Abstract
Purpose
To evaluate conventional brachytherapy (BT) plans using dose-volume parameters and high resolution (3 Tesla) MRI datasets, and to quantify dosimetric benefits and limitations when MRI-guided, conformal BT (MRIG-CBT) plans are generated.
Material and methods
Fifty-five clinical high-dose-rate BT plans from 14 cervical cancer patients were retrospectively studied. All conventional plans were created using MRI with titanium tandem-and-ovoid applicator (T&O) for delivery. For each conventional plan, a MRIG-CBT plan was retrospectively generated using hybrid inverse optimization. Three categories of high risk (HR)-CTV were considered based on volume: non-bulky (< 20 cc), low-bulky (> 20 cc and < 40 cc) and bulky (≥ 40 cc). Dose-volume metrics of D90 of HR-CTV and D2cc and D0.1cc of rectum, bladder, and sigmoid colon were analyzed.
Results
Tumor coverage (HR-CTV D90) of the conventional plans was considerably affected by the HR-CTV size. Sixteen percent of the plans covered HR-CTV D90 with the prescription dose within 5%. At least one OAR had D2cc values over the GEC-ESTRO recommended limits in 52.7% of the conventional plans. MRIG-CBT plans showed improved target coverage for HR-CTV D90 of 98 and 97% of the prescribed dose for non-bulky and low-bulky tumors, respectively. No MRIG-CBT plans surpassed the D2cc limits of any OAR. Only small improvements (D90 of 80%) were found for large targets (> 40 cc) when using T&O applicator approach.
Conclusions
MRIG-CBT plans displayed considerable improvement for tumor coverage and OAR sparing over conventional treatment. When the HR-CTV volume exceeded 40 cc, its improvements were diminished when using a conventional intracavitary applicator.
Keywords: brachytherapy, cervical cancer, high-dose-rate brachytherapy, MRI-guided brachytherapy
Purpose
Brachytherapy (BT) has played a central role in radiation therapy for cervical cancer since 1903. The standard of care for cervical cancer is a combination of external beam radiation therapy (EBRT) with concomitant cisplatinum-based chemotherapy and BT. The standard BT treatment technique has been unchanged for decades: 2D orthogonal radiographs are used to specify a dose to a fixed reference point, point A, relative to the intracavitary applicator. The patient anatomy and tumor location, not radiographically visible, are unused in the treatment planning process [1, 2]. Recent advances in treatment planning based on 3D imaging enables the delineation of the target volumes and critical structures allowing volume-based inverse planning [3]. As magnetic-resonance imaging (MRI) provides superior soft tissue differentiation relative to computed tomography (CT) [4], MRI-based treatment planning prior to each BT fraction is preferred. The patient's clinical target volume (CTV) and organs at risk (OARs) can be clearly identified and contoured on MRI datasets [4], allowing radiation delivery to macroscopic tumor volumes, and adaptive planning to account for tumor regression.
The American Brachytherapy Society (ABS) task group for cervical cancer [2] has adopted the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) recommendations for 3-D imaging based BT planning [5]. The planning method takes the pear-shaped isodose lines of conventional treatment and adjusts dwell times to optimize the minimum dose received by 90%, D90, of the High Risk (HR)-CTV, while restricting the minimum dose to the hottest 2 cc of each OAR, D2cc. Several studies [6–9] have investigated correlations between dose-volume-histogram (DVH) parameters from these optimized treatments and conventional point doses from conventional planning. For example point A and International Commission on Radiation Units and measurements (ICRU) report #38-defined rectum and bladder points [1] using pulsed-dose-rate (PDR) and high-dose-rate (HDR) BT. The goal is to understand the correlation between ICRU report #38-defined points & point A and DVH metrics [6–9]. One center, performing MRI-guided, conformal BT (MRIG-CBT) [10] reports that the mean HR-CTV volume for patients with local recurrence was significantly larger (50 ± 24 cc) than for patients without local recurrence (34 ± 23 cc, p < 0.05). Only D90 and D100 of the HR-CTV had a statistically significant effect on local tumor control, while the doses to intermediate-risk CTV and gross target volume did not [10]. This result implies that more analysis is needed into the dosimetric benefits and limitations of MRIG-CBT plans for different HR-CTV sizes. Previous studies considering HR-CTV size have only classified large and small volumes, and employed lower resolution MRI (< 2 Tesla) [8, 9]. There is a need for improved contouring precision [4], accomplished with a high-resolution (3 Tesla) MRI [11]. Even though high-resolution (3 Tesla) MRI is being increasingly used in diagnosis, its use in MRI-guided BT has only recently been introduced in the literature [12–15].
In this study, we retrospectively evaluated conventional plans using GEC-ESTRO recommended [5, 16]. DVH parameters, and quantified the dosimetric benefits of MRIG-CBT, both in general and with respect to three different HR-CTV sizes.
