Abstract
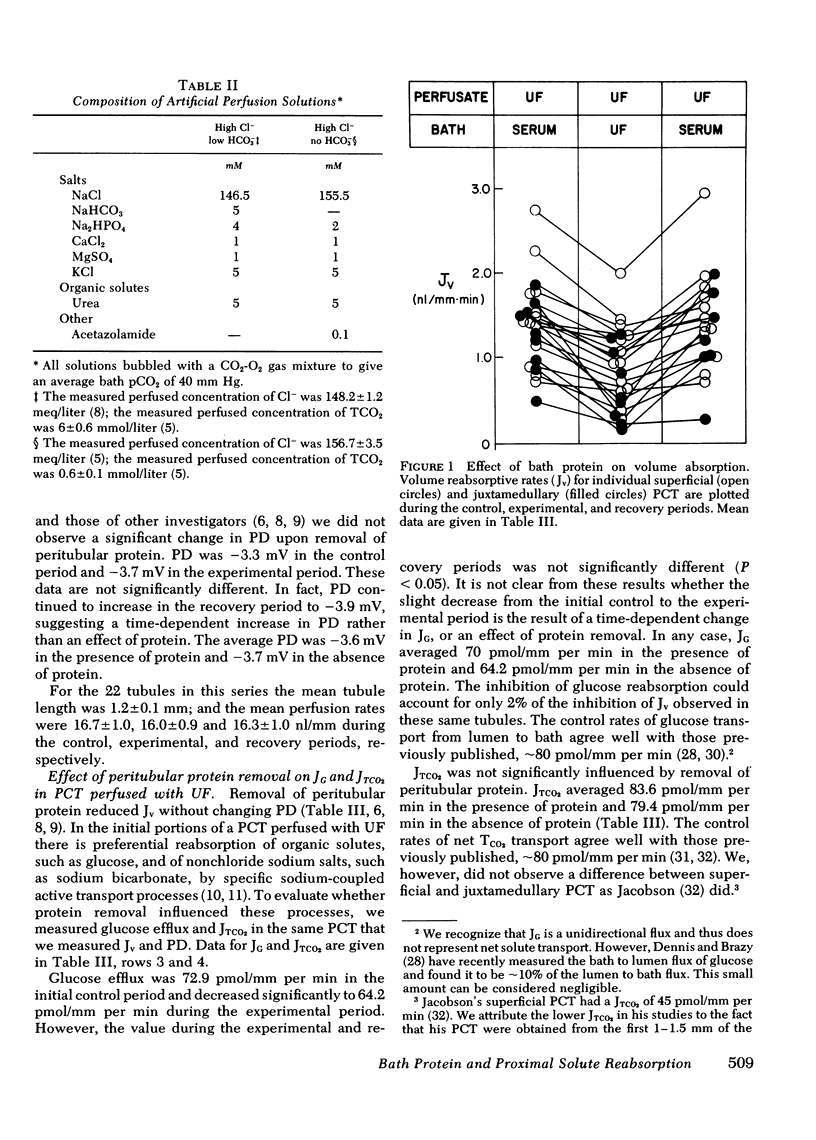
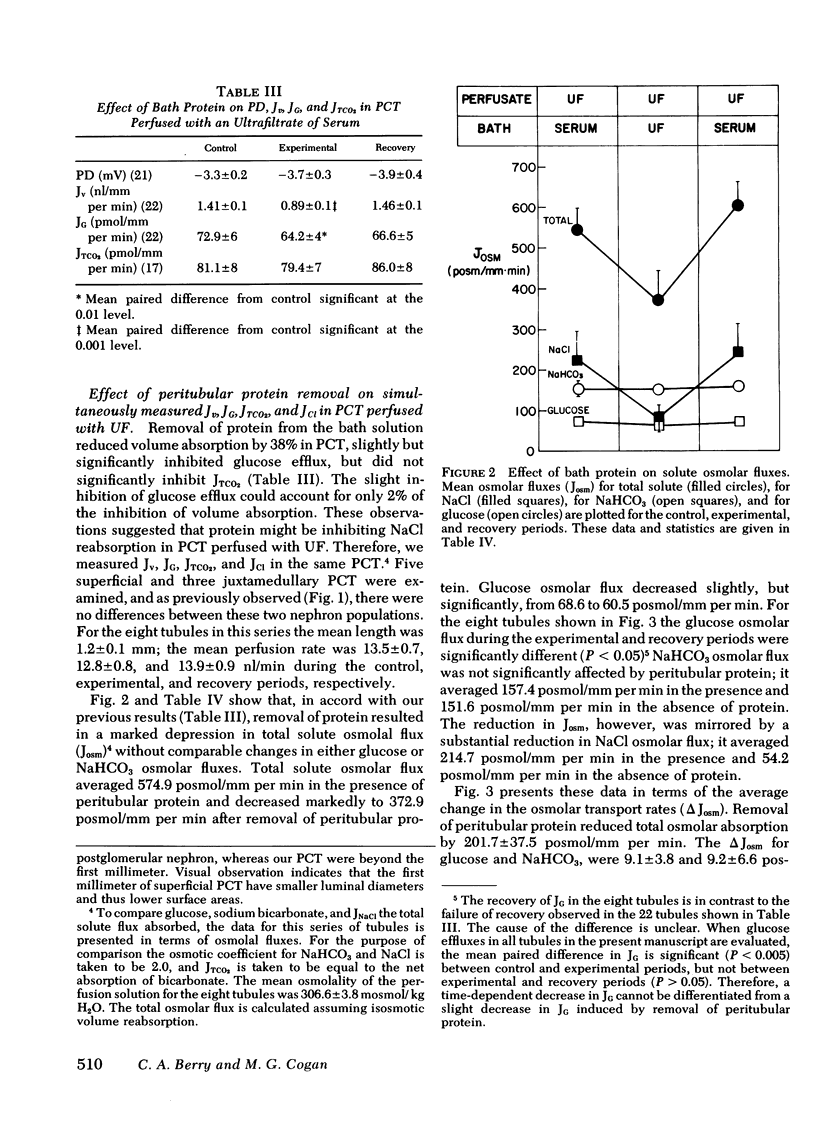
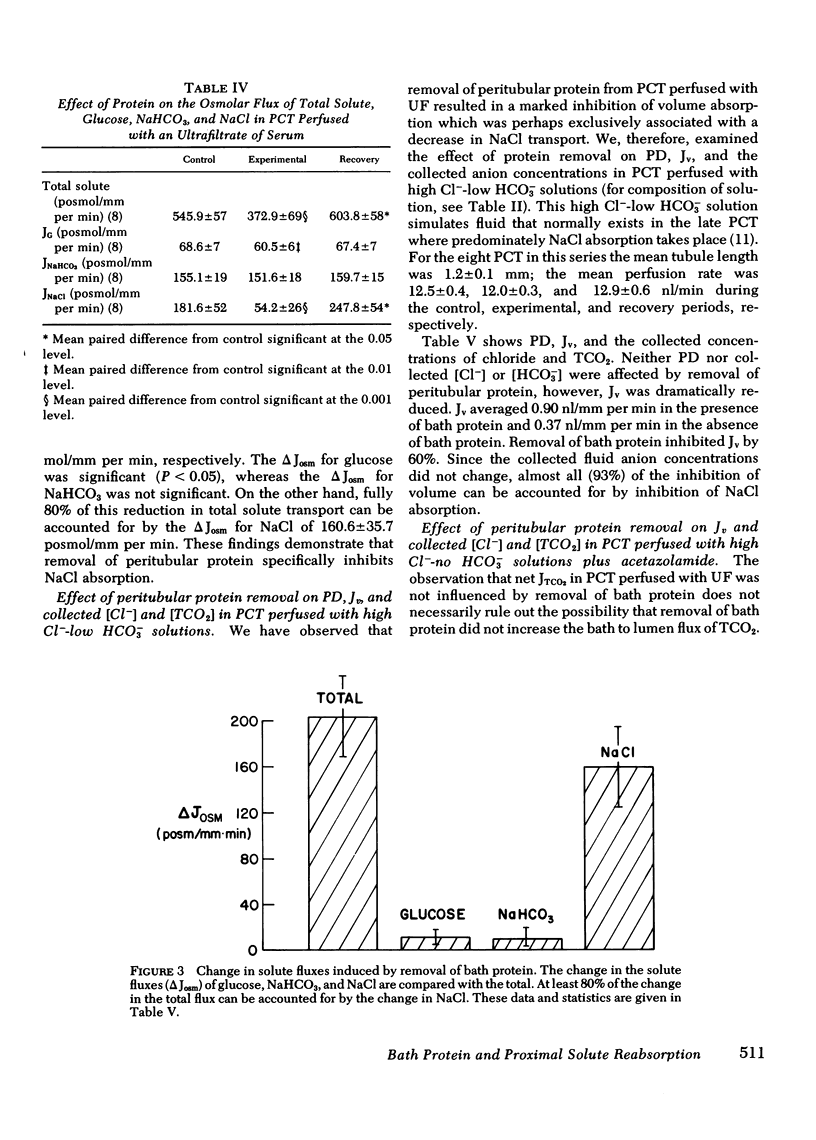
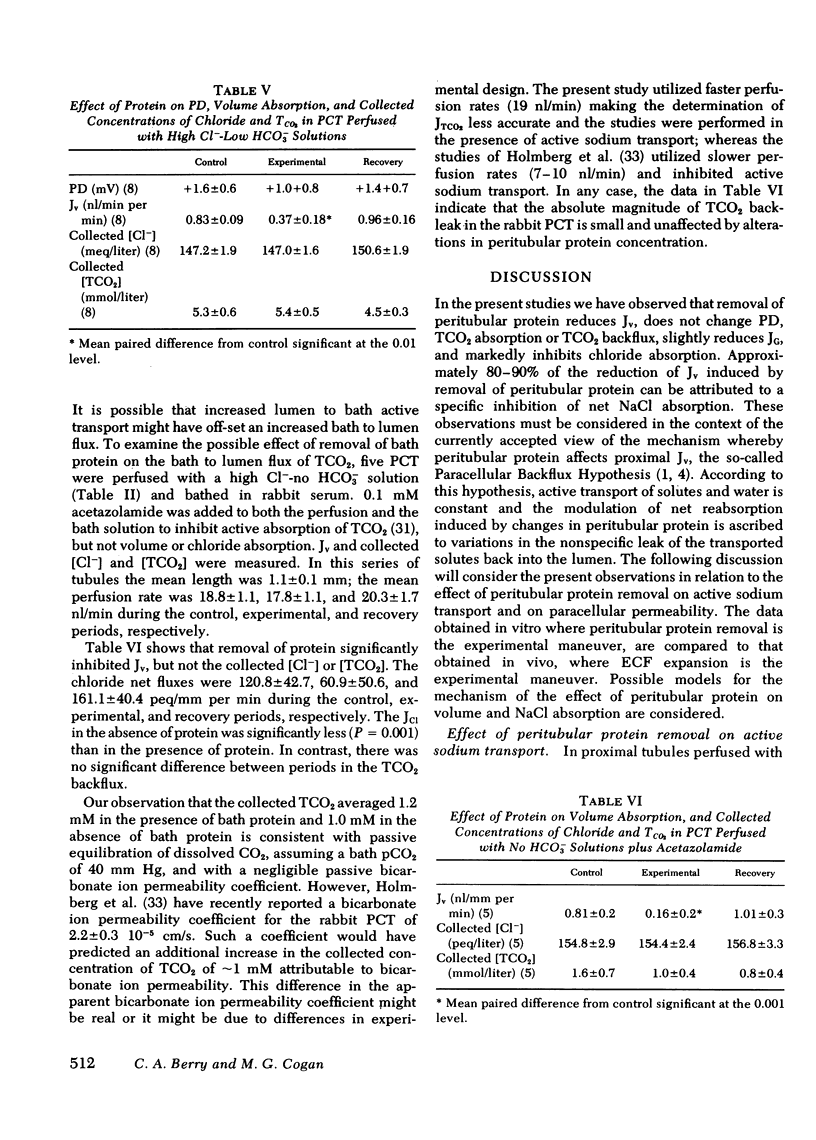
The effect of removal of peritubular protein on the reabsorption of various solutes and water was examined in isolated rabbit proximal convoluted tubules (PCT) perfused in vitro. In 22 PCT perfused with ultrafiltrate (UF) and bathed in serum, volume absorption (Jv) was 1.44 nl/mm per min and potential difference (PD) was -3.6 mV. When these same PCT were bathed in a protein-free UF, Jv was reduced 38% without a change in PD. Simultaneous measurements of total CO2 net flux (JTCO2) and glucose efflux (JG) showed that less than 2% of the decrease in JV could be accounted for by a reduction in JTCO2 and JG, suggesting that removal of peritubular protein inhibited sodium chloride transport (JNaCl). Therefore, in eight additional PCT, JNaCl was measured, in addition to PD, Jv, JG, and JTCO2. In these PCT, the decrease in total solute transport induced by removal of bath protein was 201.7 +/- 37.5 posmol/mm per min. JG decreased slightly (9.1 +/- 3.9 posmol/mm per min); NaHCO3 transport did not change (9.2 +/- 6.6 posmol/mm per min); but JNaCl decreased markedly (160.6 +/- 35.7 posmol/mm per min). 80% of the decrease in Jv could be accounted for by a decrease in JNaCl. In 13 additional PCT perfused with simple NaCl solutions, a comparable decrease in Jv and JNaCl was observed when peritubular protein was removed without an increase in TCO2 backleak. In summary, removal of peritubular protein reduced Jv and JNacl, but did not significantly alter PD, JG, JTCO2, or TCO2 backleak. The failure to inhibit JG and JTCO2, known sodium-coupled transport processes, indicates that protein removal does not primarily affect the Na-K ATPase pump