Abstract
Cardiovascular disease (CVD) is the primary cause of death in Korea. Hyperhomocysteinemia confers an independent risk for CVD comparable to the risk of smoking and hyperlipidemia. The purpose of this study was to assess the effect of cardiovascular risk factors and body composition change on homocysteine (Hcy) levels in Korean men and women. The association between body composition and Hcy levels was investigated in a 2-yr prospective cohort study of 2,590 Koreans (mean age 45.5±9.6 yr). There were 293 cases of hyperhomocysteinemia (>14 µM/L) at follow-up. Increases in total body fat proportion and decreases in lean body mass (LBM) were significantly associated with increases in Hcy concentration after controlling for confounding factors. Further adjustments for behavioral factors showed that decreases in LBM were associated with Hcy increase. Decrease in LBM also predicted hyperhomocysteinemia at follow-up, after controlling for confounding factors. There was no significant association between change in body mass index (BMI) and Hcy concentrations over time. Hcy changes over time were related to change in LBM and body fat content, whereas BMI or weight change did not predict change in Hcy levels. Changes in ratio of LBM to total fat mass may contribute to hyperhomocysteinemia.
Keywords: Body Composition, Lean Body Mass, Hyperhomocysteinemia
INTRODUCTION
It has been suggested that hyperhomocysteinemia confers an independent risk for cardiovascular disease (CVD) comparable to that of smoking and hyperlipidemia (1). The atheropathology of hyperhomocysteinemia has been proposed to result from oxidizing effects on low-density lipoprotein (LDL) (1) as well as auto-oxidation effects of Hcy, leading to increases in endothelium-damaging hydrogen peroxide (2).
Homocysteine (Hcy) concentrations increase with age (3). Other causes of elevated Hcy include low intake or deficiencies of B vitamins, genetic defects, polymorphisms of enzymes involved in Hcy metabolism, impaired renal function, and lifestyle factors such as smoking, heavy coffee consumption, and lack of exercise (4). Research in rat models has proposed that insulin alters the activity of metabolic enzymes involved in the turnover of Hcy (5). In 1998, the first study in humans exploring the connection of Hcy to insulin showed a positive association between serum insulin and Hcy levels (6).
A recent study has shown that body weight was also a significant factor influencing plasma concentrations of total Hcy; an inverse association between gain in body weight and change in Hcy concentration was seen, even after adjustment for vitamin status, smoking, and coffee consumption (7). The mechanism by which weight affects Hcy is difficult to discern, because various factors with partly opposite effects may be involved. These include alterations in vitamin B status by diet or physical activity (8, 9), insulin effects (10), and decreased creatinine synthesis and Hcy formation by reduction in muscle mass (11). Although the importance of body composition was previously explored (12), the effects of body composition change in association to changes in Hcy and the development of hyperhomocysteinemia have not been previously investigated. Moreover, the association between Hcy and long-term body composition change in non-Caucasian populations is less clear. To address these shortcomings, we conducted a prospective cohort study to assess effects of body composition change on Hcy in Korean men and women.
MATERIALS AND METHODS
Patients
We enrolled 2,782 Koreans over the age of 20 yr who had undergone medical evaluation two times or more at Ajou University Hospital between January 2005 and March 2008. To avoid reverse causation of the association between body composition and the risk of pre-existing hyperhomocysteinemia, 179 subjects with hyperhomocysteinemia during the enrollment period were excluded. In addition, 13 subjects with missing information about Hcy level were excluded. The final sample included 2,590 subjects.
Data collection
The sociodemographic characteristics of subjects (smoking status, alcohol drinking status, and activity status) were surveyed by questionnaire. Height, weight, and body composition were measured using bioelectrical impedance analysis (Inbody 3.0; Biospace, Seoul, Korea) following an overnight fast. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2) (13). Waist circumference was measured at the middle part between the lower rib and iliac crest by a trained nurse. Blood pressure was measured with a model TM-2650A semiautomated blood pressure monitor (PMS Instruments, Tokyo, Japan) after a rest of at least 15 min. Venous blood was drawn following an 8 hr overnight fast and 24 hr abstinence from vigorous activity. Levels of hemoglobin, albumin, creatinine, glucose, total cholesterol, triglyceride, and high density lipoprotein cholesterol were measured with a model TBA-200FR enzymatic colorimetric apparatus (Toshiba, Tokyo, Japan); serum insulin was measured with an immunoradiometric assay kit (Dnabot, Tokyo, Japan). Homocysteine was measured with an IMX® homocysteine assay kit (Abbott, Abbott Park, IL, USA) by fluorescence polarization immunoassay (FPIA).