Material and methods
Patients and EBRT treatments
We retrospectively studied 55 plans from 14 patients with biopsy-proven cervical cancer, with approval from the institutional review board. The malignancies were rated FIGO (International Federation of Gynecology and Obstetrics) stage Ib1 to IV and were treated in 2009 or 2010. The treatments employed EBRT with concomitant chemotherapy (for all but one patient in which chemotherapy was contraindicated), followed by 3-5 fractions of BT with 5.5-8 Gy fraction size. The institutional protocol specifies eight weeks from the beginning of EBRT to the end of BT; though this time was extended in five cases due to clinical complications. Other pertinent characteristics of the patients, tumors, and treatments are summarized in Table 1. EBRT treatment plans used either a 3-D conformal technique or intensity-modulated radiotherapy (IMRT) generated using the Pinnacle3® treatment planning system (version 7.0, Philips Medical Systems Inc., Madison, WI, USA). The prescription dose of EBRT was 45 Gy in 25 fractions, and, when necessary, a 5.4 to 10 Gy boost in 3 to 5 fractions to the involved paraortic or parametrial regions.
Table 1.
Patient and plan characteristics
| Patient characteristics | N | % | |
|---|---|---|---|
| Patients | 14 | ||
| FIGO stage | |||
| IB-IIB | 8 | 57 | |
| III-IV | 6 | 43 | |
| Histopathology | |||
| Squamous cell carcinoma | 11 | 79 | |
| Adenocarcinoma | 2 | 14 | |
| Other | 1 | 7 | |
| Lymph node involvement | 4 | 29 | |
| 3 BT fractions (5.3-7.5 Gy) | 5 | 36 | |
| 4 BT fractions (5.0-6.0 Gy) | 2 | 14 | |
| 5 BT fractions (5.5-6.0 Gy) | 7 | 50 | |
| Patient age | Median | Range | |
| 58 | 33-79 | ||
| EBRT dose (Gy) | 50 | 45-62 | |
| BT dose (Gy) | 26 | 16-30 | |
| Plan characteristics | N | Median | Range |
| BT (plans) | |||
| Total fractions | 58 | ||
| Usable fractions† | 55 | ||
| HR-CTV size (cc) | 32 | 8-145 | |
| Non-bulky (cc) | 15 | 15 | 8.3-20.0 |
| Low-bulky (cc) | 19 | 27 | 8.3-39.0 |
| Bulky (cc) | 21 | 74 | 8.3-145 |
FIGO – International Federation of Gynecology and Obstetrics, BT – brachytherapy, EBRT – external beam radiotherapy
For 3 fractions the MRI scanner was unavailable, and patients were imaged by CT instead. These fractions were excluded from the study
HDR treatments
All 55 plans were treated using a conventional Point A planning technique. Intracavitary HDR started after 3-4 weeks of EBRT, using the 192Ir radioisotope (VariSource iX®, Varian Medical Systems Inc., Palo Alto, CA, USA). Patients were prescribed a total dose of 33-36 Gy10 in 5-7 fractions, where Gy10 is the equivalent dose in 2 Gy fractions of EBRT (EQD2) using an α/β of 10 Gy. For each fraction, clinical examination was performed under general anesthesia, and a Fletcher-Suit-Declos® T&O applicator (Varian Medical Systems Inc.) was inserted under ultrasound guidance. A titanium applicator was chosen for advantages previously described in the literature [12, 17]. Orthogonal digital radiographs were acquired before and after 3.0 Tesla MRI scan to identify applicator displacements during patient transport to the MR scanner (MAGNETOM Trio™, Siemens Medical System Inc., Erlangen, Germany). All conventional plans were clinically generated on MRI not on radiographs, using a previously reported scan protocol [12]. T2- and T1-weighted MR images were used for contouring and source-pathway reconstruction, respectively.
Conventional and MRIG-CBT HDR treatment planning
Conventional point A based planning followed the institute's clinical protocol developed according to ABS guidelines [2] (Fig. 1). The point A was defined by drawing a line through the center of each ovoid, the radius of the ovoid + 2 cm superiorly along the tandem from the intersection of this line, and 2 cm lateral from the center of the tandem. Optimization lines were generated 0.5 cm lateral from each ovoid surface to specify vaginal doses, in addition to the lines of 2 cm lateral from the center of the tandem to specify point A doses. Source pathway reconstruction [12, 17] for T&O was done on T1-weighted MRI (1 mm slice thickness) by importing predetermined shapes from the applicator library (SolidApplicator, BrachyVision™, Varian Medical System Inc.). According to the GEC-ESTRO recommendations [5, 18], the HR-CTV, rectum, bladder and sigmoid colon were retrospectively contoured on T2-weighted MRI of 3 mm slice thickness.
