Abstract
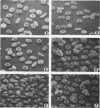
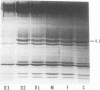
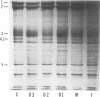
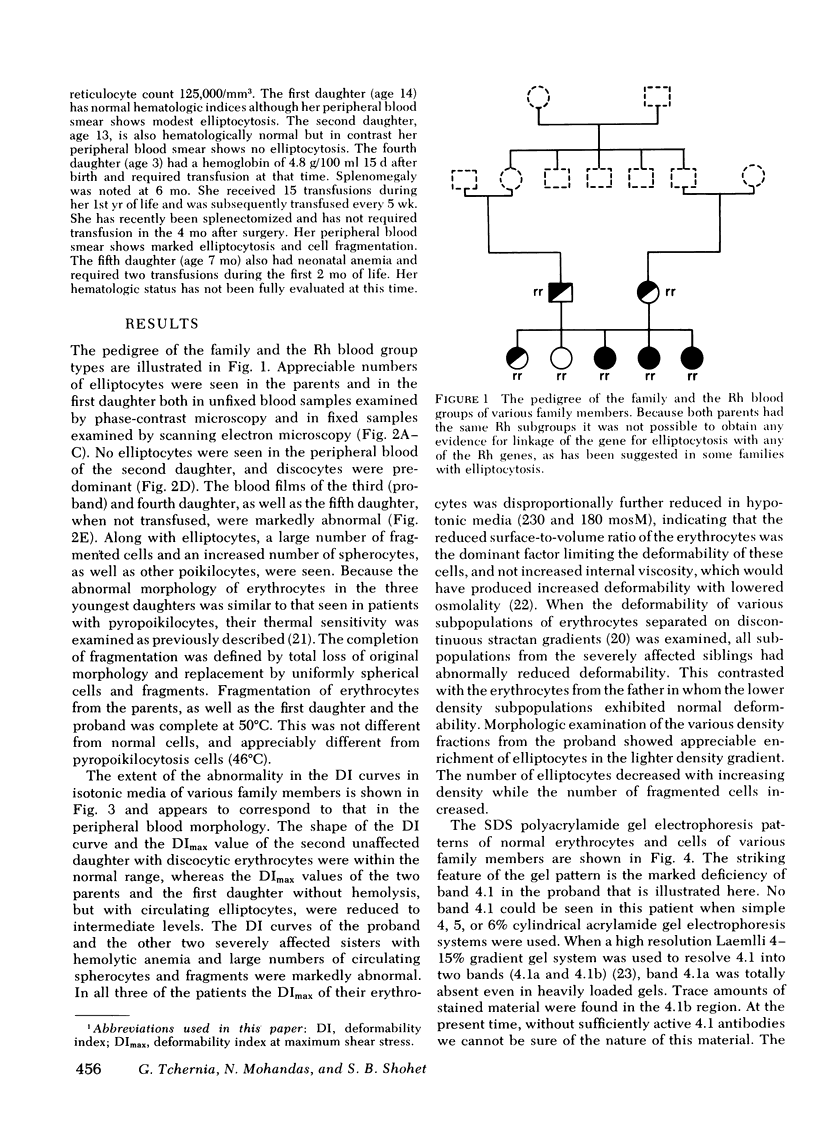
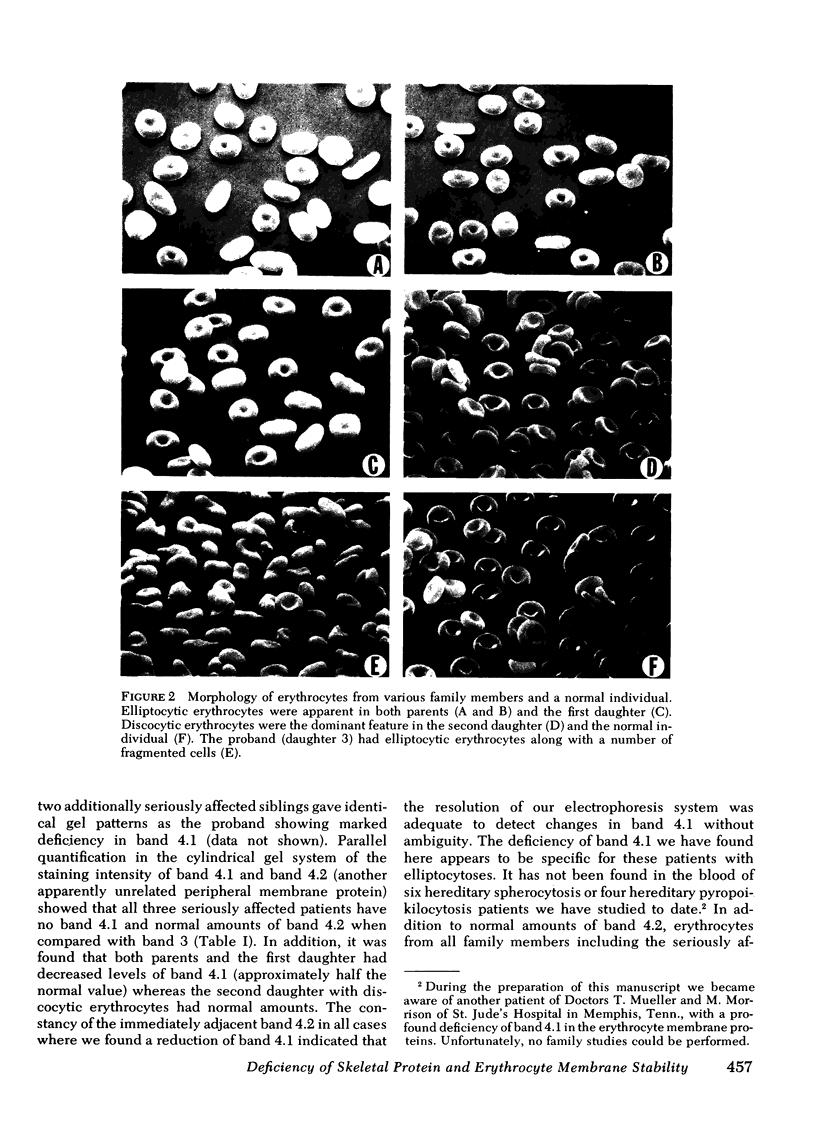
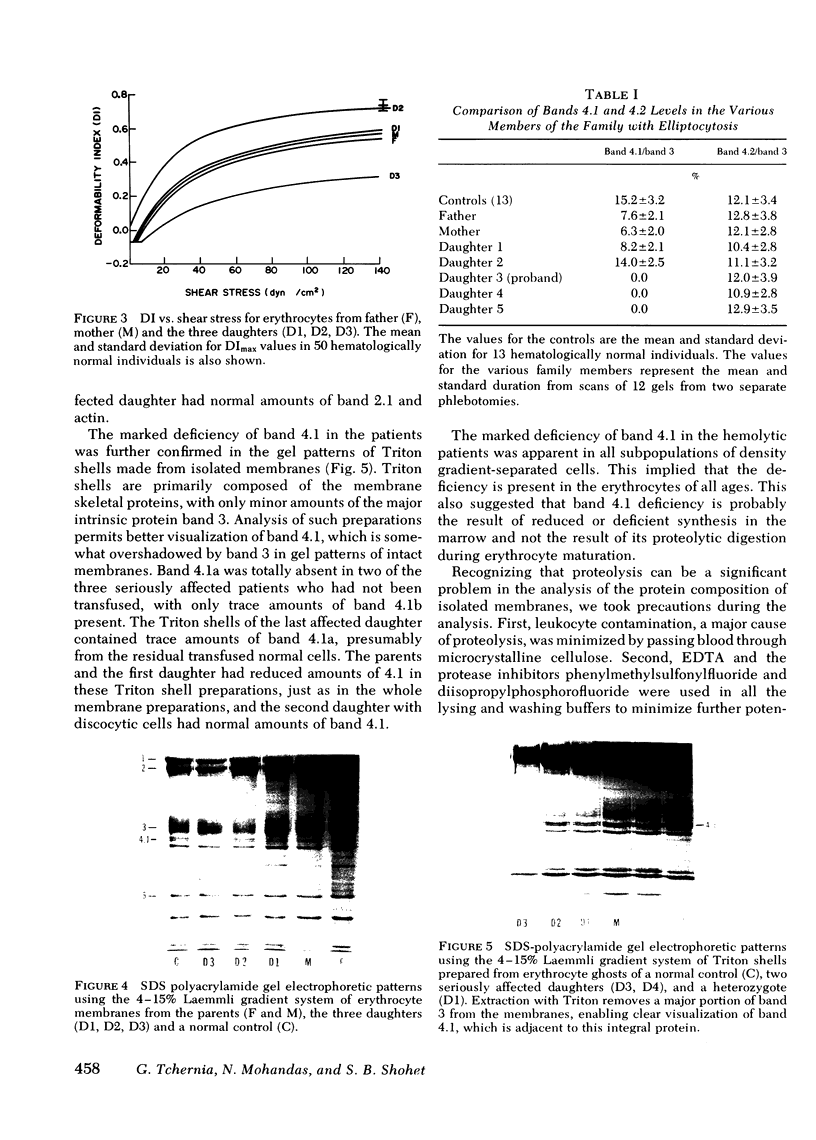
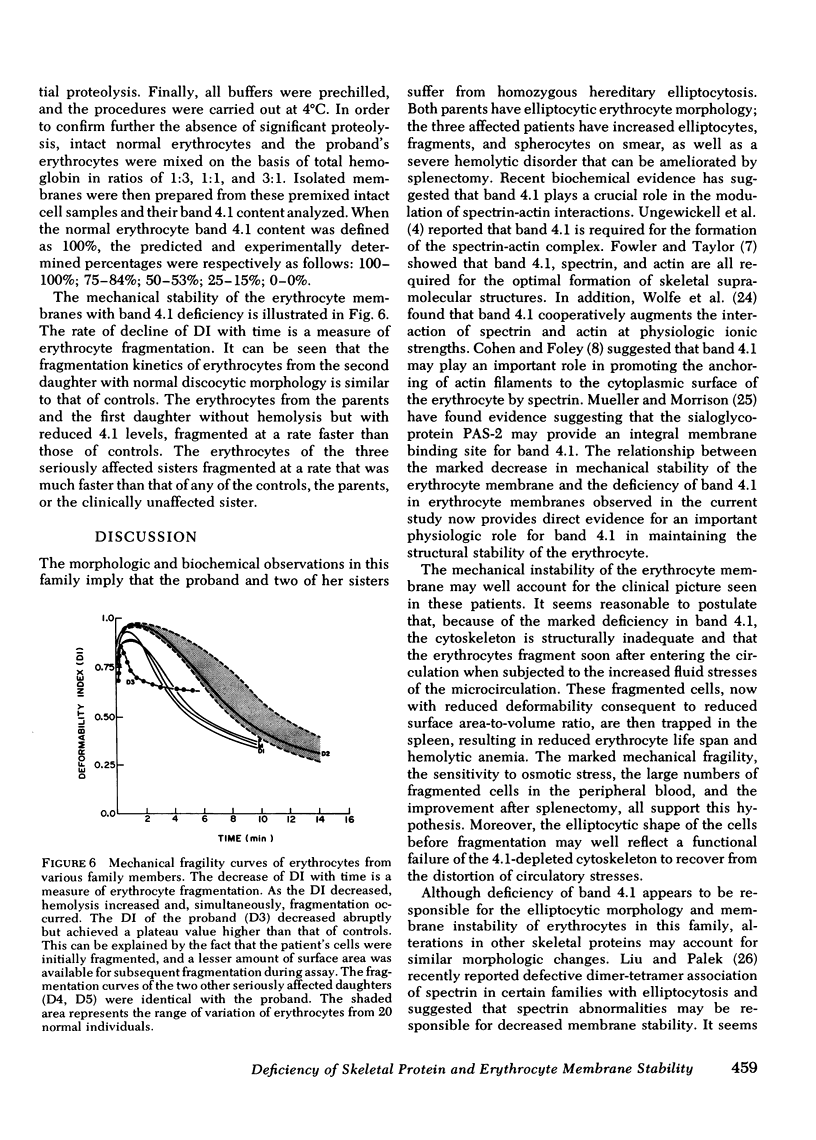
Erythrocytes from three patients with severe hemolytic anemia, marked erythrocyte fragmentation, and elliptocytic poikilocytosis, were studied in terms of both their membrane protein composition and their mechanical characteristics. Erythrocytes from the patients' parents and one minimally affected and one normal sibling were also studied. Morphologic observations implied that the severely affected patients suffered from homozygous hereditary elliptocytosis because erythrocytes of both parents and the one minimally affected sibling showed moderate elliptocytosis on smear, whereas those of an unaffected sibling had normal morphology. The parallel findings of markedly reduced levels of band 4.1 in the erythrocyte membrane proteins of the patients and an intermediate reduction in the cells of the parents and the putative heterozygous sibling, suggest that the elliptocytic shape of the cells was related to the reduced levels of band 4.1. Additional studies showed