Outcome definition and follow-up procedures
We defined hyperhomocysteinemia as Hcy levels >14.0 µM/L compared to the normal reference range of 3.7-13.9 µM/L. The last follow-up was conducted on 28 February 2009, and the mean follow-up period was 2.1 yr.
Statistics
We examined the relationship between body composition, cardiovascular risk factors, and Hcy levels at baseline, adjusting for age, gender, smoking status, alcohol intake, exercise habits, and serum creatinine. Linear regression analyses were used to assess effects of body composition as predictors of changes in plasma Hcy concentration, controlling for age, gender, smoking status, alcohol intake, exercise habits, serum creatinine, and baseline Hcy and body composition levels. Logistic regression analyses were used to evaluate associations of changes in body composition to hyperhomocysteinemia at follow-up, while controlling for age, gender, and baseline Hcy and body composition levels. In all analyses, a two-sided α level of 0.05 was considered statistically significant. All analyses were conducted with the use of SPSS version 12 software (SPSS, Chicago, IL, USA).
Ethics statement
The institutional review board of Ajou University approved the study (AJIRB-MED-MDB-10-032). The board waived informed consent from the survey participants.
RESULTS
Demographic characteristics
Demographic characteristics are presented in Table 1. The total population of 2,590 individuals comprised 1,570 (60.6%) men and 1,020 (39.4%) women. The mean Hcy concentration was 9.36±1.95 µM/L for men and 7.01±1.79 µM/L for women. Men had higher BMI and lean body mass levels than women. In contrast, women had higher total body fat levels than men. Men, in comparison to women, had higher rates of smoking and alcohol intake (Table 1). Over a 2-yr follow-up, there were 293 incident cases of hyperhomocysteinemia (273 in men, 20 in women). The mean Hcy concentration in normal group was 10.07±2.02 µM/L and 16.33±3.52 µM/L in the hyperhomocysteinemia group.
Table 1.
General characteristics of the study population at baseline and follow-up
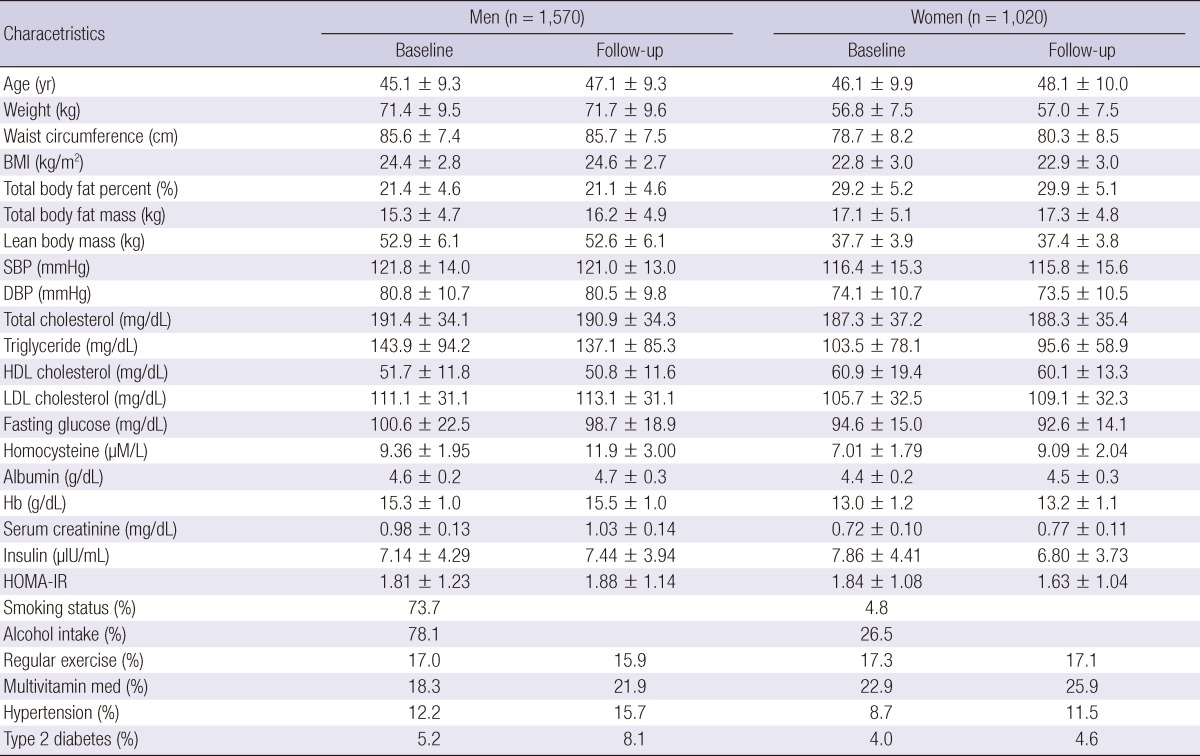
Values are mean±SD unless otherwise indicated. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HG, hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance. Smoking status, current or former smoker; alcohol intake, % of people taking at least one drink in the last week; regular exercise, moderate or severe vs none or sedentary; hypertension and type 2 diabetes, self reported.