system. Furthermore, since PD and TCO2 backleak were not influenced, it is unlikely that protein removal increased the permeability of the paracellular pathway. We conclude that protein removal specifically inhibits active transcellular or passive paracellular NaCl transport.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Sacktor B. The Na+ gradient-dependent transport of D-glucose in renal brush border membranes. J Biol Chem. 1975 Aug 10;250(15):6032–6039. [PubMed] [Google Scholar]
- BARTHELMAI W., CZOK R. [Enzymatic determinations of glucose in the blood, cerebrospinal fluid and urine]. Klin Wochenschr. 1962 Jun 1;40:585–589. doi: 10.1007/BF01478633. [DOI] [PubMed] [Google Scholar]
- BRESLER E. H. The problem of the volume component of body fluid homeostasis. Am J Med Sci. 1956 Jul;232(1):93–104. doi: 10.1097/00000441-195607000-00014. [DOI] [PubMed] [Google Scholar]
- Barac-Nieto M., Murer H., Kinne R. Lactate-sodium cotransport in rat renal brush border membranes. Am J Physiol. 1980 Nov;239(5):F496–F506. doi: 10.1152/ajprenal.1980.239.5.F496. [DOI] [PubMed] [Google Scholar]
- Bentzel C. J. Proximal tubule structure-function relationships during volume expansion in necturus. Kidney Int. 1972 Dec;2(6):324–335. doi: 10.1038/ki.1972.116. [DOI] [PubMed] [Google Scholar]
- Berry C. A., Boulpaep E. L. Nonelectrolyte permeability of the paracellular pathway in Necturus proximal tubule. Am J Physiol. 1975 Feb;228(2):581–595. doi: 10.1152/ajplegacy.1975.228.2.581. [DOI] [PubMed] [Google Scholar]
- Berry C. A. Electrical effects of acidification in the rabbit proximal convoluted tubule. Am J Physiol. 1981 May;240(5):F459–F470. doi: 10.1152/ajprenal.1981.240.5.F459. [DOI] [PubMed] [Google Scholar]
