Abstract
To evaluate the incidence of delayed enteral nutrition (EN) and identify avoidable causes of delay, we retrospectively reviewed medical records of 200 children (median age [range]; 37.5 [1-216] months) who stayed in the intensive care unit (ICU) for a minimum of 3 days. Among 200 children, 115 received EN following ICU admission with a median time of EN initiation of 5 days after admission. Of these, only 22 patients achieved the estimated energy requirement. A significant decrease in the final z score of weight for age from the initial assessment was observed in the non-EN group only (-1.3±2.17 to -1.57±2.35, P<0.001). More survivors than non-survivors received EN during their ICU stay (61.2% vs 30.0%, P=0.001) and received EN within 72 hr of ICU admission (19.8% vs 3.3%, P=0.033). The most common reason for delayed EN was gastrointestinal (GI) bleeding, followed by altered GI motility and hemodynamic instability. Only eight cases of GI bleeding and one case of altered GI motility were diagnosed as active GI bleeding and ileus, respectively. This study showed that the strategies to reduce avoidable withholding EN are necessary to improve the nutrition status of critically ill children.
Keywords: Enteral Nutrition, Parenteral Nutrition, Critical Illness, Child
INTRODUCTION
Enteral nutrition (EN) is the preferred mode of nutrient intake in critically ill patients with a functional gastrointestinal (GI) system because of its lower complication rate and cost compared with parenteral nutrition (PN) (1). To realize the potential benefits of EN in the pediatric intensive care unit, early initiation and maintenance of EN must be ensured. In adults, successful commencement of EN within 48 hr of mechanical ventilation (MV) is associated with up to 20% decrease in mortality in the intensive care unit (ICU) and a 25% decrease in hospital mortality in artificially ventilated patients (2). Early initiation of EN is particularly important in infants and children because they are at risk of rapid nutritional depletion that can lead to muscle wasting, impairment of vital organ function, compromised wound healing, and decreased immune function (3). Studies undertaken in children with burns and critical illness have shown that early EN is well tolerated without detrimental effects (4, 5).
Unfortunately, the delivery of EN is fraught with practical difficulties. There is often a delay in the initiation of EN or hesitation in increasing enteral caloric intake, even if patients are eligible for tube feeding while waiting for bowel sounds, and the managing physicians often underestimate nutritional needs incritically ill patients or prescribe an inadequate amount of nutrients. In addition, EN is frequently interrupted because of nursing interventions or delayed gastric emptying (6, 7).
We hypothesized that a significant proportion of delayed EN in critically ill children is avoidable. The objective of this study was to evaluate the current nutrition support behavior in the care of critically ill children and to identify avoidable reasons for delayed EN.
MATERIALS AND METHODS
A retrospective chart review was conducted on pediatric patients admitted to the ICU at Severance Hospital between January 2008 and December 2011. Children were included in the review if they were younger than 19 yr and had been in the ICU for a minimum of 3 days. Patients who stayed for longer than 30 days or were admitted to the general surgery and trauma services were excluded from the study because decisions regarding nutrition in these patients are made by the surgical, rather than critical care, staff. The following patient characteristics were recorded: sex, age, diagnosis on admission, and severity of illness at admission rated by three scoring systems, pediatric index of mortality 2 (PIM2), pediatric risk of mortality (PRISM), and pediatric logistic organ dysfunction (PELOD). Outcomes such as duration of mechanical ventilation support, length of ICU stay, and time of achieving caloric goal were abstracted retrospectively from patient charts. We compared patients receiving EN (EN group) with those not receiving any EN (non-EN group) during the ICU course.
Changes in nutritional status
For nutrition assessment, we obtained the following indices using the data for age (A), weight (W), height (H), and length (L): W/A, H/A or L/A, and W/H or W/L. We calculated body mass index with the following equation: BMI=W (kg)/H (m2). Nutrition status was determined by the z score for W/A. We used reference values from the Korean Centers for Disease Control and Prevention and the Korean Pediatric Society (8).
