Abstract
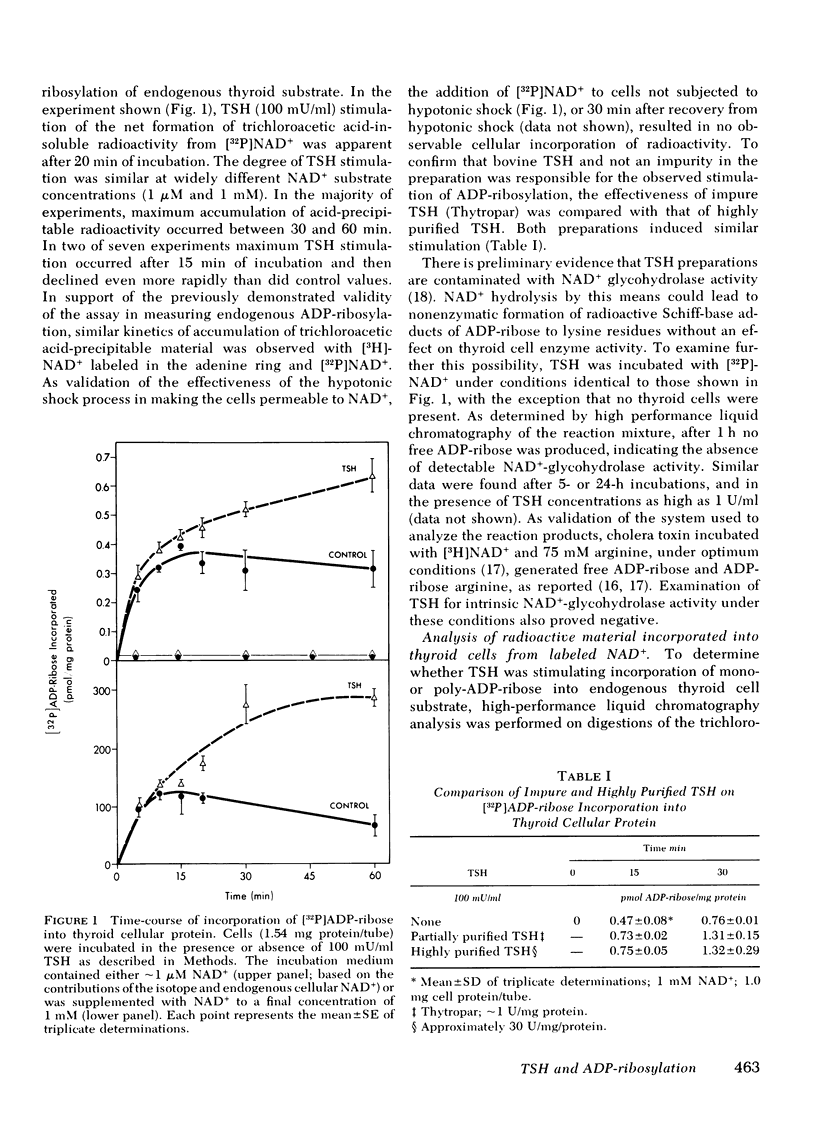
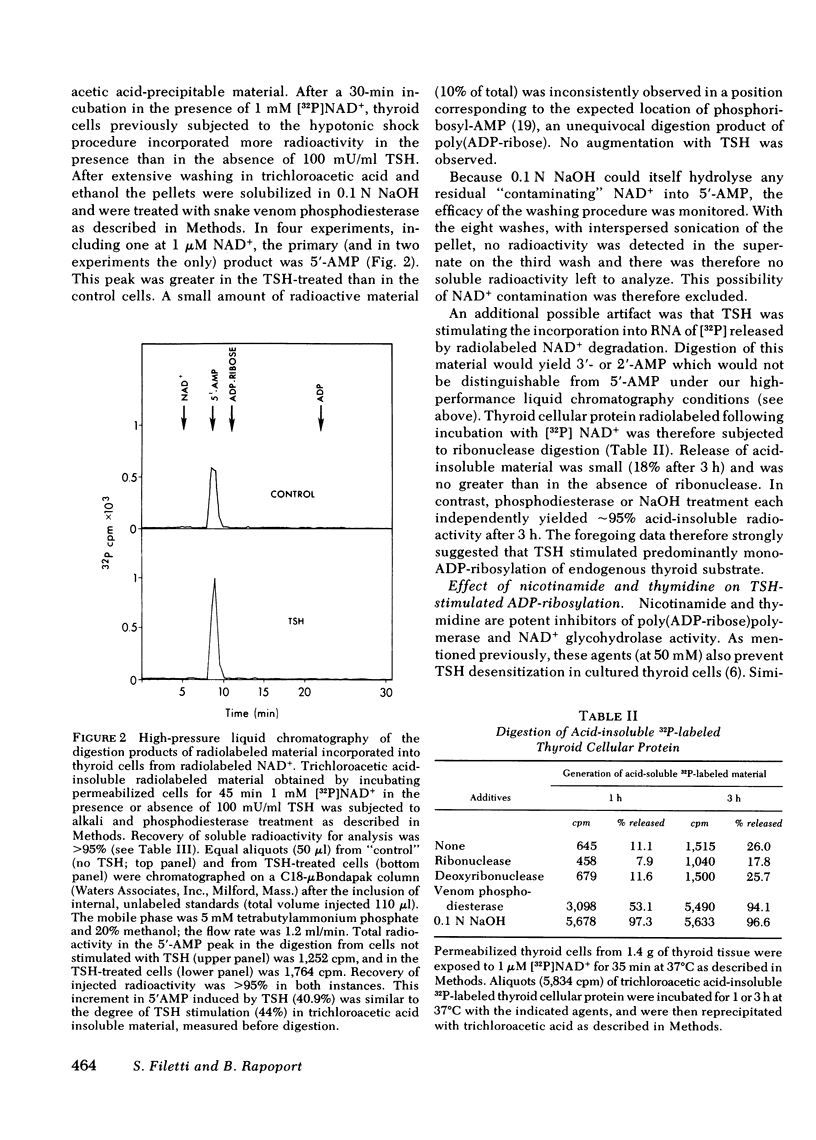
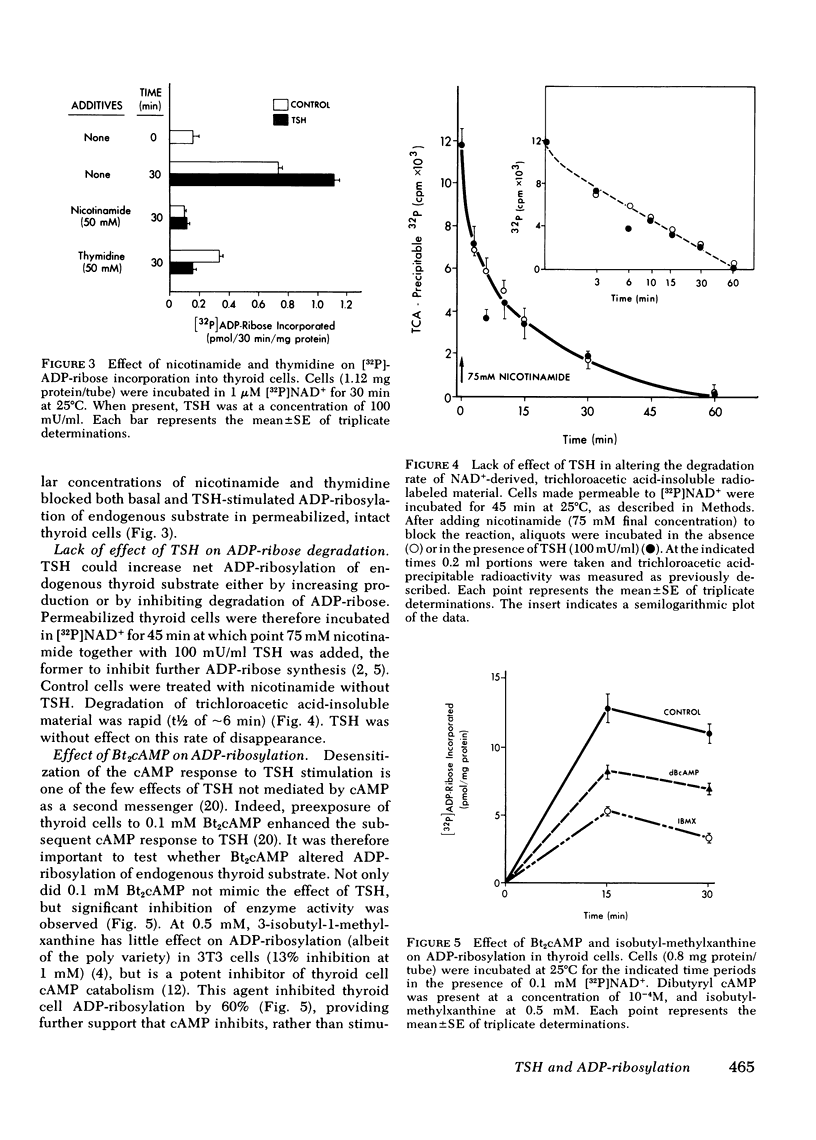
The effect of thyrotropin (TSH) on the ADP-ribosylation of endogenous thyroid cell acceptor proteins was examined. Cells were “permeabilized” at 4°C in hypotonic medium and then exposed to [32P]- or [3H-adenine]NAD+. The net incorporation of labeled ADP-ribose was measured by trichloroacetic acid precipitation. TSH (100 mU/ml) enhanced ADP-ribosylation with a maximum effect after 30-60 min in the majority of experiments. TSH stimulation was observed even when the incubation contained 1,000-fold more exogenous NAD+ than the amount of NAD+ contributed by the permeabilized cells, indicating an effect on enzymatic activity rather than an alteration in NAD+ pool size or specific activity. No incorporation of radioactivity from labeled NAD+ was observed in cells not rendered permeable to NAD+ by hypotonic shock. TSH did not increase the rate of disappearance of trichloroacetic-precipitable radioactivity and did not contain intrinsic NAD+ glycohydrolase activity. Alkali and snake venom phosphodiesterase, but not ribonuclease or deoxyribonuclease digestion of trichloroacetic acid precipitable thyroid cell radioactivity, revealed primarily 5′-AMP, consistent with an effect of TSH on mono-ADP ribosylation. Nicotinamide and thymidine (50 mM) inhibited both basal and TSH-stimulated ADP-ribosylation of thyroid cell protein. Dibutyryl cyclic (c)AMP (0.1 mM) inhibited endogenous ADP-ribosylation by ∼35% but had no effect at lower concentrations. 0.5 mM isobutylmethylxanthine inhibited this reaction by ∼60%.