- Berry C. A., Rector F. C., Jr Relative sodium-to-chloride permeability in the proximal convoluted tubule. Am J Physiol. 1978 Dec;235(6):F592–F604. doi: 10.1152/ajprenal.1978.235.6.F592. [DOI] [PubMed] [Google Scholar]
- Berry C. A., Warnock D. G., Rector F. C., Jr Ion selectivity and proximal salt reabsorption. Am J Physiol. 1978 Sep;235(3):F234–F245. doi: 10.1152/ajprenal.1978.235.3.F234. [DOI] [PubMed] [Google Scholar]
- Boulpaep E. L. Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol. 1972 Mar;222(3):517–531. doi: 10.1152/ajplegacy.1972.222.3.517. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler E. H. Ludwig's theory of tubular reabsorption: the role of physical factors in tubular reabsorption. Kidney Int. 1976 Apr;9(4):313–322. doi: 10.1038/ki.1976.37. [DOI] [PubMed] [Google Scholar]
- Burg M., Green N. Bicarbonate transport by isolated perfused rabbit proximal convoluted tubules. Am J Physiol. 1977 Oct;233(4):F307–F314. doi: 10.1152/ajprenal.1977.233.4.F307. [DOI] [PubMed] [Google Scholar]
- Burg M., Patlak C., Green N., Villey D. Organic solutes in fluid absorption by renal proximal convoluted tubules. Am J Physiol. 1976 Aug;231(2):627–637. doi: 10.1152/ajplegacy.1976.231.2.627. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Maddox D. A., Lucci M. S., Rector F. C., Jr Control of proximal bicarbonate reabsorption in normal and acidotic rats. J Clin Invest. 1979 Nov;64(5):1168–1180. doi: 10.1172/JCI109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis V. W., Brazy P. C. Sodium, phosphate, glucose, bicarbonate, and alanine interactions in the isolated proximal convoluted tubule of the rabbit kidney. J Clin Invest. 1978 Aug;62(2):387–397. doi: 10.1172/JCI109140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp A., Boulpaep E. L. Pressure control of sodium reabsorption and intercellular backflux across proximal kidney tubule. J Clin Invest. 