Abstract
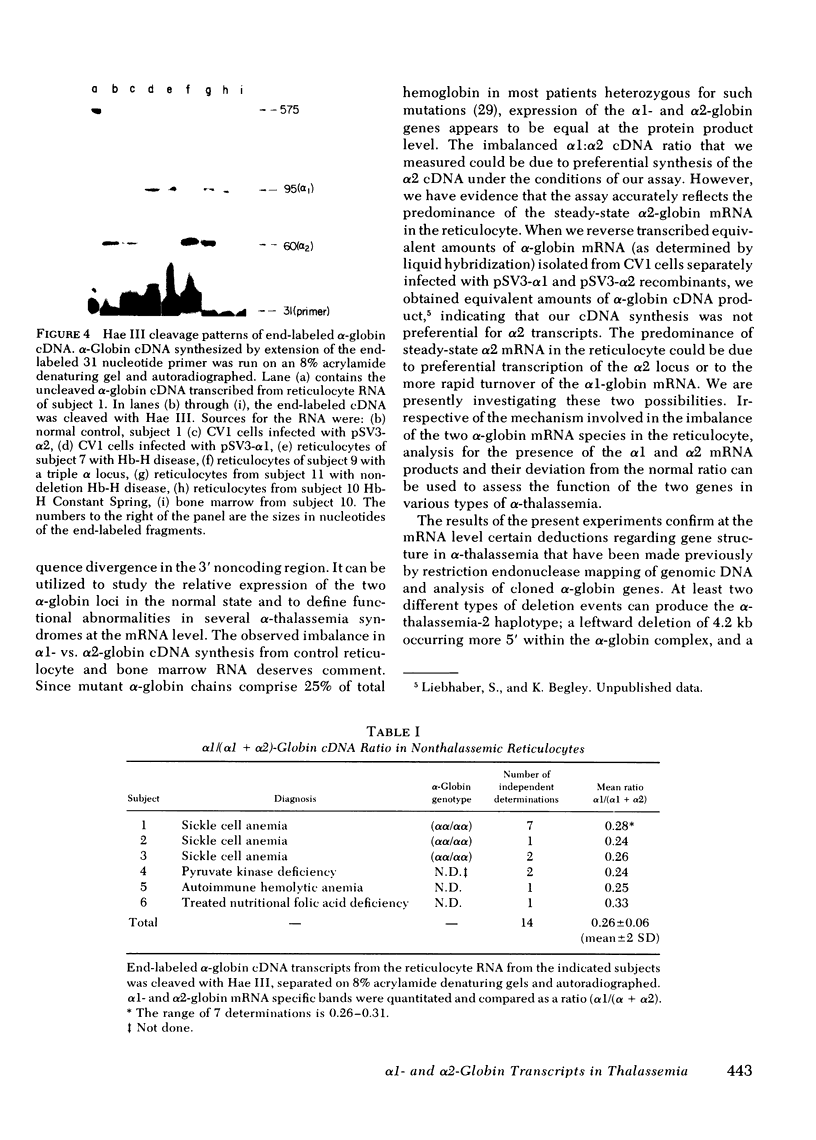
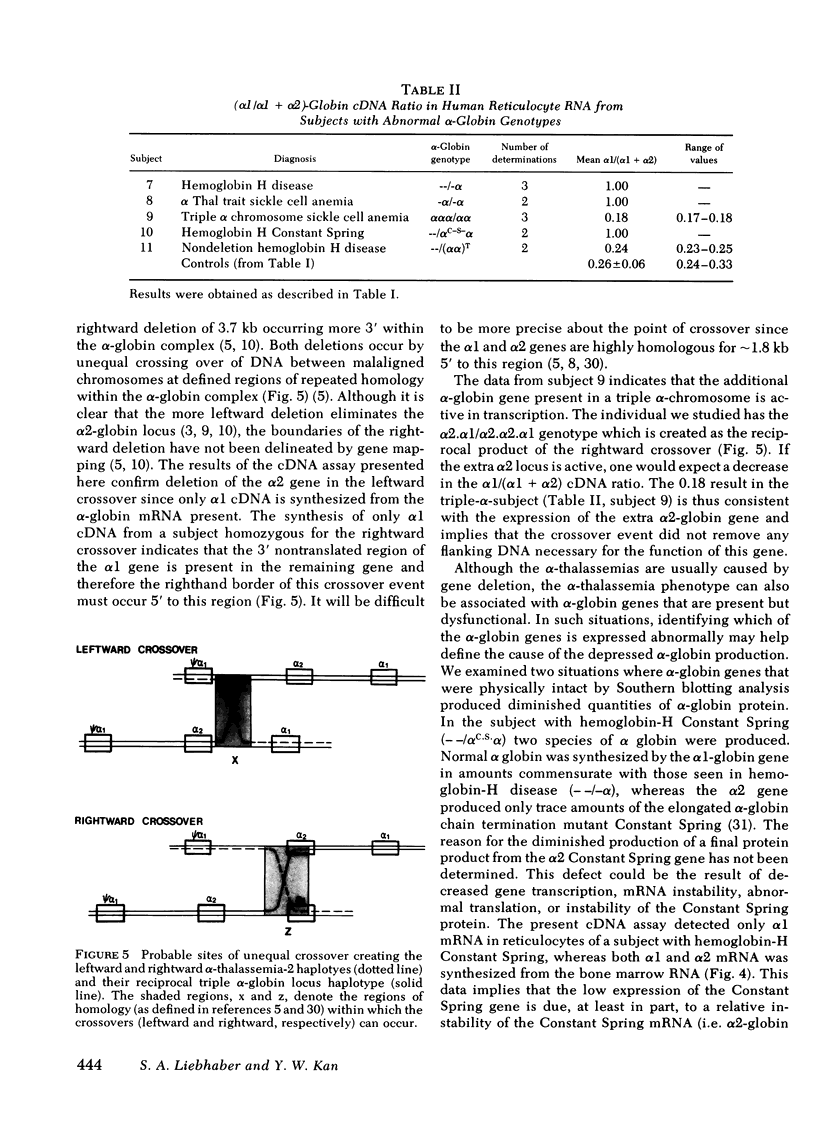
The alpha-globin polypeptide is encoded by two adjacent genes, alpha 1 and alpha 2. In the normal diploid state (alpha alpha/alpha alpha) all four alpha-globin genes are expressed. Loss or dysfunction of one or more of these genes leads to deficient alpha-globin production and results in alpha-thalassemia. We present a technique to differentially assess the steady-state levels of the alpha 1- and alpha-2-globin messenger RNA (mRNA) transcripts and thus delineate the relative level of expression of the two alpha-globin loci in a variety of alpha-thalassemia states. Only alpha 1 mRNA was produced in the alpha-thalassemia-2 haplotype (-alpha) (one of the two alpha-globin genes deleted from chromosome 16). This confirms previous gene mapping data which demonstrate deletion of the alpha 2 gene. The triple alpha-globin gene haplotype (alpha alpha alpha) is the reciprocal of the alpha-thalassemia-2 haplotype and thus contains an extra alpha 2-globin gene. RNA from this haplotype contained a greater than normal level of alpha 2-relative to alpha 1-globin mRNA. This data implies that the extra alpha 2 gene in the triple alpha-globin haplotype is functional. We detected a relative instability of the alpha 2-globin mRNA encoding the alpha-globin structural mutant Constant Spring. This instability may contribute to the low level of expression of the alpha-Constant Spring protein. In a Chinese patient with nondeletion hemoglobin-H disease (- -/alpha alpha T) (both alpha-globin genes are present but not fully functional) a normal ratio was maintained between the levels of alpha 1- and alpha 2-globin mRNA, implying that mRNA production from both alpha-globin genes is suppressed in a balanced manner. These observations extended previous findings concerning the structural rearrangements in the deletion types of alpha-thalassemia and the pathophysiology of two nondeletion variants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Milner P. F. Haemoglobin Constant Spring--a chain termination mutant? Nature. 1971 Dec 10;234(5328):337–340. doi: 10.1038/234337a0. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Nienhuis A., Turner P., Velez R., Anderson W. F., Ruddle F., Lawrence J., Creagan R., Kucherlapati R. Localization of the human alpha-globin structural gene to chromosome 16 in somatic cell hybrids by molecular hybridization assay. Cell. 1977 Sep;12(1):205–218. doi: 10.1016/0092-8674(77)90198-2. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Embury S. H., Dozy A. M., Kan Y. W. Molecular mechanisms in alpha thalassemia: racial differences in alpha-globin gene organization. Ann N Y Acad Sci. 1980;344:31–40. doi: 10.1111/j.1749-6632.1980.tb33646.x. [DOI] [PubMed] [Google Scholar]
- Embury S. H., Lebo R. V., Dozy A. M., Kan Y. W. Organization of the alpha-globin genes in the Chinese alpha-thalassemia syndromes. J Clin Invest. 1979 Jun;63(6):1307–1310. doi: 10.1172/JCI109426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury S. H., Miller J. A., Dozy A. M., Kan Y. W., Chan V., Todd D. Two different molecular organizations account for the single alpha-globin gene of the alpha-thalassemia-2 genotype. J Clin Invest. 1980 Dec;66(6):1319–1325. doi: 10.1172/JCI109984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E. Y., Rosbash M. The syntheiss of high yields of full-length reverse transcripts of globin mRNA. Nucleic Acids Res. 1977 Oct;4(10):3455–3471. doi: 10.1093/nar/4.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens M., Dozy A. M., Embury S. H., Zachariades Z., Hadjiminas M. G., Stamatoyannopoulos G., Kan Y. W. Triplicated alpha-globin loci in humans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):518–521. doi: 10.1073/pnas.77.1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M., Trecartin R., Todd D. Identification of a nondeletion defect in alpha-thalassemia. N Engl J Med. 1977 Nov 17;297(20):1081–1084. doi: 10.1056/NEJM197711172972002. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M., Varmus H. E., Taylor J. M., Holland J. P., Lie-Injo L. E., Ganesan J., Todd D. Deletion of alpha-globin genes in haemoglobin-H disease demonstrates multiple alpha-globin structural loci. Nature. 1975 May 15;255(5505):255–256. doi: 10.1038/255255a0. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M. J., Kan Y. W. Cloning and complete nucleotide sequence of human 5'-alpha-globin gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7054–7058. doi: 10.1073/pnas.77.12.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M., Kan Y. W. Homology and concerted evolution at the alpha 1 and alpha 2 loci of human alpha-globin. Nature. 1981 Mar 5;290(5801):26–29. doi: 10.1038/290026a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. The 3' untranslated regions of the duplicated human alpha-globin genes are unexpectedly divergent. Cell. 1980 Nov;22(2 Pt 2):371–377. doi: 10.1016/0092-8674(80)90347-5. [DOI] [PubMed] [Google Scholar]