Nutrition support
We recorded the time when PN or EN was initiated and the reasons why EN was withheld for patients who started EN more than 72 hr after ICU admission or were maintained nil per os (NPO). Causes of delayed EN were categorized into the following groups: 1) gastrointestinal bleeding; 2) altered gastrointestinal motility; 3) hemodynamic instability; and 4) other reasons such as prone position or failure to insert feeding tube.
Patients received expressed breast milk, standard infant formula, or enteral feeding formula, unless a more specialized feed was indicated. Gastric (nasogastric or gastrostomy) feeding tubes were placed in all patients. Feeds were administered as 2-hr or 3-hr boluses or by continuous infusion.
Statistical analysis
Patient characteristics were described using frequency tables for categorical variables. Variables that were normally distributed were described using mean (standard deviation [SD]), whereas those displaying a high degree of skew were characterized by their median (range). We compared patient characteristics between EN and non-EN groups. Tests of significance for two-group comparisons included chi-square test for categorical variables, and Student's t-test and the Mann-Whitney rank sum test for normal and skewed distributions, respectively. Tests of significance were two-sided, and all statistical analyses were conducted with PWAS Statistics 18.0-August 2009 (SPSS in IBM, Armonk, NY, USA).
Ethics statement
This study protocol was approved by the institutional review board of Severance Hospital, Yonsei University Health System (IRB No. 4-2012-0369). The board waived informed consent because this study was a retrospective chart review.
RESULTS
Medical records were available for 200 children (112 males, 88 females) who met the study criteria. The median age was 37.5 (range 1-216) months and 41.5% of the patients were younger than 2 yr. Respiratory problems were the most common causes of ICU admission, followed by neurologic problems and sepsis/septic shock. The median length of ICU stay was 9 (3-30) days. One hundred and fifteen children required mechanical ventilation and the median duration of mechanical ventilation was 6.5 (0-30) days. During hospitalization in the ICU 30 deaths occurred, corresponding to 15% of the cases (Table 1).
Table 1.
Comparison of patient characteristics between EN group and non-EN group
