Abstract
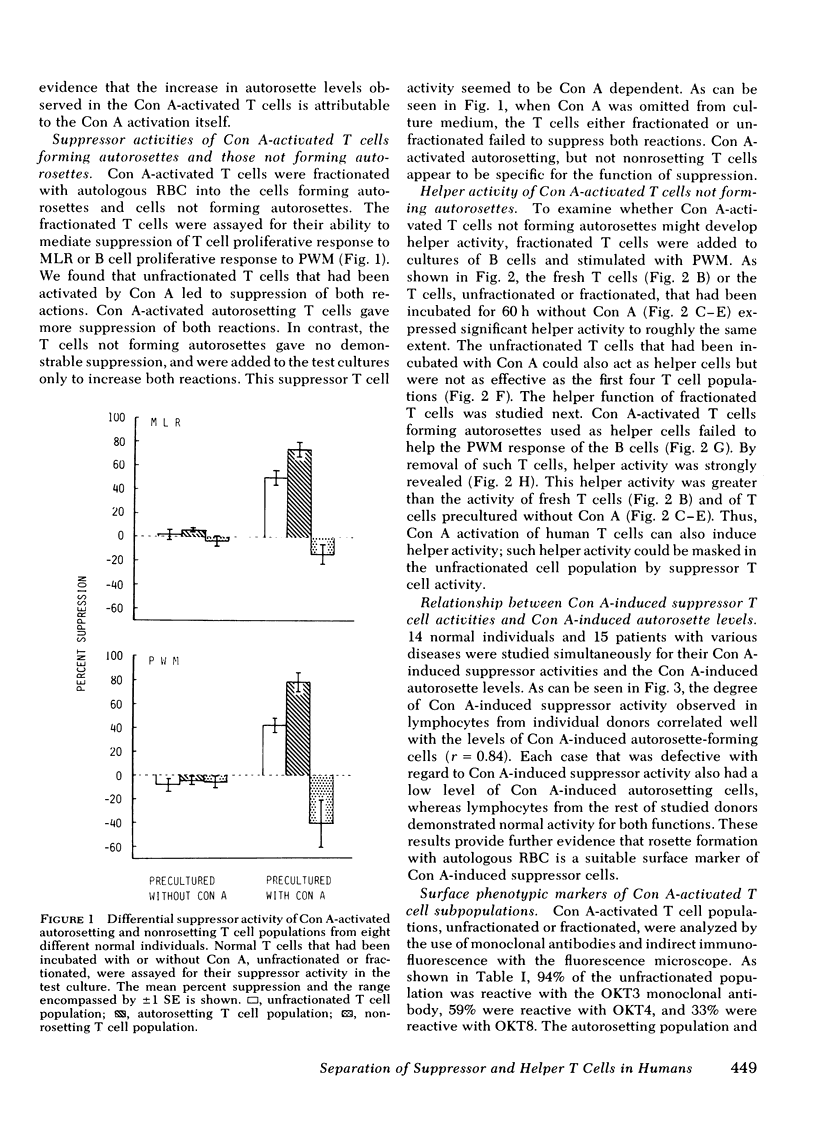
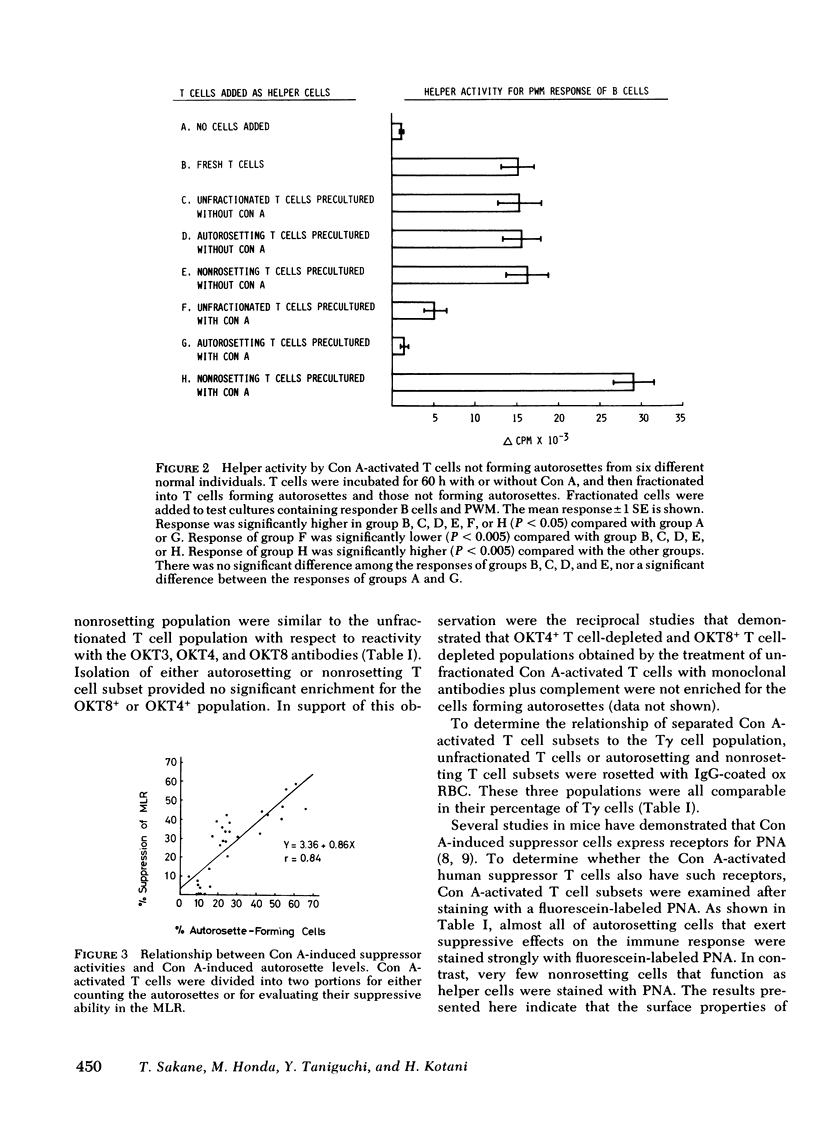
Very few normal human peripheral blood T cells are capable of binding autologous erythrocytes to form rosettes, whereas in the T cell population activated by concanavalin A (Con A) the autorosette levels are markedly enhanced. Fractionation of the Con A-activated T cells with autologous erythrocytes into autorosetting and nonrosetting cells demonstrates that suppressor, but not helper, activity resides in the autorosetting population, whereas the reverse is true of the nonrosetting population. Both these activities are found to be Con A dependent. The Con A-induced human suppressor cells can be identified and separated from the Con A-induced human helper cells by the autorosette technique. Studies on the surface properties of autorosetting and nonrosetting T cells indicate that there is little correlation between the activated suppressor and helper T cell subsets defined by autorosette technique and either those defined by monoclonal antibodies (which are able to distinguish these subsets in the resting but not activated T cells) or those defined by Fc receptors. Since the autorosetting T cell population (which acts as suppressor cells) bears receptors for peanut agglutinin, the nature of Con A-induced human suppressor cells appears to be analogous to that of Con A-induced murine suppressor cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erb P., Meier B., Kraus D., von Boehmer H., Feldmann M. Nature of T cell-macrophage interaction in helper cell induction in vitro. I. Evidence for genetic restriction of T cell-macrophage interactions prior to T cell priming. Eur J Immunol. 1978 Nov;8(11):786–792. doi: 10.1002/eji.1830081107. [DOI] [PubMed] [Google Scholar]
- Fournier C., Charreire J. Activation of a human T cell subpopulation bearing receptors for autologous erythrocytes by concanavalin A. J Immunol. 1978 Aug;121(2):771–776. [PubMed] [Google Scholar]
- Imai Y., Oguchi T., Nakano T., Osawa T. Separation of mouse T cell subsets by a fluorescent activated cell sorter using fluorescence-labeled peanut agglutinin. Immunol Commun. 1979;8(5-6):495–503. doi: 10.3109/08820137909063248. [DOI] [PubMed] [Google Scholar]