1974 Jul;54(1):69–82. doi: 10.1172/JCI107751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Qualizza P. B., Welling L. W. Influence of serum proteins on net fluid reabsorption of isolated proximal tubules. Kidney Int. 1972 Aug;2(2):66–75. doi: 10.1038/ki.1972.73. [DOI] [PubMed] [Google Scholar]
- Green R., Windhager E. E., Giebisch G. Protein oncotic pressure effects on proximal tubular fluid movement in the rat. Am J Physiol. 1974 Feb;226(2):265–276. doi: 10.1152/ajplegacy.1974.226.2.265. [DOI] [PubMed] [Google Scholar]
- Higgins J. T., Jr, Meinders A. E. Quantitative relationship of renal glucose and sodium reabsorption during ECF expansion. Am J Physiol. 1975 Jul;229(1):66–71. doi: 10.1152/ajplegacy.1975.229.1.66. [DOI] [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Humphreys M. H., Earley L. E. The mechanism of decreased intestinal sodium and water absorption after acute volume expansion in the rat. J Clin Invest. 1971 Nov;50(11):2355–2367. doi: 10.1172/JCI106734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Effect of peritubular protein concentration on reabsorption of sodium and water in isolated perfused proxmal tubules. J Clin Invest. 1972 Feb;51(2):314–325. doi: 10.1172/JCI106816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Transtubular oncotic pressure gradients and net fluid transport in isolated proximal tubules. Kidney Int. 1974 Sep;6(3):138–145. doi: 10.1038/ki.1974.92. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R. Characteristics of volume reabsorption in rabbit superficial and juxtamedullary proximal convoluted tubules. J Clin Invest. 1979 Mar;63(3):410–418. doi: 10.1172/JCI109317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman N. A. Regulation of renal bicarbonate reabsorption by extracellular volume. J Clin Invest. 1970 Mar;49(3):586–595. doi: 10.1172/JCI106269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy J. E., Windhager E. E. Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol. 1968 May;214(5):943–954. doi: 10.1152/ajplegacy.1968.214.5.943. [DOI] [PubMed] [Google Scholar]