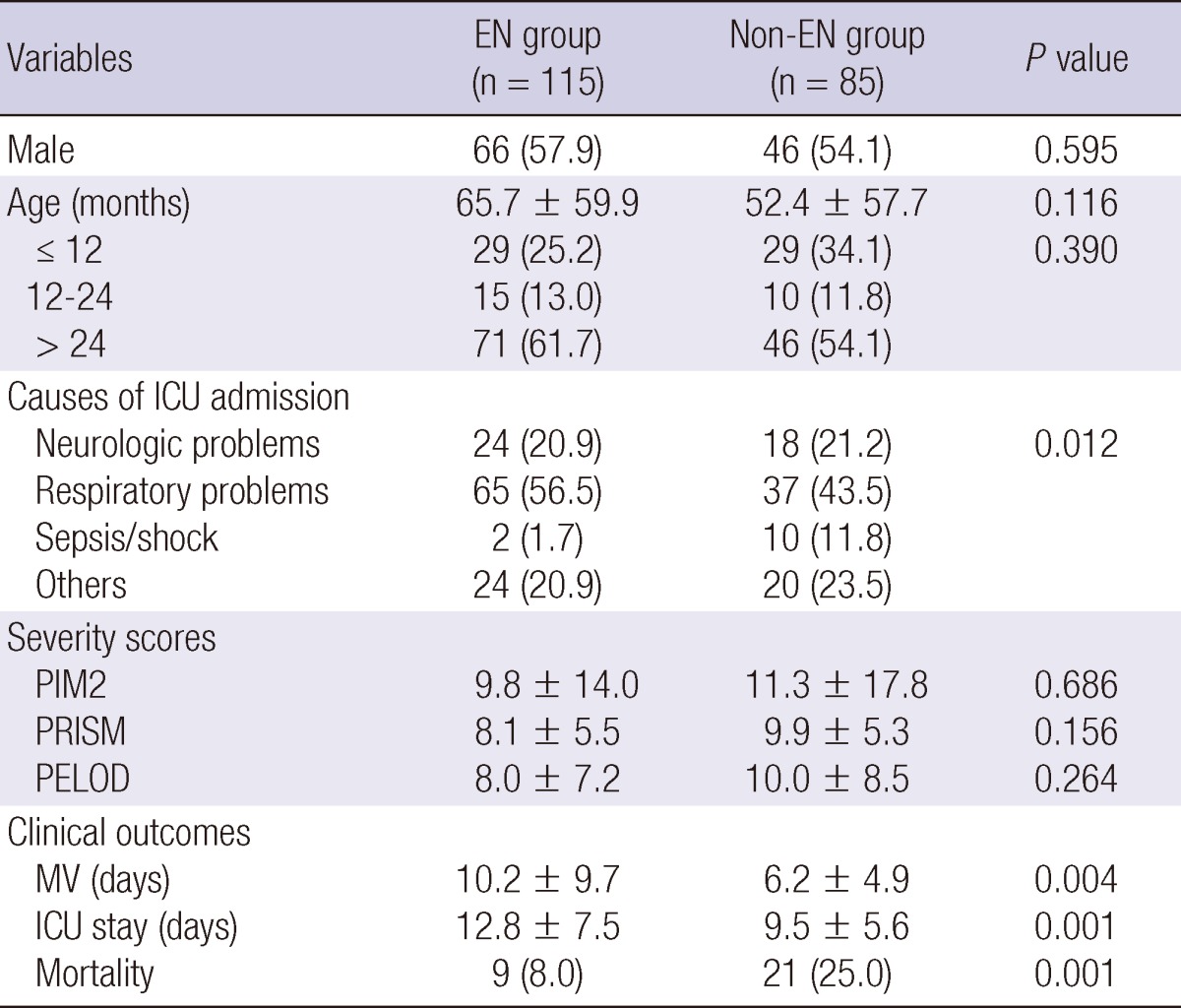
Data are expressed as mean±standard deviation or number (%). EN, enteral nutrition; ICU, intensive care unit; PIM2, pediatric index of mortality; PRISM, pediatric risk of mortality; PELOD, pediatric logistic organ dysfunction; MV, mechanical ventilation.
Nutritional therapy in any form was started on the day of admission in 74% of the patients. Seven patients did not receive any nutrients during their ICU stay. PN was used for 88.5% of the children and the median time to initiation was 1 day after admission to the ICU. EN was administered to 115 patients, with a median time to EN initiation of 5 days. Only 17% of the patients received EN within 72 hr of ICU admission. The median duration from EN initiation to achievement of calorie goal via EN was 8.5 days in 22 patients.
Comparison between EN group and non-EN group
The non-EN group contained 85 (42.5%) patients (Table 1). Distribution of gender and age was similar between the two groups. Although respiratory problems were the most common cause of ICU admission in both groups, a higher proportion of patients were admitted to ICU because of sepsis or septic shock in the non-EN group than in the EN group (11.8% vs 1.7%, P=0.012). Average PIM2, PRISM, and PELOD scores were identical between the two groups. Duration of mechanical ventilation and length of ICU stay was significantly shorter, and mortality was significantly higher, in the non-EN group than in the EN group.
Compared with the non-EN group, both initial and final z score of W/A during ICU stay were significantly lower in the EN group than in the non-EN group (-2.34±3.06 vs -1.30±2.17, P=0.008; -2.41±3.08 vs -1.57±2.35, respectively; P=0.036) (Table 2). Although there was a significant decrease in the z score of W/A from the initial to the final assessment in the non-EN group (-1.3±2.17 to -1.57±2.35, P<0.001), there was no significant change in the EN group. The proportion of patients with z score <-2 was not significantly different between the two groups (45.6% in EN group vs 34.1% in non-EN group, P=0.167). More patients in the non-EN group than in the EN group experienced a decrease in z score between admission and discharge from ICU (74.1% in non-EN group vs 61.4% in EN group, P=0.060) and a decrease in BMI (71.8% in non-EN group vs 57.9% in EN group, P=0.044).
Table 2.