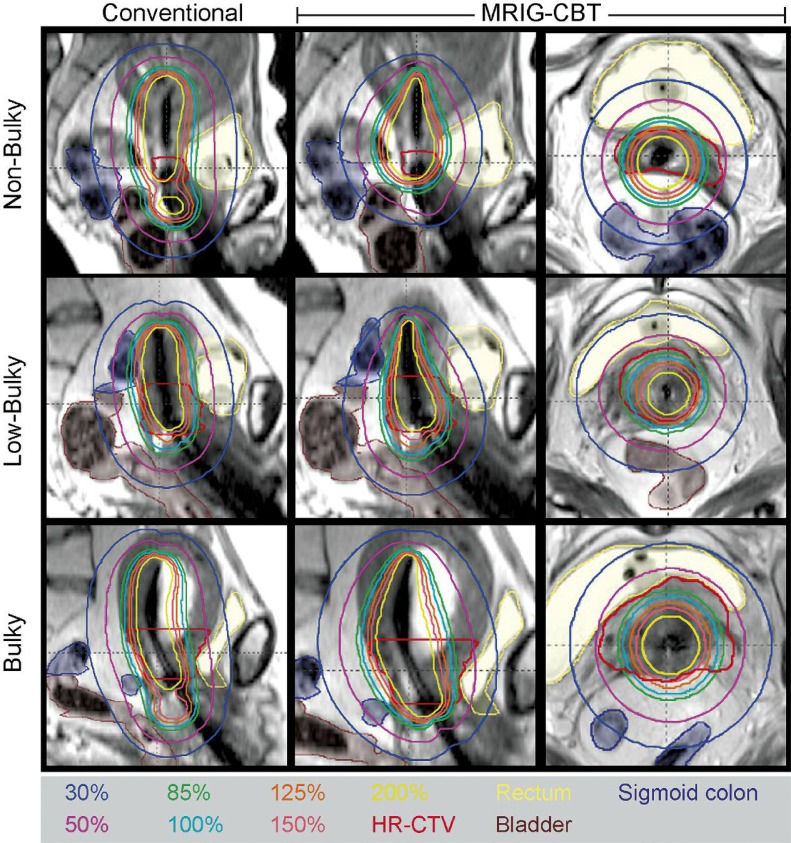
Fig. 1.
Isodose lines for conventional and MRIG-CBT planning of all three HR-CTV sizes: non-bulky (HR-CTV < 20 cc), low-bulky (20 cc = HR-CTV < 40 cc) and bulky (HR-CTV = 40 cc)
The strategy for volume-optimization was based on a study by Kim et al. [12] (Fig. 1) using an hybrid-inverse planning process. Hybrid-inverse planning represents a 3-D volume-optimization plan not solely generated by inverse volume optimization, but generated in a hybrid manner using a conventional point A plan and inverse volume-optimization. The first step is the generation of a conventional point A plan. A planner evaluates the DVH parameters according to the total dose limit according to the GEC-ESTRO recommendations [19], the D2cc of an OAR are ≤ 75 Gy3 of EQD2 for the rectum and sigmoid colon, and ≤ 90 Gy3 of EQD2 for the bladder. The dose-volume constraints in inverse-volume optimization are set so that all OARs receive equal or lower doses than their limit. Afterward the optimization is iteratively altered with updated dose-volume optimization constraints until three goals are achieved. The three goals are: 1) OAR D2cc values below limits of equation (1) (defined below), 2) HR-CTV D90 below 105% of the prescription dose, and 3) if HR-CTV D90 was below the prescription dose, at least one OAR D2cc value within 5% of its limit. The isodose lines for each slice on coronal, sagittal, and axial plans were reviewed, and final dose shaping using graphical optimization was performed if necessary.
As a “worst-case scenario”, it was assumed that the HR-CTV and all OARs received the EBRT prescription dose, and that each OAR D2cc was in the same location for all BT treatment fractions [20]. The OAR D2cc values from the EBRT boosts were also accounted for and used to determine an OAR limit for each BT fraction:
| 1 |
where L i is the D2cc limit for BT fraction i, D L is the total dose limit [19] to D2cc of an OAR, D EBRT – the total EBRT dose, D boost – the D2cc dose to the OAR from EBRT boost, D i is the prescribed BT dose for fraction i, and D BT is the total prescribed BT dose. All OAR quantities were converted to EQD2 values using an α/β of 3 Gy [18].
Analysis
Each plan was analyzed by recording the D90 and D100 of the HR-CTV, the D2cc and D0.1cc of each OAR, and the doses to point A and the ICRU bladder and rectum points in contrast to those with MRIG-CBT. It has been reported that DVH measurements in MRIG-CBT are highly-dependent on a number of variables, including applicator reconstruction, MRI artifacts, and contouring variation [17]. As both conventional and MRIG-CBT cases employ the same applicator location and contours, the comparison of the two methods was unaffected by these variables. All plans were divided into three volume categories: non-bulky (HR-CTV < 20 cc), low-bulky (20 cc ≤ HR-CTV < 40 cc) and bulky (HR-CTV ≥ 40 cc). The p values were calculated using a two-tailed t-test assuming homoscedasticity.