marked abnormalities in cellular deformability and membrane fragility in the erythrocytes from the homozygous patients. Importantly, these changes were also closely proportional to the reduced levels of band 4.1, suggesting a central role for this protein in the maintenance of normal membrane stability and normal cell shape. It seems likely that this role for band 4.1 is intimately related to its known biochemical connection to the "membrane skeleton" through its linkage with spectrin and actin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Stenbuck P. J. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979 Apr 10;254(7):2533–2541. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Bessis M., Weed R. I. The structure of normal and pathologic erythrocytes. Adv Biol Med Phys. 1973;14:35–91. doi: 10.1016/b978-0-12-005214-1.50006-6. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Unger R. C., Shohet S. B. Monovalent cation composition and ATP and lipid content of irreversibly sickled cells. Blood. 1978 Jun;51(6):1169–1178. [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F. Spectrin-dependent and -independent association of F-actin with the erythrocyte membrane. J Cell Biol. 1980 Aug;86(2):694–698. doi: 10.1083/jcb.86.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Tyler J. M., Branton D. Spectrin-actin associations studied by electron microscopy of shadowed preparations. Cell. 1980 Oct;21(3):875–883. doi: 10.1016/0092-8674(80)90451-1. [DOI] [PubMed] [Google Scholar]
- Feo C. J., Fischer S., Piau J. P., Grange M. J., Tchernia G. Première observation de l'absence d'une protéine de la membrane érythrocytaire (bande 4(1)) dans un cas d'anémie elliptocytaire familiale. Nouv Rev Fr Hematol. 1980;22(4):315–325. [PubMed] [Google Scholar]
- Fowler V., Taylor D. L. Spectrin plus band 4.1 cross-link actin. Regulation by micromolar calcium. J Cell Biol. 1980 May;85(2):361–376. doi: 10.1083/jcb.85.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRECH J. L., CACHIA E. A., CALLEJA F., PULLICINO F. Hereditary elliptocytosis in two Maltese families. J Clin Pathol. 1961 Jul;14:365–373. doi: 10.1136/jcp.14.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenquist A. C., Shohet S. B., Bernstein S. E. Marked reduction of spectrinin hereditary spherocytosis in the common house mouse. Blood. 1978 Jun;51(6):1149–1155. [PubMed] [Google Scholar]
- King L. E., Jr, Morrison M. Calcium effects on human erythrocyte membrane proteins. Biochim Biophys Acta. 1977 Nov 15;471(1):162–168. doi: 10.1016/0005-2736(77)90404-7. [DOI] [PubMed] [Google Scholar]