Comparison of nutrition support and changes in nutritional status between EN group and non-EN group
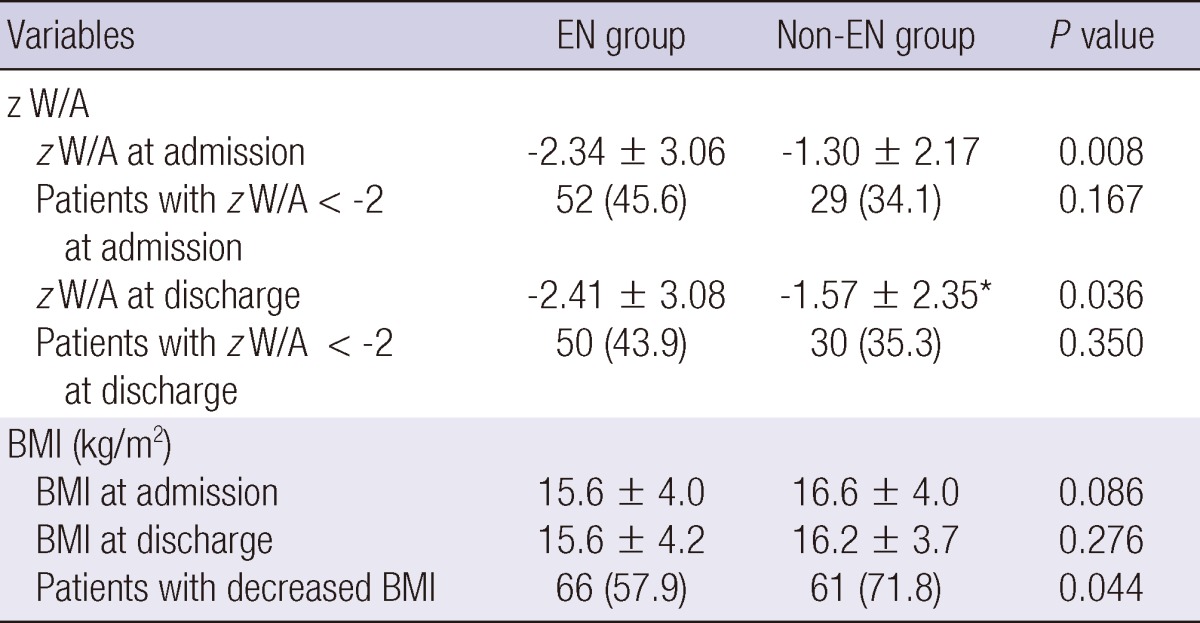
Data are expressed as mean±standard deviation or number (%). *P<0.001 between time of admission and discharge. W, weight; A, age; z, z score; BMI, body mass index.
Comparison between the survivor and non-survivor groups
More patients in the survivor group than in the non-survivor group received EN during ICU stay (61.2% vs 30.0%, P=0.001) and the percentage of the patients who received EN within 72 hr after ICU admission was higher in the survivor group than in the non-survivor group (19.8% vs 3.3%, P=0.033) (Table 3). Although BMI at admission to ICU was significantly higher in the non-survivor group than in the survivor group, BMI at discharge was not significantly different between the two groups. More patients in the non-survivor group experienced a decrease in the W/A percentile of more than 1 level (18.9% for survivor group vs 47.1% for non-survivor group, P=0.024).
Table 3.