- Lucci M. S., Warnock D. G. Effects of anion-transport inhibitors on NaCl reabsorption in the rat superficial proximal convoluted tubule. J Clin Invest. 1979 Aug;64(2):570–579. doi: 10.1172/JCI109495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Chapman L. W., Kleeman C. R. Effect of NaCl infusion on urinary Ca++ and Mg++ during reduction in their filtered loads. Am J Physiol. 1967 Nov;213(5):1218–1224. doi: 10.1152/ajplegacy.1967.213.5.1218. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B., Boulpaep E. L. Hydrostatic pressure changes related to paracellular shunt ultrastructure in proximal tubule. Kidney Int. 1980 Jun;17(6):732–748. doi: 10.1038/ki.1980.86. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976 Mar 15;154(3):597–604. [PMC free article] [PubMed] [Google Scholar]
- Neumann K. H., Rector F. C., Jr Mechanism of NaCl and water reabsorption in the proximal convoluted tubule of rat kidney. J Clin Invest. 1976 Nov;58(5):1110–1118. doi: 10.1172/JCI108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson A. E., Schnermann J., Agerup B., Eriksson N. E. The hydraulic conductivity of the rat proximal tubular wall determined with colloidal solutions. Pflugers Arch. 1975 Oct 16;360(1):25–44. doi: 10.1007/BF00584324. [DOI] [PubMed] [Google Scholar]
- Purkerson M. L., Lubowitz H., White R. W., Bricker N. S. On the influence of extracellular fluid volume expansion on bicarbonate reabsorption in the rat. J Clin Invest. 1969 Sep;48(9):1754–1760. doi: 10.1172/JCI106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson A. M., Srivastava P. L., Bricker N. S. The influence of saline loading on renal glucose reabsorption in the rat. J Clin Invest. 