Results
Conventional, point A based plans
All 55 conventional plans delivered the prescribed dose to point A with little variation (Table 2). The average doses to right and left point A were 100 ± 1% (mean ± standard deviation) of the respective prescription doses for non-bulky and low-bulky tumors. The plans for bulky tumors showed increased variation in point A dose, at 100 ± 2% of the respective prescription doses. The D90 of the HR-CTV for conventional plans was considerably affected by the HR-CTV volume. On average, D90 for the HR-CTV for the low-bulky tumors was favorable, at 104 ± 17% (Table 2). However, we found the HR-CTV D90 for 47% of the low-bulky tumor plans corresponded to more than 5% overdose, while 42% of the plans received more than 5% under-dose (Table 3). For non-bulky or bulky tumors, the average HR-CTV D90 values were either considerable overdoses (+10.5%) or under-doses (–28.2%), respectively. Regardless of their tumor size, only 16% of conventional plans had HR-CTV D90 values within 5% of the prescription dose (Table 3). Of the conventional plans, 52.7%, had at least one OAR with a D2cc value above the GEC-ESTRO limit. The D2cc limits for the rectum, bladder, and sigmoid colon were exceeded in 20%, 29%, and 18% of the conventional plans, respectively (Table 3). The bladder was the most frequently overdosed OAR (29% of plans, Table 3), despite having a higher recommended dose limit than the rectum or sigmoid colon. The mean doses of each OAR across all plans remained below limits for all HR-CTV sizes. When normalized to the fraction D2cc limits of equation 1, mean OAR D2cc ranged from 66 ± 21% for the sigmoid colon in non-bulky geometries to a high of 94 ± 34% for bladder near low-bulky HR-CTVs (Table 4). When the dose distributions for all HDR and EBRT fractions were considered cumulatively, four of the twelve patients for which all HDR fractions were available showed at least one OAR D2cc value over the limit for the entire treatment: one patient had D2cc values over the limit for both rectum and bladder, one patient exceeded only the rectum limit, one only the bladder limit, and one only the limit of the sigmoid colon.
Table 2.
Conventional plan results
| A: Conventional plan results, target coverage | |||||
| Target (% Rx) | Pt. A dose | HR-CTV D 90 | Diff. | ||
| All plans | 100 ± 1 | 93 ± 24 | –7.1% | ||
| Non-bulky | 100 ± 1 | 111 ± 16 | 10.6% | ||
| Low-bulky | 100 ± 1 | 103 ± 16 | 3.0% | ||
| Bulky | 100 ± 2 | 72 ± 17 | –28.8% | ||
| B: Conventional plan results, OAR sparring | |||||
| ICRU (Gy) Pt. | D 0.1cc (Gy) | D 0.1 cc /D ICRU | D 2cc (Gy) | D 2cc /D ICRU | |
| Rectum | |||||
| All plans | 3.9 ± 1.5 | 5.0 ± 1.7 | 1.3 ± 0.4 | 3.7 ± 1.1 | 1.0 ± 0.3 |
| Non-bulky | 4.2 ± 1.8 | 5.1 ± 1.7 | 1.4 ± 0.5 | 3.8 ± 1.3 | 1.0 ± 0.4 |
| Low-bulky | 3.9 ± 1.6 | 5.0 ± 2.0 | 1.3 ± 0.3 | 3.7 ± 1.3 | 1.0 ± 0.3 |
| Bulky | 3.8 ± 1.1 | 4.9 ± 1.5 | 1.3 ± 0.5 | 3.7 ± 0.9 | 1.0 ± 0.3 |
| Bladder (% Lim) | |||||
| All plans | 4.5 ± 1.8 | 6.7 ± 2.2 | 1.7 ± 0.8 | 5.1 ± 1.4 | 1.3 ± 0.6 |
| Non-bulky | 3.8 ± 1.7 | 5.9 ± 1.7 | 1.7 ± 0.4 | 4.6 ± 1.5 | 1.3 ± 0.3 |
| Low-bulky | 4.6 ± 1.9 | 7.0 ± 1.8 | 1.8 ± 0.9 | 5.3 ± 0.9 | 1.4 ± 0.7 |
| Bulky | 4.7 ± 1.7 | 7.1 ± 2.8 | 1.7 ± 0.9 | 5.4 ± 1.6 | 1.3 ± 0.7 |
Rx – prescribed dose, Pt – point, HR-CTV – high-risk clinical target volume, D90 – minimum dose to the hottest 90% of the target volume, OAR – organ-at-risk, ICRU Pt – point recommended by ICRU report #38 [1], D0.1cc/D2cc – minimum dose to the hottest 0.1 cc or 2 cc of the OAR volume, DICRU – dose to an ICRU Point. All values are mean ± one standard deviation
Table 3.
Conventional and MRIG-CBT plans. Analysis with respect to tumor coverage and organs-at-risk sparing
| Plan characteristics | All sizes (%) | Non-bulky (%) | Low-bulky (%) | Bulky (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Conv. | MRIG. | Conv. | MRIG. | Conv. | MRIG. | Conv. | MRIG. | |
| Point A | ||||||||
| Over Rx +5% | 2 | 35 | 0 | 13 | 0 | 37 | 5 | 48 |
| Within Rx ±5% | 98 | 16 | 100 | 13 | 100 | 11 | 95 | 24 |
| Under Rx –5% | 0 | 49 | 0 | 73 | 0 | 53 | 0 | 29 |
| ICRU point over 80% Rx | ||||||||
| Rectum ICRU | 22 | 22 | 33 | 13 | 16 | 21 | 19 | 29 |
| Bladder ICRU | 42 | 47 | 27 | 13 | 58 | 47 | 38 | 71 |
| HR-CTV D90 | ||||||||
| Over Rx +5% | 33 | 0 | 53 | 0 | 47 | 0 | 5 | 0 |
| Within Rx ±5% | 16 | 56 | 33 | 87 | 11 | 68 | 10 | 24 |
| Under Rx –5% | 51 | 44 | 13 | 13 | 42 | 32 | 86 | 76 |
| D2cc over OAR limit | ||||||||
| Rectum D2cc | 20 | 0 | 13 | 0 | 26 | 0 | 19 | 0 |
| Bladder D2cc | 29 | 0 | 13 | 0 | 37 | 0 | 33 | 0 |
| Sigmoid D2cc | 18 | 0 | 7 | 0 | 5 | 0 | 38 | 0 |
| D2cc within ±5% of OAR limit | ||||||||
| Rectum D2cc | 7 | 9 | 7 | 0 | 0 | 0 | 14 | 24 |
| Bladder D2cc | 13 | 44 | 20 | 13 | 5 | 47 | 14 | 62 |
| Sigmoid D2cc | 11 | 9 | 13 | 0 | 11 | 0 | 10 | 24 |
OAR – organ-at-risk, D2cc, D0.1cc – minimum dose to the hottest 0.1 cc or 2 cc of the OAR volume, Rx – prescribed dose, HR-CTV – high-risk clinical target volume, D90 – minimum dose to the hottest 90% of the volume
Table 4.