Comparison of nutritional status and nutrition support between survivors and non-survivors
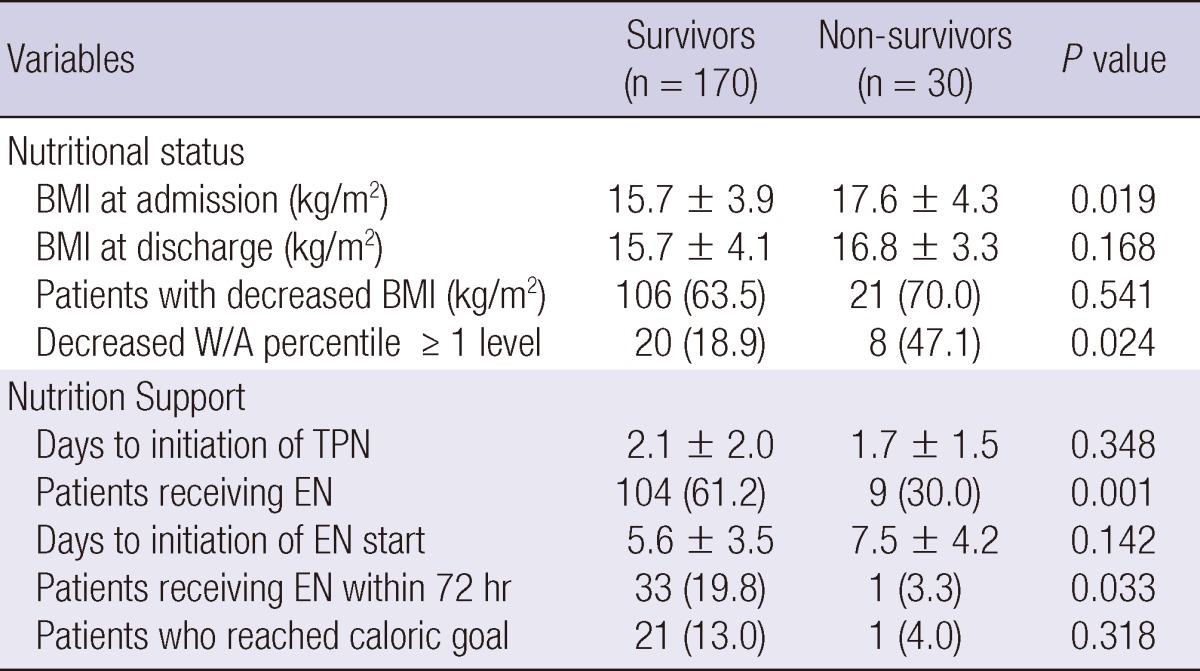
Data are expressed as mean±standard deviation or number (%). BMI, body mass index; W, weight; A, age; TPN, total parenteral nutrition; EN, enteral nutrition.
Causes of delayed EN
In both the survivor and the non-survivor groups the most common reason for delayed EN was gastrointestinal bleeding, of which approximately 33% was caused by old blood clots and only 6% was due to active bleeding (Table 4). Among the cases of altered gastrointestinal motility, only one was medically diagnosed ileus. A higher percentage of the non-survivor group were withheld EN because of hemodynamic instability than among the survivors, but this was not statistically significant (17.9% vs 7.8%, P=0.109). For 43 patients we were unable to find any explanation in the medical records for why EN was withheld or contraindicated.
Table 4.
Causes of delayed initiation of EN or NPO
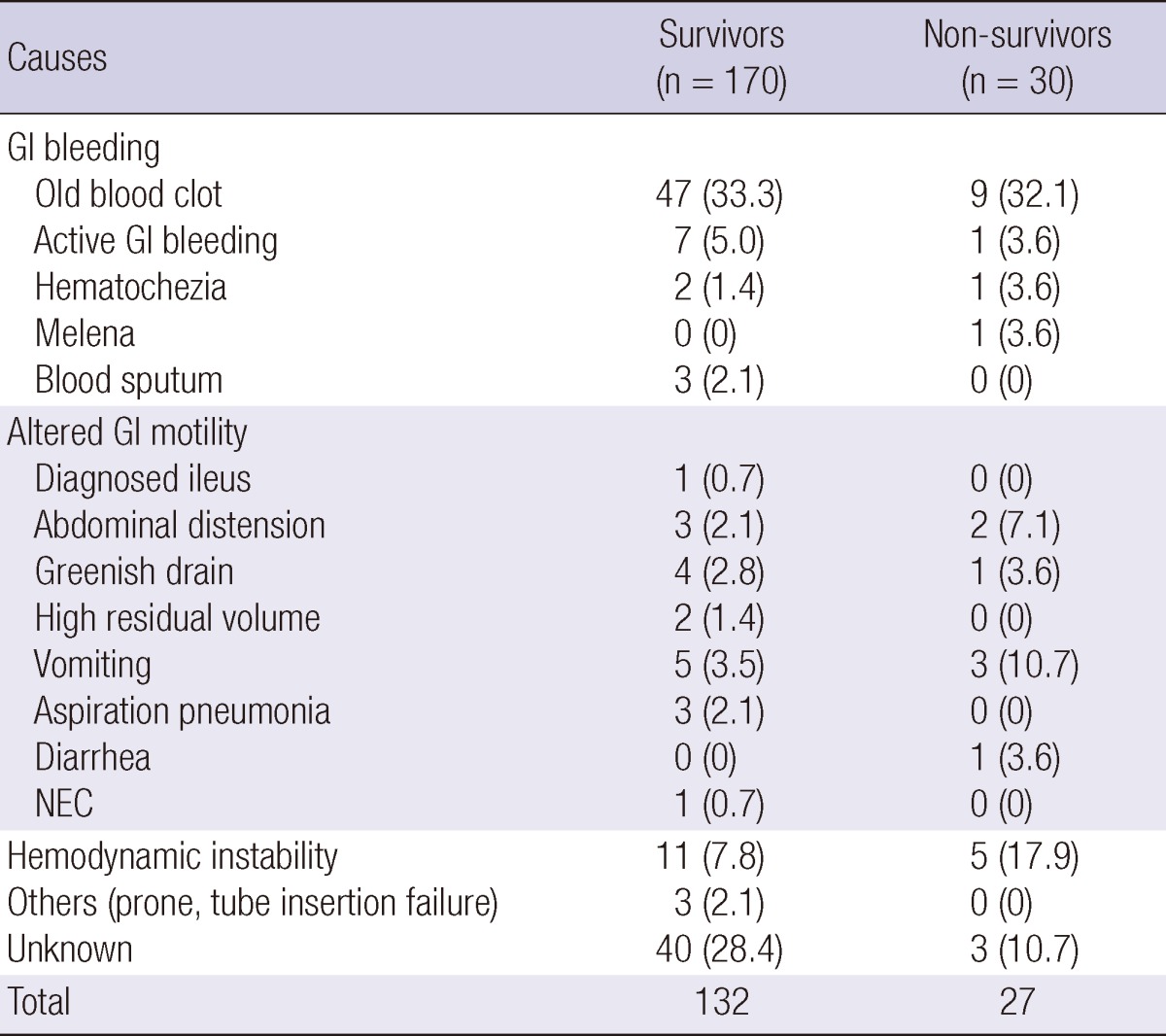
Data are expressed as number (%). NPO, nil per os; GI, gastrointestinal; NEC, necrotizing enterocolitis.
DISCUSSION
Among the patients in this study, EN was often initiated as late as day 5 after admission and only 34 patients received EN within 72 hr of admission despite the fact that early EN is known to improve clinical outcomes, decrease infection rates, decrease length of stay, and be cost-effective (1, 9-11). The current clinical guidelines for nutritional support in critically ill adults recommend early initiation of EN within the first 24-48 hr following admission (12, 13). Because there are currently no clinical guidelines for nutritional support in critically ill children, we defined delayed EN as initiation of EN later than 72 hr after admission to ICU - a looser criterion than for adults. The delivery of EN in the ICU may be delayed for several reasons, including GI dysfunction, elective discontinuation of procedures, and physicians' ignorance of nutritional requirements (7, 14). It is important that physicians understand the importance of feeding for appropriate nutritional support because the physician's order is the first step in nutrition delivery. However, many physicians are reluctant to start EN, especially in critically ill patients, because it is known to be associated with an increased risk of aspiration pneumonia (2). In our study, there was no description of the reasons for withholding EN in the medical records of 27% of the patients who received EN late or never received it.
In the current study, the most common reason for delayed EN was gastrointestinal bleeding, in most cases caused by gastric drainage of old blood. Specific definition of bleeding is very important in the ICU setting. Clinically important hemorrhage is defined as overt bleeding plus either hemodynamic changes or the need for blood transfusion. Hemodynamic changes mean that the patient is hypotensive, tachycardic, or orthostatic and the need for transfusion is usually defined by a requirement for two units of blood (15). Only 8 of 71 patients with gastrointestinal bleeding sign met these criteria.