Correlation between cardiovascular risk factors and homocysteine concentrations at baseline by gender
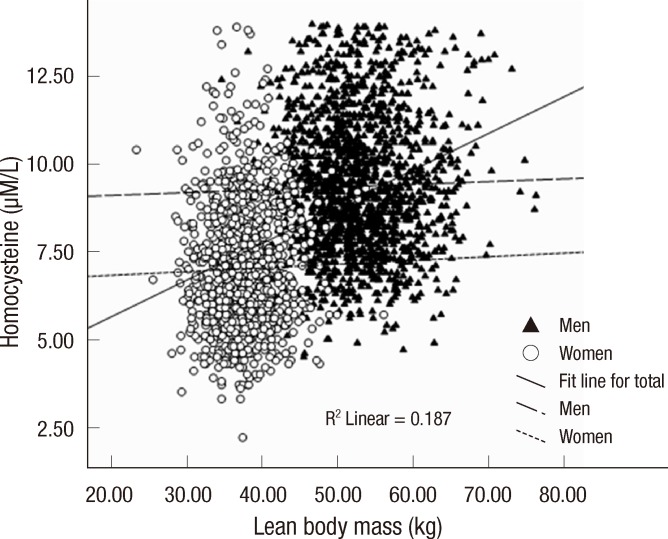
There were associations between Hcy and body composition measures at baseline, especially between Hcy and lean body mass (r=0.433, P<0.001). Consistent with previous research (14), this association was largely moderated by gender (Fig. 1). Therefore, the data of the baseline Hcy to CVD risk factors and body composition measures was presently investigated separately for men and women. In women, Hcy levels were associated with total body fat percent and total body fat mass, associations that remained significant after adjustment for age, serum creatinine, smoking status, alcohol intake, and exercise habits (Table 2). Hcy levels were also associated with systolic blood pressure, diastolic blood pressure, total cholesterol, and low density lipoprotein cholesterol levels, but these associations were reduced to non-significance after adjustment. As shown in Table 2, there were no associations between Hcy levels and body composition measures or other cardiovascular risk factors in the men after adjustment.
Fig. 1.
Scatter plot depicting the association of lean body mass to homocysteine levels in women (open circles) and men (closed triangles).
Table 2.
Pearson correlations between homocysteine levels and cardiovascular risk factors at baseline
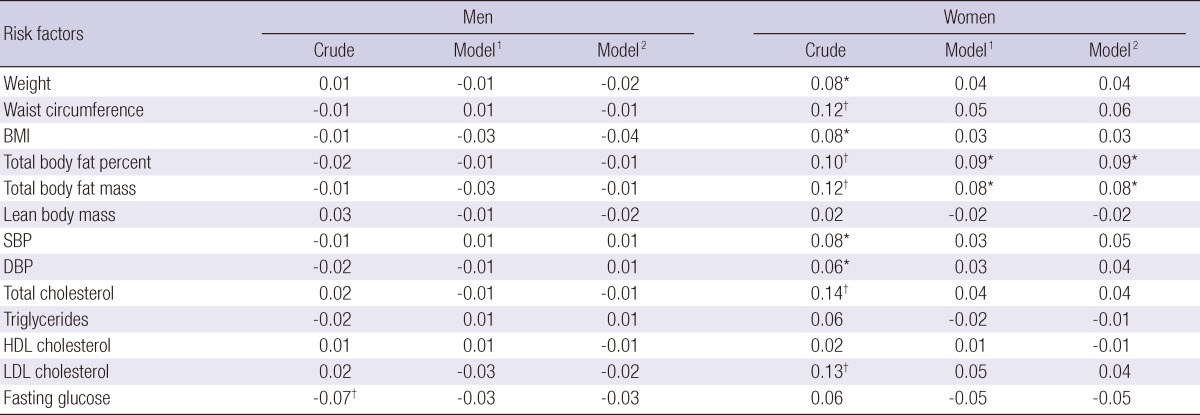
Correlation coefficients were calculated using partial correlation analysis. *P<0.05, †P<0.01. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein. 1Adjusted for age and serum creatinine, 2Adjusted for age, serum creatinine, smoking status, alcohol intake and exercise.