1968 Feb;47(2):329–335. doi: 10.1172/JCI105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. F. Effects of peritubular oncotic pressure on rat proximal tubule electrical resistance. Kidney Int. 1973 Jul;4(1):28–35. doi: 10.1038/ki.1973.77. [DOI] [PubMed] [Google Scholar]
- Tisher C. C., Kokko J. P. Relationship between peritubular oncotic pressure gradients and morphology in isolated proximal tubules. Kidney Int. 1974 Sep;6(3):146–156. doi: 10.1038/ki.1974.93. [DOI] [PubMed] [Google Scholar]
- Tune B. M., Burg M. B. Glucose transport by proximal renal tubules. Am J Physiol. 1971 Aug;221(2):580–585. doi: 10.1152/ajplegacy.1971.221.2.580. [DOI] [PubMed] [Google Scholar]
- Usberti M., Federico S., Cianciaruso B., Costanzo R., Russo D., Andreucci V. E. Relationship between serum albumin concentration and tubular reabsorption of glucose in renal disease. Kidney Int. 1979 Nov;16(5):546–551. doi: 10.1038/ki.1979.164. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]
- WINDHAGER E. E., WHITTEMBURY G., OKEN D. E., SCHATZMANN H. J., SOLOMON A. K. Single proximal tubules of the Necturus kidney. III. Dependence of H2O movement on NaCl concentration. Am J Physiol. 1959 Aug;197:313–318. doi: 10.1152/ajplegacy.1959.197.2.313. [DOI] [PubMed] [Google Scholar]
- Weinman E. J., Kashgarian M., Hayslett J. P. Role of peritubular protein concentration in sodium reabsorption. Am J Physiol. 1971 Nov;221(5):1521–1528. doi: 10.1152/ajplegacy.1971.221.5.1521. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Welling D. J. Physical properties of isolated perfused basement membranes from rabbit loop of Henle. Am J Physiol. 1978 Jan;234(1):F54–F58. doi: 10.1152/ajprenal.1978.234.1.F54. [DOI] [PubMed] [Google Scholar]