Twenty patients received EN between 3 and 19 days following admission because of signs of decreased bowel motility such as abdominal distension, high gastric residual volume, or vomiting. An excess of gastric residues is the most common complication in critically ill patients receiving gastric nutrition. There is no evidence that the volume of gastric residues affects the risk of pulmonary aspiration and recently published clinical guidelines do not recommend withholding EN for gastric residual volumes <500 mL in the absence of other signs of intolerance (12). However, there are no reference values for children, and primary physicians have to decide when to withhold EN. A survey of physicians showed substantial discordance over this issue (16). Although most physicians believed that nutrition is important in the ICU, they did all not feel confident in their knowledge of the role of nutrition support in the critically ill. Several factors may contribute to this lack of confidence, including a general lack of awareness and poor familiarity with current guidelines, and difficulty integrating previous dogma with recent clinical practice guidelines. It has been reported that most cases of withholding EN, including delayed initiation of EN, could be avoided by better education on standardized feeding processes (17, 18).
In our study, EN was withheld for 16 patients because of hemodynamic instability. Clinical guidelines recommend that EN should be withheld in patients requiring significant hemodynamic support including high-dose catecholamine agents because of an increased risk for subclinical ischemia/reperfusion injury involving the intestinal microcirculation (12). However, there is no consensus among clinicians on the definition of hemodynamic instability; and King et al. (19) reported that pediatric patients receiving cardiovascular medications tolerated EN without adverse events. Medications such as opioids and bowel edema resulting from fluid resuscitation in critically ill patients may cause decreased gastrointestinal motility. Implementation of an EN protocol has been demonstrated to increase nutritional delivery and minimize the risks of tube feeding (7, 20, 21). In our study, there was no significant difference in the percentage of the patients who received delayed EN between the survivor and the non-survivor group.
Upon admission to the ICU, 40.5% of patients had preexisting malnutrition defined by z score of W/A <-2, similar to results of a previous study (22). Although the prevalence of malnourishment was not significantly changed at the time of discharge from ICU, the z score of W/A significantly decreased during ICU stay in the non-EN group but not in the EN group. Briassoulis et al. (5) reported that nutritional status and clinical outcomes were improved by providing early enteral nutrition to critically ill children within 8 hr of ICU admission. As this was a retrospective study, we could not obtain data about daily nutrient intake from EN and/or PN. Although the z score significantly decreased and mortality was higher in the non-EN group, we cannot conclude that EN had a direct positive effect on nutritional status or mortality. Moreover, the duration of mechanical ventilation and ICU stay was significantly longer in the EN group than in the non-EN group. It is possible that a longer stay in the ICU increased the likelihood of receiving EN, and under such conditions delayed EN would be predominant. Moreover, the clinical effects of EN might have been biased by the presence of healthier patients in the EN group, although there was no significant difference in severity scores between the two groups.
Although this study had some limitations due to its retrospective nature, our data showed that EN was often initiated as late as day 5 after admission. We also found that a significant proportion of delayed EN might be avoidable, for example in cases with old blood clots and lower gastrointestinal bleeding. Identification of avoidable reasons for delaying EN will allow educational intervention and modification of clinical practice with the aim of decreasing the incidence of delayed EN in critically ill children.
In conclusion, this study revealed that EN was often initiated as late as day 5 after admission and that such delays might be avoidable in a significant proportion of cases. Strategies for reducing avoidable delayed EN are required to improve enteral nutrition delivery and nutritional status in the critically ill children.
Footnotes
The authors have no conflicts of interest to disclose.
This work was supported by the Korea Research Foundation Grant funded by the Republic of Korean Government (KRF-2010-0017338).