Change in body composition in the prediction of changes in homocysteine concentrations
To assess the associations between body composition and Hcy concentrations, linear regression models were conducted, controlling for age, gender, serum creatinine, baseline Hcy level, and baseline body composition level. Higher total body fat percent and lower weight, waist circumference, and lean body mass were significantly associated with changes in Hcy concentrations (Table 3). Further adjustments for smoking status, alcohol intake, and exercise habits revealed that changes in waist circumference and lean body mass were still negatively associated with changes in Hcy concentrations.
Table 3.
Summary of results from six standardized multiple linear regression coefficients predicting change in total homocysteine concentration
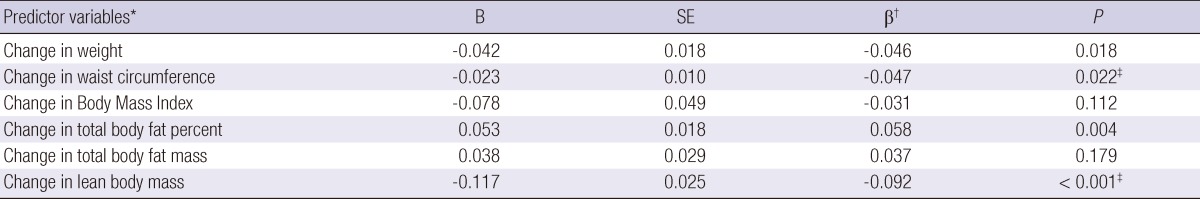
*Computed as the difference between baseline and follow-up levels. Results are based on 2,590 individuals with complete data; †Adjusted for age, gender, serum creatinine, baseline homocysteine level, and baseline body composition level; ‡P<0.05 controlling for age, gender, serum creatinine, baseline homocysteine level, baseline body composition level, smoking status, alcohol intake, and exercise.
Associations of changes in body composition to change in homocysteine levels
The associations of changes in body composition to hyperhomocysteinemia were examined in separate logistic regression models that controlled for age, gender, and baseline levels of Hcy and body composition measures (Table 4). Hyperhomocysteinemia was significantly associated with a decrease in lean body mass over the study period, after controlling for baseline Hcy levels and baseline lean body mass level. BMI, waist circumference, weight, total body fat percent, and total body fat mass did not predict hyperhomocysteinemia after controlling for baseline levels.
Table 4.
Summary of results for six independent logistic regression analyzes of different body composition measures predicting hyperhomocysteinemia
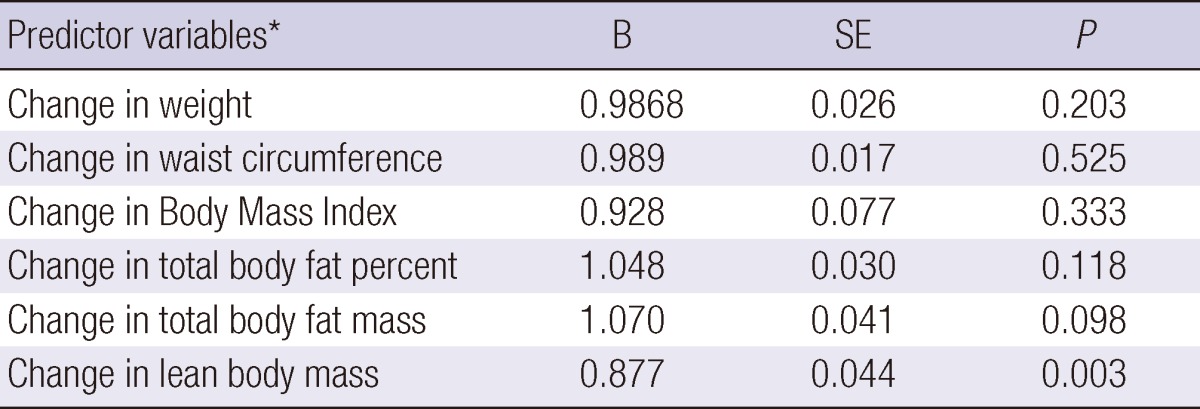
*Controlled for age, gender, baseline levels of homocysteine, and the baseline level of each body composition measure.