- Molloy P. L., Symons R. H. Cleavage of DNA.RNA hybrids by type II restriction enzymes. Nucleic Acids Res. 1980 Jul 11;8(13):2939–2946. doi: 10.1093/nar/8.13.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C. The duplicated human alpha-globin genes: their relative expression as measured by RNA analysis. Cell. 1981 May;24(2):345–351. doi: 10.1016/0092-8674(81)90324-x. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Old J., Lazarus H., Altay C., Gurgey A., Weatherall D. J., Nathan D. G. The molecular basis of alpha-thalassemias: frequent occurrence of dysfunctional alpha loci among non-Asians with Hb H disease. Cell. 1979 May;17(1):33–42. doi: 10.1016/0092-8674(79)90292-7. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. The duplicated human alpha globin genes lie close together in cellular DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5950–5954. doi: 10.1073/pnas.75.12.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Phillips J. A., 3rd, Scott A. F., Smith K. D., Young K. E., Lightbody K. L., Jiji R. M., Kazazian H. H., Jr A molecular basis for hemoglobin-H disease in American blacks. Blood. 1979 Dec;54(6):1439–1445. [PubMed] [Google Scholar]
- Pressley L., Higgs D. R., Clegg J. B., Perrine R. P., Pembrey M. E., Weatherall D. J. A new genetic basis for hemoglobin-H disease. N Engl J Med. 1980 Dec 11;303(24):1383–1388. doi: 10.1056/NEJM198012113032402. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Maniatis T. The structure of a human alpha-globin pseudogene and its relationship to alpha-globin gene duplication. Cell. 1980 Sep;21(2):537–544. doi: 10.1016/0092-8674(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Palacios R., Sullivan D., Kiely M. L., Gonzales C., Taylor J. M. Immunoadsorption of ovalbumin synthesizing polysosmes and partial purification of ovalbumin messenger RNA. Methods Enzymol. 1974;30:631–648. doi: 10.1016/0076-6879(74)30061-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Temple G. F., Chang J. C., Kan Y. W. Authentic beta-globin mRNA sequences in homozygous betaO-thalassemia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3047–3051. doi: 10.1073/pnas.74.7.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]