We suggest that TSH enhances thyroid cell ADP-ribosylation by a mechanism independent of cAMP as a second messenger, and that ADP-ribosylation plays a role in the expression of TSH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger N. A., Weber G., Kaichi A. S. Characterization and comparison of poly(adenosine dephosphoribose) synthesis and DNA synthesis in nucleotide-permeable cells. Biochim Biophys Acta. 1978 Jun 22;519(1):87–104. doi: 10.1016/0005-2787(78)90064-3. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Filetti S., Takai N. A., Rapoport B. Prevention by nicotinamide of desensitization to thyrotropin stimulation in cultured human thyroid cells. J Biol Chem. 1981 Feb 10;256(3):1072–1075. [PubMed] [Google Scholar]
- Gill D. M. Poly (adenosine diphosphate ribose) synthesis in soluble extracts of animal organs. J Biol Chem. 1972 Sep 25;247(18):5964–5971. [PubMed] [Google Scholar]
- Halldorsson H., Gray D. A., Shall S. Poly (ADP-ribose) polymerase activity in nucleotide permeable cells. FEBS Lett. 1978 Jan 15;85(2):349–352. doi: 10.1016/0014-5793(78)80489-x. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hilz H., Stone P. Poly(ADP-ribose) and ADP-ribosylation of proteins. Rev Physiol Biochem Pharmacol. 1976;76:1-58, 177. doi: 10.1007/BFb0027686. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi V., Jacobson E. L., Jacobson M. K. Inhibition of poly(ADP-ribose) polymerase by methylated xanthines and cytokinins. FEBS Lett. 1978 Apr 1;88(1):144–146. doi: 10.1016/0014-5793(78)80627-9. [DOI] [PubMed] [Google Scholar]
- MAAYAN M. L. NAD+-GLYCOHYDROLASE OF THYROID HOMOGENATES. Nature. 1964 Dec 19;204:1169–1170. doi: 10.1038/2041169a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Ross P. S., Agosto G., Birken S., Canfield R. E., Vaughan M. Mechanism of action of choleragen and the glycopeptide hormones: is the nicotinamide adenine dinucleotide glycohydrolase activity observed in purified hormone preparations intrinsic to the hormone? Endocrinology. 1978 Feb;102(2):415–419. doi: 10.1210/endo-102-2-415. [DOI] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Watkins P. A. Isolation and properties of an NAD- and guanidine-dependent ADP-ribosyltransferase from turkey erythrocytes. J Biol Chem. 1980 Jun 25;255(12):5838–5840. [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Müller W. E., Totsuka A., Nusser I., Obermeier J., Rhode H. J., Zahn R. K. Poly(adenosine diphosphate-ribose) polymerase in quail oviduct. Changes during estrogen and progesterone induction. Nucleic Acids Res. 1974 Oct;1(10):1317–1327. doi: 10.1093/nar/1.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer N. J. Structural determination and stereospecificity of the choleragen-catalyzed reaction of NAD+ with guanidines. J Biol Chem. 1978 Jul 25;253(14):4907–4910. [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Preiss J., Schlaeger R., Hilz H. Specific inhibition of poly adpribose polymerase by thymidine and nicotinamide in HeLa cells. FEBS Lett. 1971 Dec 15;19(3):244–246. doi: 10.1016/0014-5793(71)80524-0. [DOI] [PubMed] [Google Scholar]
- Rapoport B., Adams R. J. Induction of refractoriness to thyrotropin stimulation in cultured thyroid cells. Dependence on new protein synthesis. J Biol Chem. 1976 Nov 10;251(21):6653–6661. [PubMed] [Google Scholar]
- Rapoport B. Dog thyroid cells in monolayer tissue culture: adenosine 3', 5'-cyclic monophosphate response to thyrotropic hormone. Endocrinology. 1976 May;98(5):1189–1197. doi: 10.1210/endo-98-5-1189. [DOI] [PubMed] [Google Scholar]
- Skidmore C. J., Davies M. I., Goodwin P. M., Halldorsson H., Lewis P. J., Shall S., Zia'ee A. A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979 Nov 1;101(1):135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Stone P. R., Lorimer W. S., 3rd, Kidwell W. R. Properties of the complex between histone H1 and poly(ADP-ribose synthesised in HeLa cell nuclei. Eur J Biochem. 1977 Nov 15;81(1):9–18. doi: 10.1111/j.1432-1033.1977.tb11921.x. [DOI] [PubMed] [Google Scholar]