- LIPTON E. L. Elliptocytosis with hemolytic anemia: the effects of splenectomy. Pediatrics. 1955 Jan;15(1):67–83. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. A., Praktitioner S. Homozygous hereditary elliptocytosis as the cause of haemolytic anemia in infancy. Scand J Haematol. 1968;5(6):486–496. doi: 10.1111/j.1600-0609.1968.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Pryor D. S., Pitney W. R. Hereditary elliptocytosis: a report of two families from new guinea. Br J Haematol. 1967 Jan;13(1):126–134. doi: 10.1111/j.1365-2141.1967.tb08701.x. [DOI] [PubMed] [Google Scholar]
- Shohet S. B. Reconstitution of spectrin-deficient, spherocytic mouse erythrocyte membranes. J Clin Invest. 1979 Aug;64(2):483–494. doi: 10.1172/JCI109486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Reinhardt B. N., Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem. 1980 Jul 25;255(14):7034–7039. [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- Zarkowsky H. S., Mohandas N., Speaker C. B., Shohet S. B. A congenital haemolytic anaemia with thermal sensitivity of the erythrocyte membrane. Br J Haematol. 1975 Apr;29(4):537–543. doi: 10.1111/j.1365-2141.1975.tb02740.x. [DOI] [PubMed] [Google Scholar]