MRIG-CBT plan results
| A: Conventional plan results, target coverage | ||||||||
| HR-CTV D 90 | Conventional (% Rx) | MRIG-CBT (% Rx) | Diff. (%) | p -value | ||||
| All plans | 93 ± 24 | 91 ± 15 | –7.6 | 0.421 | ||||
| Non-bulky | 111 ± 16 | 98 ± 9 | –9.2 | 0.002 | ||||
| Low-bulky | 103 ± 16 | 97 ± 9 | –6.2 | 0.101 | ||||
| Bulky | 72 ± 17 | 80 ± 16 | –7.7 | 0.028 | ||||
| B: Planning comparison, OAR sparring | ||||||||
| Conventional | MRIG-CBT | Diff. of % OAR limits | p -value | |||||
| D 0.1cc (Gy) | D 2cc (Gy) | % OAR limit | D 0.1cc (Gy) | D 2cc (Gy) | % OAR limit | |||
| Rectum | ||||||||
| All plans | 5.0 ± 1.7 | 3.7 ± 1.1 | 75 ± 34 | 4.2 ± 1.4 | 3.1 ± 1.0 | 56 ± 25 | –19.0% | < 0.001 |
| Non-bulky | 5.1 ± 1.7 | 3.8 ± 1.3 | 80 ± 43 | 3.6 ± 1.2 | 2.5 ± 0.7 | 41 ± 16 | –38.7% | < 0.001 |
| Low-bulky | 5.0 ± 2.0 | 3.7 ± 1.3 | 74 ± 32 | 3.9 ± 1.3 | 2.9 ± 0.8 | 50 ± 19 | –24.1% | < 0.001 |
| Bulky | 4.9 ± 1.5 | 3.7 ± 0.9 | 72 ± 29 | 4.9 ± 1.4 | 3.7 ± 1.0 | 72 ± 26 | –0.1% | 0.893 |
| Bladder | ||||||||
| All plans | 6.7 ± 2.2 | 5.1 ± 1.4 | 86 ± 36 | 6.3 ± 1.7 | 4.7 ± 1.3 | 75 ± 25 | –11.4% | 0.006 |
| Non-bulky | 5.9 ± 1.7 | 4.6 ± 1.5 | 78 ± 40 | 4.9 ± 1.6 | 3.6 ± 1.2 | 53 ± 28 | –25.4% | < 0.001 |
| Low-bulky | 7.0 ± 1.8 | 5.3 ± 0.9 | 94 ± 34 | 6.5 ± 1.3 | 4.8 ± 0.9 | 78 ± 21 | –15.2% | 0.009 |
| Bulky | 7.1 ± 2.8 | 5.4 ± 1.6 | 85 ± 34 | 7.1 ± 1.6 | 5.5 ± 1.0 | 87 ± 16 | 2% | 0.502 |
| Sigmoid | ||||||||
| All plans | 5.1 ± 2.2 | 3.6 ± 1.1 | 73 ± 31 | 4.3 ± 1.9 | 3.0 ± 0.9 | 54 ± 23 | –18.4% | < 0.001 |
| Non-bulky | 4.8 ± 1.4 | 3.5 ± 1.0 | 66 ± 21 | 4.0 ± 1.6 | 2.7 ± 0.9 | 44 ± 14 | –22.0% | < 0.001 |
| Low-bulky | 4.7 ± 1.4 | 3.5 ± 1.1 | 72 ± 30 | 3.9 ± 1.3 | 2.9 ± 0.8 | 52 ± 21 | –19.4% | < 0.001 |
| Bulky | 5.6 ± 3.1 | 3.8 ± 1.3 | 78 ± 38 | 4.9 ± 2.4 | 3.4 ± 1.0 | 64 ± 28 | –14.8% | 0.006 |
OAR – organ-at-risk, D2cc, D0.1cc – minimum dose to the hottest 0.1 cc or 2 cc of the OAR volume, Rx – prescribed dose, HR-CTV – high-risk clinical target volume, D90 – minimum dose to the hottest 90% of the volume
Statistically significant correlation was found between the rectum ICRU point and its D2cc measurements (r 2 = 0.5449, p < 0.001) (Table 2). The mean ratio of D2cc to DICRU for the rectum was 1.0 ± 0.3. However, no significant correlation was seen between the bladder ICRU point and D2cc (r 2 = 0.210, p = 0.125). The bladder ICRU point underestimated maximal dose (D2cc), i.e. the mean ratio of D2cc to DICRU was 1.3 ± 0.6.