DISCUSSION
Results from the present study showed that change in lean body mass was associated with change in Hcy concentrations. Previous evidence that moderate protein-energy depletion leads to hyperhomocysteinemia (15) disagrees with the view that Hcy and methionine concentrations are positively related to the abundance of fat-free mass. It was recently suggested that in protein energy malnutrition, hyperhomocysteinemia arised from the activation of sulphur sparing mechanisms based on the suppression of the trans-sulphuration pathway, that was not coupled to a compensatory increment in remethylation, leading to an incremental change in the homocysteine/methionine ratio (16). This assumption is consistent with the assumption that increased methionine concentration raises the concentration of S-adenosylmethionine (17), thus leading to the inhibition of methylenetetrahydrofolate reductase activity (18), a key enzyme in the remethylation pathway of Hcy.
On the other hand, preliminary evidence exists that Hcy levels are positively linked to creatine turnover rates and muscle mass, as suggested by a study on German individuals where lean body mass was estimated using skinfold thickness or single-frequency bioelectrical impedance analysis (19). Currently, there was a positive cross-sectional correlation between lean body mass and Hcy levels at base line. This was mainly driven by an interaction effect of sex and lean body mass on Hcy levels. It has been suggested that the effect of muscle mass may be related to the amount of Hcy formed in conjunction with creatine-creatinine synthesis (20). In addition, sex steroid-induced change in plasma Hcy levels could conceivably be explained by their anabolic/catabolic effects (14).
Our study also revealed that an increase in total body fat percent is associated with increase in Hcy concentrations. Reductions in muscle mass and physical activity levels decreases total energy expenditures, which results in the accumulation of fat mass, especially visceral fat (21). Along with visceral fat accumulation, loss of skeletal muscle, which is the largest insulin-responsive target tissue, produces insulin resistance (IR) promoting metabolic syndrome (22). In addition, an association between IR and Hcy similar to that of insulin was found, consistent with the existence of a vicious circle. Thus, part of the dangers of Hcy might be explained by its link with IR (23). Also, IR was associated with elevated plasma tHcy levels in healthy, non-obese subjects suggesting that elevated Hcy levels may be a biological link between IR and atherothrombosis (24).
On the other hand, another study found no significant association between levels of Hcy and IR in a population with an increased risk for type 2 diabetes. Despite a successful lifestyle intervention resulting in a significant decrease in body mass index, body fat content, and improved insulin sensitivity, no differences in Hcy concentrations were achieved (10). Also, our results did not reveal a significant relation of Hcy levels with homeostatic model assessment-IR using subgroup analysis (data not shown). A recent study found that Hcy concentration was significantly higher in hyperinsulinemic obese children than in the normoinsulinemic group. There was no significant difference in Hcy levels between the obese and non-obese children (25). Therefore, increased plasma Hcy, particularly in hyperinsulinemic obese children, may be causally involved in the pathogenesis of atherosclerosis and/or CVD, both of which are common in obesity (26).
In our study, a decrease in waist circumference was significantly related to increased Hcy levels. Also, an overall weight gain tended to show a negative relation to change in Hcy levels, although the association was not significant after further adjustment. A previous study reported that weight gain was inversely related to Hcy levels (7). Another study assessed the renal effects of high vs low-protein low-fat diets in overweight subjects, and found that weight loss after dietary intervention was significantly different between the high-protein group and the low-protein group. Fat loss was also significantly different between the 2 groups, whereas there were not significant changes of fat-free mass between the two groups (27). Our results thus suggest that the change in Hcy concentrations is related to not only the change in lean body mass but also the change in body fat content.
