186/1471-2407-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MS, Burk RD. Primary cilium formation requires von hippel-lindau gene function in renal-derived cells. Cancer Res. 2006;66:6903–6907. doi: 10.1158/0008-5472.CAN-06-0501. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Ohh M, Yauch RL, Lonergan KM, Whaley JM, Stemmer-Rachamimov AO, Louis DN, Gavin BJ, Kley N, Kaelin WG, Jr, Iliopoulos O. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol. Cell. 1998;1:959–968. doi: 10.1016/s1097-2765(00)80096-9. [DOI] [PubMed] [Google Scholar]
- Paraf F, Chauveau D, Chretien Y, Richard S, Grunfeld JP, Droz D. Renal lesions in von Hippel-Lindau disease: immunohistochemical expression of nephron differentiation molecules, adhesion molecules and apoptosis proteins. Histopathology. 2000;36:457–465. doi: 10.1046/j.1365-2559.2000.00857.x. [DOI] [PubMed] [Google Scholar]
- Roe JS, Kim H, Lee SM, Kim ST, Cho EJ, Youn HD. p53 stabilization and transactivation by a von Hippel-Lindau protein. Mol. Cell. 2006;22:395–405. doi: 10.1016/j.molcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Schoenfeld A, Davidowitz EJ, Burk RD. A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8817–8822. doi: 10.1073/pnas.95.15.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld AR, Davidowitz EJ, Burk RD. Elongin BC complex prevents degradation of von Hippel-Lindau tumor suppressor gene products. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8507–8512. doi: 10.1073/pnas.97.15.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickle NH, Chung J, Klco JM, Hill RP, Kaelin WG, Jr, Ohh M. pVHL modification by NEDD8 is required for fibronectin matrix assembly and suppression of tumor development. Mol. Cell. Biol. 2004;24:3251–3261. doi: 10.1128/MCB.24.8.3251-3261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jin G, Miao H, Li JY, Usami S, Chien S. Integrins regulate VE-cadherin and catenins: dependence of this regulation on Src, but not on Ras. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1774–1779. doi: 10.1073/pnas.0510774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumor suppressor by mRNA differential expression profiling. Oncogene. 2000;19:6297–6305. doi: 10.1038/sj.onc.1204012. [DOI] [PubMed] [Google Scholar]
- Yamada A, Fujita N, Sato T, Okamoto R, Ooshio T, Hirota T, Morimoto K, Irie K, Takai Y. Requirement of nectin, but not cadherin, for formation of claudin-based tight junctions in annexin II-knockdown MDCK cells. Oncogene. 2006;25:5085–5102. doi: 10.1038/sj.onc.1209525. [DOI] [PubMed] [Google Scholar]
- Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]