MRIG-CBT plans
MRIG-CBT plans exhibited significantly higher HR-CTV D90 values for non-bulky (p = 0.002) and bulky tumors (p = 0.028) (Table 4). No MRIG-CBT plans had a D90 greater than 5% above the prescription dose. No MRIG-CBT plans exhibited D2cc of any OARs over their GEC-ESTRO limit (Table 3). Thus, no patients showed overall D2cc values over the limits throughout their full HDR treatments. The effect of this restriction was seen in the HR-CTV coverage result: for all plans where HR-CTV D90 was less than the prescription dose, at one or more OAR D2cc was within 5% of its limit: this OAR was the bladder in 96% of these plans, the rectum in 20%, and the sigmoid colon in 20%. The average D2cc values of MRIG-CBT plans ranged from 41 ± 16% of the fraction limit for rectum of non-bulky cases and 87 ± 16% for bladder in bulky plans (Table 4). The bladder was the only structure limiting dose in non-bulky and low-bulky plans. For the 16 bulky plans with HR-CTV D90 less than the prescribed dose, the bladder D2cc was within 5% of its limit in 81% of the plans, and the rectum and sigmoid D2cc were each dose-restricting in 31% of such cases.
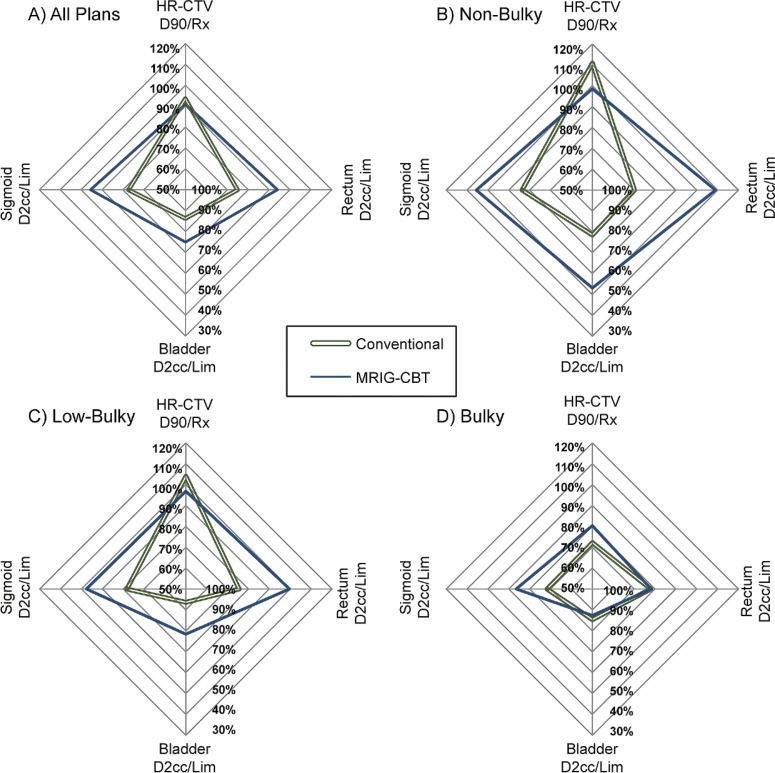
To visually compare volumetric measurements (Fig. 2), the mean success of each plan attribute was plotted on a separate axis; HR-CTV D90 is plotted on the vertical axis relative to the prescription dose (Rx). Therefore, the 100% value of HR-CTV D90/prescription dose (HR-CTV D90/Rx) on the vertical axis is ideal. For instance, in B panel, conventional plans have 111% Rx at HR-CTV D90, while MRIG-CBT plans deliver 99% Rx. The D2cc of each OAR relative to the OAR limit (D2cc/Lim) is plotted on the other three axes, thus smaller % values represent better OAR sparing; these axes were inverted such that OAR sparing increased as the values of sigmoid, bladder, rectum move away from the origin. Thus a larger area of the plotted “diamonds” indicates improved plan characteristics. Large improvements were seen for both low- and non-bulky cases, while mean treatment results of bulky HR-CTV plans remained comparable (Table 3).
Fig. 2.