Hyperhomocysteinemia may result from dietary deficiency in any of the hydrosoluble vitamins operating as cofactors of the Hcy-metabolizing machinery. However, recent studies using stepwise multiple regression analysis concluded that folate and cobalamine deficiencies provide only a partial explanation because they accounted for only 28% of the variance in total Hcy concentrations (28), very close to the 35% to 40% attributed to folate, cobalamin, and pyridoxine deprivation as a whole (29). Moreover, further aspects beyond the adequacy of vitamin factors have been much less intensely investigated up to now.
This study had several limitations. The study population consisted of patients who visited a single university hospital. Therefore, the prevalence of hyperhomocysteinemia in our data did not reflect the general population. Another limitation was that we did not evaluate the nutritional status of this study subjects. However, we performed this study as a follow-up study so that the baseline characteristics of the study population were considered to be similar until the end of follow-up.
In conclusion, changes in Hcy concentrations were associated with changes in lean body mass and body fat content in this cohort of Korean men and women. The change in lean body mass significantly contributed to hyperhomocysteinemia. These results suggest that a decrease in lean body mass determines increase in Hcy levels, rather than overall body mass index or weight change.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Graham IM, Daly LE, Refsum HM, Robinson K, Brattström LE, Ueland PM, Palma-Reis RJ, Boers GH, Sheahan RG, Israelsson B, et al. Plasma homocysteine as a risk factor for vascular disease: the European Concerted Action Project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 2.Upchurch GR, Jr, Welch GN, Fabian AJ, Pigazzi A, Keaney JF, Jr, Loscalzo J. Stimulation of endothelial nitric oxide production by homocyst(e)ine. Atherosclerosis. 1997;132:177–185. doi: 10.1016/s0021-9150(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 3.Ventura E, Durant R, Jaussent A, Picot MC, Morena M, Badiou S, Dupuy AM, Jeandel C, Cristol JP. Homocysteine and inflammation as main determinants of oxidative stress in the elderly. Free Radic Biol Med. 2009;46:737–744. doi: 10.1016/j.freeradbiomed.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Vollset SE, Refsum H, Nygård O, Ueland PM. Lifestyle factors associated with hyperhomocysteinemia. In: Carmel R, Jacobsen DW, editors. Homocysteine in health and disease. Cambridge: Cambridge University Press; 2001. pp. 341–355. [Google Scholar]
- 5.Weiss N. Mechanisms of increased vascular oxidant stress in hyperhomocys-teinemia and its impact on endothelial function. Curr Drug Metab. 2005;6:27–36. doi: 10.2174/1389200052997357. [DOI] [PubMed] [Google Scholar]
- 6.Stühlinger MC, Stanger O. Asymmetric dimethyl-L-arginine (ADMA): a possible link between homocyst(e)ine and endothelial dysfunction. Curr Drug Metab. 2005;6:3–14. doi: 10.2174/1389200052997393. [DOI] [PubMed] [Google Scholar]
- 7.Nurk E, Tell GS, Vollset SE, Nygård O, Refsum H, Nilsen RM, Ueland PM. Changes in lifestyle and plasma total homocysteine: the Hordaland Homocysteine Study. Am J Clin Nutr. 2004;79:812–819. doi: 10.1093/ajcn/79.5.812. [DOI] [PubMed] [Google Scholar]
- 8.Henning BF, Tepel M, Riezler R, Gillessen A, Doberauer C. Vitamin supplementation during weight reduction: favourable effect on homocysteine metabolism. Res Exp Med (Berl) 1998;198:37–42. doi: 10.1007/s004330050087. [DOI] [PubMed] [Google Scholar]
- 9.Manore MM. Effect of physical activity on thiamine, riboflavin, and vitamin B-6 requirements. Am J Clin Nutr. 2000;72:598S–606S. doi: 10.1093/ajcn/72.2.598S. [DOI] [PubMed] [Google Scholar]