- Innes J. B., Kuntz M. M., Kim Y. T., Weksler M. E. Induction of suppressor activity in the autologous mixed lymphocyte reaction and in cultures with concanavalin A. J Clin Invest. 1979 Dec;64(6):1608–1613. doi: 10.1172/JCI109622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandinski J., Cantor H., Tadakuma T., Peavy D. L., Pierce C. W. Separation of helper T cells from suppressor T cells expressing different Ly components. I. Polyclonal activation: suppressor and helper activities are inherent properties of distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1382–1390. doi: 10.1084/jem.143.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish A. S., Tansey F. A., Yu G. S., Doyle A. T., Bloom B. R. Interferon as a mediator of human lymphocyte suppression. J Exp Med. 1980 Mar 1;151(3):637–650. doi: 10.1084/jem.151.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Schlossman S. F., Schur P. H., Mills J. A., Steinberg A. D. Alterations in immunoregulatory T cell subsets in active systemic lupus erythematosus. J Clin Invest. 1980 Nov;66(5):1171–1174. doi: 10.1172/JCI109948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Imai Y., Naiki M., Osawa T. Characterization of mouse helper and suppressor T cell subsets separated by lectins. J Immunol. 1980 Nov;125(5):1928–1932. [PubMed] [Google Scholar]
- Nakano T., Oguchi Y., Imai Y., Osawa T. Induction and separation of mouse helper T cells by lectins. Immunology. 1980 Jun;40(2):217–222. [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt R. B., Cevario S. J., Gery I. Altered suppressor-cell activities in uveitis. Lancet. 1980 Oct 4;2(8197):722–724. doi: 10.1016/s0140-6736(80)91938-8. [DOI] [PubMed] [Google Scholar]
- Palacios R., Llorente L., Alarcón-Segovia D., Ruíz-Arguelles A., Díaz-Jouanen E. Autologous rosette-forming T cells as the responding cells in human autologous mixed-lymphocyte reaction. J Clin Invest. 1980 Jun;65(6):1527–1530. doi: 10.1172/JCI109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Biniaminov M., Rosenthal E., Sharon N., Ramot B. Interaction of peanut agglutinin with normal human lymphocytes and with leukemic cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):447–451. doi: 10.1073/pnas.76.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Sakane T., Green I. Specificity and suppressor function of human T cells responsive to autologous non-T cells. J Immunol. 1979 Aug;123(2):584–589. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Complement-dependent immunoglobulin M anti-thymus-derived cell antibodies preferentially inactivate suppressor cells. J Clin Invest. 1979 May;63(5):954–965. doi: 10.1172/JCI109396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse H., Dutton R. W. Separation of helper and suppressor T lymphocytes on a ficoll velocity sedimentation gradient. J Exp Med. 1976 May 1;143(5):1199–1210. doi: 10.1084/jem.143.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]