Average plan attributes for each HR-CTV size. HR-CTV D90 is plotted on the vertical axis relative to the prescription dose. Therefore, the 100% of HR-CTV D90/prescription dose (Rx) represents HR-CTV D90 receives Rx. The D2cc of each OAR relative to the OAR limit (D2cc/Lim) is plotted on the other three axes, thus smaller % values represent better OAR sparing; these axes are inverted such that OAR sparing increased as the values of sigmoid, bladder, rectum move away from the origin
Discussion
The dosimetric improvements of MRIG-CBT plans have, when compared to conventional plans, been published for HDR [19], and PDR [6–9] BT when using tandem-and-ring applicator (T&R) [7, 9], T&R plus needles [7, 9], T&O [6], or tandem only [8]. However, previous studies analyzed the benefits of MRIG-CBT without tumor size classification or with only two tumor size classifications, large and small volumes. In this study, the dosimetric benefits of MRIG-CBT were analyzed for three different HR-CTV groups when using conventional T&O applicators without the use of supplementary needles. For bulky tumors, however, neither planning method was able to increase HR-CTV D90 to the prescribed dose when the T&O was used. MRIG-CBT resulted in only small improvements for bulky tumors. Therefore, the only use of conformal volume optimization and MRI guidance are limited to appropriately cover bulky tumors when a conventional intracavitary applicator is used. MRIG-CBT applicators for bulky tumors have been developed: T&R plus needles (Vienna applicator) [7, 9] and T&O plus needles (Utrecht applicator) [21] and their use is recommended for bulky tumors. Transperineal interstitial implants for MRIG-CBT can be an alternative technique for bulky tumors. As a less invasive approach for bulky tumors, intensity-modulated brachytherapy using intracavitary applicators with electronic brachytherapy source has been under development [22, 23].
The MRIG-CBT treatment planning approach showed good HR-CTV D90 in all cases where it was not restricted by OAR dose limits. Most of the optimization results were highly dependent on the OAR D2cc limits set by equation (1). In cases where the HR-CTV D90 did not reach the prescription dose, one of the three OARs restricted the HR-CTV D90, and increasing the limit or allowing a slight overdose could have a large effect on HR-CTV D90. This may be possible by shifting more of the allotted OAR dose to earlier HDR fractions and relying on tumor regression to enable sparing in the corresponding OAR in later treatment fractions. Such a shift in OAR limits would also affect the relative results of the two planning methods. In the present study, the optimization was able to reduce systematic overdoses to the HR-CTV in non-bulky plans, and reduce the D90 variance seen in the conventional coverage of low-bulky tumors. Especially, for non-bulky tumors, MRIG-CBT can result in the dose reduction on side wall and nodes. Future studies on nodal doses of MRIG-CBT need to be performed. The studies on how to deliver appropriate radiation dose to nodes through EBRT also are expected to be followed. For instance, a simultaneous boosting to pelvic lymph nodes can be an option using image guided IMRT technique.
As emphasized by Trnková et al. [3], large-scale modifications to conventional distributions and/or solely inverse planning should be avoided until an understanding of the relationship between DVH-values and treatment efficacy is established. Thus, hybrid inverse optimization [24] was utilized in this study. What constitutes a “large-scale” modification and how to find improved inverse planning objectives are still open questions.
A low-magnetic-field MR system of 1.5 or 2.0 Tesla has been used for MR-guided BT. However, the signal-to-noise ratio (SNR) of a 1.5 Tesla MR is suboptimal, particularly its ability to accurately delineate the uterine cervix and the macroscopic tumor regions [14, 24]. In addition, increased intra-scan motion artifacts exist when using 1.5 Tesla MR due to the extended acquisition time when compared to a 3 Tesla MR (see Fig. 3 in reference [11]). The high resolution (3 Tesla) MR system used in this study has approximately double the SNR of a 1.5 Tesla system, resulting in an image with a higher contrast in the uterine cervix and vagina [11] and a substantially faster image acquisition speed [12, 25]. However, caution must be exercised when metal based applicators are used in 3 Tesla MRI-guided BT scans. In this study, a titanium T&O applicator was used with a 3 Tesla MRI. Before any clinical use of 3 Tesla MRI-guided BT, the safety concerns, especially radiofrequency [RF] heat- had been verified first [26]. Once assured of the safety of the process, the artifacts and distortions of the titanium applicator were quantified [12], since the artifact to noise ratio also increases in 3 Tesla MR systems.
Although it has been suggested to replace the BT component of cervical cancer treatment with stereotactic body RT, the unique advantages of BT should not be overlooked. BT offers a significantly higher biologically-effective dose due to the short distance between radiation sources and target volumes, and the sharp fall-off in radiation dose in space allows for higher dose to non-bulky and low-bulky tumor volumes while still retaining adequate sparing of nearby healthy tissues.
Conclusions
HR-CTV D90 was considerably affected by tumor size. Only 16% of conventional plans had HR-CTV D90 values within 5% of the prescription dose. Mean D2cc values of rectum, bladder, and sigmoid colon were found to be below the GEC-ESTRO recommended limits for the conventional plans. However, in 52.7% of conventional plans at least one of the OARs had a D2cc value over its limit. When all HDR fractions were considered cumulatively, 17% of patients had overall rectum and bladder D2cc values over their limits, while 8% of patients exceeded the limit for sigmoid colon. The MRIG-CBT treatment planning technique was found to improve treatment plans relative to those of the conventional approach with HR-CTV less that 40 cc, bringing the D90 dose closer to the prescription value while avoiding overdose to the D2cc of each OAR. Using conventional intracavitary applicators, only small improvements were seen when MRIG-CBT was used for bulky targets (> 40 cc).