References
- 1.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 2.Artinian V, Krayem H, DiGiovine B. Effects of early enteral feeding on the outcome of critically ill mechanically ventilated medical patients. Chest. 2006;129:960–967. doi: 10.1378/chest.129.4.960. [DOI] [PubMed] [Google Scholar]
- 3.Rogers EJ, Gilbertson HR, Heine RG, Henning R. Barriers to adequate nutrition in critically ill children. Nutrition. 2003;19:865–868. doi: 10.1016/s0899-9007(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 4.Chellis MJ, Sanders SV, Webster H, Dean JM, Jackson D. Early enteral feeding in the pediatric intensive care unit. JPEN J Parenter Enteral Nutr. 1996;20:71–73. doi: 10.1177/014860719602000171. [DOI] [PubMed] [Google Scholar]
- 5.Briassoulis G, Zavras N, Hatzis T. Malnutrition, nutritional indices, and early enteral feeding in critically ill children. Nutrition. 2001;17:548–557. doi: 10.1016/s0899-9007(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 6.Heyland D, Cook DJ, Winder B, Brylowski L, Van deMark H, Guyatt G. Enteral nutrition in the critically ill patient: a prospective survey. Crit Care Med. 1995;23:1055–1060. doi: 10.1097/00003246-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 7.McClave SA, Sexton LK, Spain DA, Adams JL, Owens NA, Sullins MB, Blandford BS, Snider HL. Enteral tube feeding in the intensive care unit: factors impeding adequate delivery. Crit Care Med. 1999;27:1252–1256. doi: 10.1097/00003246-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51:1–25. [Google Scholar]
- 9.Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264–2270. doi: 10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Pettignano R, Heard M, Davis R, Labuz M, Hart M. Total enteral nutrition versus total parenteral nutrition during pediatric extracorporeal membrane oxygenation. Crit Care Med. 1998;26:358–363. doi: 10.1097/00003246-199802000-00041. [DOI] [PubMed] [Google Scholar]
- 11.Kirby DF. Decisions for enteral access in the intensive care unit. Nutrition. 2001;17:776–779. doi: 10.1016/s0899-9007(01)00654-2. [DOI] [PubMed] [Google Scholar]
- 12.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 13.Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, Ebner C, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210–223. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ Southwestern Ontario Critical Care Research Network. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT) CMAJ. 2004;170:197–204. [PMC free article] [PubMed] [Google Scholar]
- 15.McClave SA, Chang WK. When to feed the patient with gastrointestinal bleeding. Nutr Clin Pract. 2005;20:544–550. doi: 10.1177/0115426505020005544. [DOI] [PubMed] [Google Scholar]
- 16.Behara AS, Peterson SJ, Chen Y, Butsch J, Lateef O, Komanduri S. Nutrition support in the critically ill: a physician survey. JPEN J Parenter Enteral Nutr. 2008;32:113–119. doi: 10.1177/0148607108314763. [DOI] [PubMed] [Google Scholar]
- 17.Adam S, Batson S. A study of problems associated with the delivery of enteral feed in critically ill patients in five ICUs in the UK. Intensive Care Med. 1997;23:261–266. doi: 10.1007/s001340050326. [DOI] [PubMed] [Google Scholar]
- 18.Mackenzie SL, Zygun DA, Whitmore BL, Doig CJ, Hameed SM. Implementation of a nutrition support protocol increases the proportion of mechanically ventilated patients reaching enteral nutrition targets in the adult intensive care unit. JPEN J Parenter Enteral Nutr. 2005;29:74–80. doi: 10.1177/014860710502900274. [DOI] [PubMed] [Google Scholar]
- 19.King W, Petrillo T, Pettignano R. Enteral nutrition and cardiovascular medications in the pediatric intensive care unit. JPEN J Parenter Enteral Nutr. 2004;28:334–338. doi: 10.1177/0148607104028005334. [DOI] [PubMed] [Google Scholar]
- 20.Mentec H, Dupont H, Bocchetti M, Cani P, Ponche F, Bleichner G. Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit Care Med. 2001;29:1955–1961. doi: 10.1097/00003246-200110000-00018. [DOI] [PubMed] [Google Scholar]
- 21.American Gastroenterological Association Medical Position Statement: guidelines for the use of enteral nutrition. Gastroenterology. 1995;108:1280–1281. doi: 10.1016/0016-5085(95)90230-9. [DOI] [PubMed] [Google Scholar]
- 22.De Souza Menezes F, Leite HP, Koch Nogueira PC. Malnutrition as an independent predictor of clinical outcome in critically ill children. Nutrition. 2012;28:267–270. doi: 10.1016/j.nut.2011.05.015. [DOI] [PubMed] [Google Scholar]