- 10.Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Insulin resistance is associated with elevated plasma total homocysteine levels in healthy, non-obese subjects. Atherosclerosis. 1998;139:197–198. doi: 10.1016/s0021-9150(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 11.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 12.Battezzati A, Bertoli S, San Romerio A, Testolin G. Body composition: an important determinant of homocysteine and methionine concentrations in healthy individuals. Nutr Metab Cardiovasc Dis. 2007;17:525–534. doi: 10.1016/j.numecd.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report: National Institutes of Health. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 14.Nakhai Pour HR, Grobbee DE, Muller M, Emmelot-Vonk M, van der Schouw YT. Serum sex hormone and plasma homocysteine levels in middle-aged and elderly men. Eur J Endocrinol. 2006;155:887–893. doi: 10.1530/eje.1.02303. [DOI] [PubMed] [Google Scholar]
- 15.Ingenbleek Y, Hardillier E, Jung L. Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrition. 2002;18:40–46. doi: 10.1016/s0899-9007(01)00783-3. [DOI] [PubMed] [Google Scholar]
- 16.Ingenbleek Y, Young VR. The essentiality of sulfur is closely related to nitrogen metabolism: a clue to hyperhomocysteinaemia. Nutr Res Rev. 2004;17:135–151. doi: 10.1079/NRR200489. [DOI] [PubMed] [Google Scholar]
- 17.Krebs HA, Hems R, Tyler B. The regulation of folate and methionine metabolism. Biochem J. 1976;158:341–353. doi: 10.1042/bj1580341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutzbach C, Stokstad EL. Mammalian methylenetetrahydrofolate reductase: partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta. 1971;250:459–477. doi: 10.1016/0005-2744(71)90247-6. [DOI] [PubMed] [Google Scholar]
- 19.Rauh M, Verwied S, Knerr I, Dörr HG, Sönnichsen A, Koletzko B. Homocysteine concentrations in a German cohort of 500 individuals: reference ranges and determinants of plasma levels in healthy children and their parents. Amino Acids. 2001;20:409–418. doi: 10.1007/s007260170037. [DOI] [PubMed] [Google Scholar]
- 20.De Laet C, Wautrecht JC, Brasseur D, Dramaix M, Boeynaems JM, Decuyper J, Kahn A. Plasma homocysteine concentration in a Belgian school-age population. Am J Clin Nutr. 1999;69:968–972. doi: 10.1093/ajcn/69.5.968. [DOI] [PubMed] [Google Scholar]
- 21.Clifton PM, Keogh JB, Noakes M. Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr. 2008;87:23–29. doi: 10.1093/ajcn/87.1.23. [DOI] [PubMed] [Google Scholar]
- 22.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 23.Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean Sarcopenic Obesity Study. Int J Obes (Lond) 2009;33:885–892. doi: 10.1038/ijo.2009.130. [DOI] [PubMed] [Google Scholar]
- 24.Björck J, Hellgren M, Råstam L, Lindblad U. Associations between serum insulin and homocysteine in a Swedish population-a potential link between the metabolic syndrome and hyperhomocysteinemia: the Skaraborg project. Metabolism. 2006;55:1007–1013. doi: 10.1016/j.metabol.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Schäfer SA, Müssig K, Stefan N, Häring HU, Fritsche A, Balletshofer BM. Plasma homocysteine concentrations in young individuals at increased risk of type 2 diabetes are associated with subtle differences in glomerular filtration rate but not with insulin resistance. Exp Clin Endocrinol Diabetes. 2006;114:306–309. doi: 10.1055/s-2006-924073. [DOI] [PubMed] [Google Scholar]
- 26.Martos R, Valle M, Morales R, Cañete R, Gavilan MI, Sánchez-Margalet V. Hyperhomocysteinemia correlates with insulin resistance and low-grade systemic inflammation in obese prepubertal children. Metabolism. 2006;55:72–77. doi: 10.1016/j.metabol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Skov AR, Toubro S, Bülow J, Krabbe K, Parving HH, Astrup A. Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord. 1999;23:1170–1177. doi: 10.1038/sj.ijo.0801048. [DOI] [PubMed] [Google Scholar]
- 28.Pancharuniti N, Lewis CA, Sauberlich HE, Perkins LL, Go RC, Alvarez JO, Macaluso M, Acton RT, Copeland RB, Cousins AL, et al. Plasma homocyst(e)ine, folate, and vitamin B-12 concentrations and risk for early-onset coronary artery disease. Am J Clin Nutr. 1994;59:940–948. doi: 10.1093/ajcn/59.4.940. [DOI] [PubMed] [Google Scholar]
- 29.Lussier-Cacan S, Xhignesse M, Piolot A, Selhub J, Davignon J, Genest J., Jr Plasma total homocysteine in healthy subjects: sex-specific relation with biological traits. Am J Clin Nutr. 1996;64:587–593. doi: 10.1093/ajcn/64.4.587. [DOI] [PubMed] [Google Scholar]