References
- 1.“ICRU-report-38”. Dose and volume specification for reporting intracavitary therapy in gynecology; International Commission on Radiation Units and Measurements; Bethesda: 1985. [Google Scholar]
- 2.Viswanathan AN, Thomadsen B, Committee ABSCCR American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy. 2012;11:33–46. doi: 10.1016/j.brachy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Trnkova P, Pötter R, Baltas D, et al. New inverse planning technology for image-guided cervical cancer brachytherapy: description and evaluation within a clinical frame. Radiother Oncol. 2009;93:331–340. doi: 10.1016/j.radonc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan AN, Dimopoulos J, Kirisits C, et al. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–498. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Haie-Meder C, Pötter R, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 6.De Brabandere M, Mousa AG, Nulens A, et al. Potential of dose optimisation in MRI-based PDR brachytherapy of cervix carcinoma. Radiother Oncol. 2008;88:217–226. doi: 10.1016/j.radonc.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Lindegaard JC, Tanderup K, Nielsen SK, et al. MRI-guided 3D optimization significantly improves DVH parameters of pulsed-dose-rate brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:756–764. doi: 10.1016/j.ijrobp.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Zwahlen D, Jezioranski J, Chan P, et al. Magnetic resonance imaging-guided intracavitary brachytherapy for cancer of the cervix. Int J Radiat Oncol Biol Phys. 2009;74:1157–1164. doi: 10.1016/j.ijrobp.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Tanderup K, Nielsen SK, Nyvang GB, et al. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol. 2010;94:173–180. doi: 10.1016/j.radonc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos JC, Lang S, Kirisits C, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2009;75:56–63. doi: 10.1016/j.ijrobp.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka M, Kido A, Koyama T, et al. MRI of the female pelvis at 3T compared to 1.5T: evaluation on high-resolution T2-weighted and HASTE images. J Magn Reson Imaging. 2007;25:527–534. doi: 10.1002/jmri.20842. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Muruganandham M, Modrick JM, et al. Evaluation of artifacts and distortions of titanium applicators on 3.0-Tesla MRI: feasibility of titanium applicators in MRI-guided brachytherapy for gynecological cancer. Int J Radiat Oncol Biol Phys. 2011;80:947–955. doi: 10.1016/j.ijrobp.2010.07.1981. [DOI] [PubMed] [Google Scholar]
- 13.Anderson J, Huang Y, Kim Y. Dosimetric impact of point A definition on high-dose-rate brachytherapy for cervical cancer: evaluations on conventional point A and MRI-guided, conformal plans. J Contemp Brachytherapy. 2012;4:241–246. doi: 10.5114/jcb.2012.32559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapur T, Egger J, Damato A, et al. 3-T MR-guided brachytherapy for gynecologic malignancies. Magn Reson Imag. 2012;30:1279–1290. doi: 10.1016/j.mri.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubley GJ, Bloch BN, Vazquez C, et al. Accuracy of endorectal magnetic resonance/transrectal ultrasound fusion for detection of prostate cancer during brachytherapy. Urology. 2013;81:1284–1290. doi: 10.1016/j.urology.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Hellebust TP, Kirisits C, Berger D, et al. Recommendations from gynaecological (GYN) GEC-ESTRO working group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol. 2010;96:153–160. doi: 10.1016/j.radonc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Haack S, Nielsen SK, Lindegaard JC, et al. Applicator reconstruction in MRI 3D image-based dose planning of brachytherapy for cervical cancer. Radiother Oncol. 2009;91:187–193. doi: 10.1016/j.radonc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Pötter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Kirisits C, Pötter R, Lang S, et al. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2005;62:901–911. doi: 10.1016/j.ijrobp.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 20.Kirisits C, Pötter R, Lang S, et al. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2005;62:901–911. doi: 10.1016/j.ijrobp.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Jurgenliemk-Schulz IM, Tersteeg RJ, Roesink JM, et al. MRI-guided treatment-planning optimisation in intracavitary or combined intracavitary/interstitial PDR brachytherapy using tandem ovoid applicators in locally advanced cervical cancer. Radiother Oncol. 2009;93:322–330. doi: 10.1016/j.radonc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Kim Y, Wu X, et al. Rotating-shield brachytherapy for cervical cancer. Phys Med Biol. 2013;58:3931–3941. doi: 10.1088/0031-9155/58/11/3931. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Flynn RT, Yang W, et al. Rapid emission angle selection for rotating-shield brachytherapy. Med Phys. 2013;40:051720. doi: 10.1118/1.4802750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y, Modrick JM, Bayouth JE, et al. Logistics of tandem and ovoids HDR plan optimization in MRI-guided brachytherapy for cervical cancer: comparisons of standard, graphical, and inverse optimization. Brachytherapy. 2009;8:121–122. [Google Scholar]
- 25.Sheu MH, Chang CY, Wang JH, et al. Preoperative staging of cervical carcinoma with MR imaging: a reappraisal of diagnostic accuracy and pitfalls. Eur Radiol. 2001;11:1828–1833. doi: 10.1007/s003300000774. [DOI] [PubMed] [Google Scholar]
- 26.Hussain SM, van den Bos IC, Oliveto JM, et al. MR imaging of the female pelvis at 3T. Magn Reson Imaging Clin N Am. 2006;14:537–544, vii. doi: 10.1016/j.mric.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Nixon E, Kim Y, Kearney WR, et al. HDR brachytherapy tandem and ovoid titanium applicator safety assessment in 3T MRI. Brachytherapy. 2008;7:135. [Google Scholar]