Abstract
Corneal blindness is the third leading cause of blindness worldwide. Gene therapy is an emerging technology for corneal blindness due to the accessibility and immune-privileged nature of the cornea, ease of vector administration and visual monitoring, and ability to perform frequent noninvasive corneal assessment. Vision restoration by gene therapy is contingent upon vector and mode of therapeutic gene introduction into targeted cells/tissues. Numerous efficacious vectors, delivery techniques, and approaches have evolved in last decade for developing gene-based interventions for corneal diseases. Maximizing the potential benefits of gene therapy requires efficient and sustained therapeutic gene expression in target cells, low toxicity, and a high safety profile. This review describes the basic science associated with many gene therapy vectors and the present progress of gene therapy carried out for various ocular surface disorders and diseases.
Keywords: adeno-associated virus, adenovirus, cornea, corneal diseases and dystrophies, gene therapy, lentivirus, nanoparticles, retrovirus
I. Introduction
Gene therapy is an attractive modality to treat ocular surfaces diseases. It is unique in its ability to correct the underlying pathological mechanism of a disease process with prolonged benefits. The cornea is exceedingly well suited for gene therapy because of its easy visualization, immune privileged status, ease of access, and ability to be maintained in culture for long periods. The significant advances made in the last decade in the field of corneal gene therapy have brought this form of molecular therapy closer to patients. The success of gene therapy for corneal diseases largely depends on the delivery of adequate levels of therapeutic genes into corneal cells, the ability to target specific cell populations, and the identification of novel targets to interrupt or intercept pathological processes. A variety of vectors, techniques, and strategies have been identified and tested for delivering gene therapy in the cornea. Each of these approaches uses a different mechanism and shows a different toxicity profile. Herein we provide an overview of various viral and nonviral vectors, delivery techniques, and strategies that have been utilized in the development of gene therapy for corneal blindness.
II. Gene Therapy Vehicles for the Cornea
Efficient vectors are the key to delivering the promise of gene therapy. A variety of vectors have been tested for their efficacy, safety, and toxicity for the cornea. An ideal gene therapy vector is one that could be easily produced at high concentration and purity using simple techniques, be capable of targeting dividing and nondividing corneal cells, and provide high levels of delivered therapeutic genes in a tissue-selective manner without toxicity, immunological response, or damage to the surrounding matrix and tissues. No such vector is currently available, and trade-offs between efficiency and safety have been the norm. Various vectors tested for the cornea can be broadly divided into two categories: viral and nonviral. The characteristics, advantages, and limitations of these vectors are summarized in Table 1. The mechanisms used by these vectors to transport and produce therapeutic gene product in host cells is discussed below.
Table-1.
Characteristics, advantages and limitations of vectors used in corneal gene therapy
| Adenovirus | Retrovirus | Lentivirus | AAV | Non-viral | Nanoparticles | |
|---|---|---|---|---|---|---|
| Family | Adenoviridae | Retrovirdae | Retrovirdae | Parvoviridae | --- | --- |
| Virion size | 70-90 nm | 80-130 nm | 80-130 nm | 18-26 nm | --- | --- |
| Genome size | 38-39 kb | 3-9 kb | 3-9 kb | 4.7 kb | --- | --- |
| Genome type | dsDNA | ssRNA | ssRNA | ssDNA | RNA or DNA | RNA or DNA |
| Virion size | 70-90 nm | 80-130 nm | 80-130 nm | 18-26 nm | --- | --- |
| Host genome integration | No | Yes | No, Yes (if impaired) | No | No | No |
| Efficiency | Low-high | Moderate-High | High | Moderate-High | Poor | Poor-moderate |
| Transgene expression | Transient (days-weeks) | Long-term (months-years) | Long-term (months-years) | Potentially long-term (months-years) | Transient (hours-days) | Variable (hours-months) |
| Immunogenicity | High | Moderate-high | Moderate-high | Low-moderate | Low | Low-high |
| Infection/tropism | Dividing cells | Dividing cells | Dividing and Non-dividing | Dividing and Non-dividing | Dividing and Non-dividing | Dividing and Non-dividing |
| Packaging capacity | ≤ 7.5 kb | ≤ 8 kb | ≤ 8 kb | ≤ 1.8 kb | No limit | No limit |
| Advantages | High titer, infect nearly all cell types, non-mutagenic | Highly efficient, sustained long gene expression | Highly efficient for all cell types sustained gene expression | Highly efficient for all cell types, stable transduction, site-specific integration | Safe, low immunogenicity, deliver any size gene | Safe, variable immunogenicity deliver any size gene |
| Limitations | Immunogenic, common human virus that reduce potency | Requires dividing cells, low titer, oncogenic, random integration | Immunogenic, HIV origin, random integration potential | Small insert size, low immunogenicity, HSV or adenovirus contamination potential | Poor efficiency, low-high immune reaction, short-term transgene expression | Poor efficiency, low-high immune reaction, short-term transgene expression |
A. Viral Vectors
1. Adenovirus Vectors
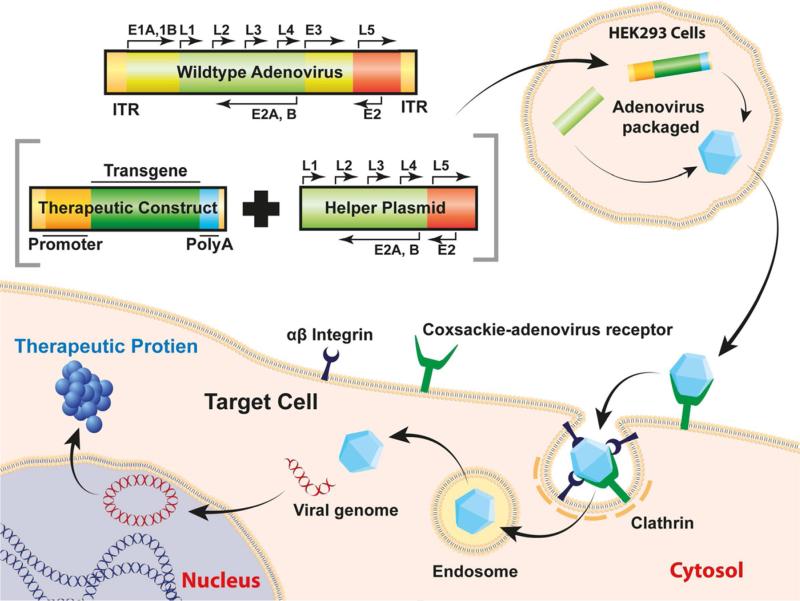
Adenovirus is a double-stranded DNA virus with a genome of 30-40 thousand base pairs.1 More than 50 human serotypes are known; serotypes 2 and 5 are most commonly used in gene therapy. Adenovirus vectors can transduce dividing and nondividing cells, carry therapeutic genes up to ~30kb, and have the natural ability to infect human tissues. Their inability to integrate into the host genome reduces risks of insertional mutagenesis. The receptor-mediated endocytosis mechanism used by adenovirus to deliver foreign genes into host cells is presented in Figure 1. Initially, adenovirus binds to the cell surface coxsackie-adenovirus receptor, interacts with αvβ3 integrin, and assembles a clathrin-coated pit to enter the cell. Once the virus reaches the cytoplasm, the viral genome is released and is transported into the nucleus. Here it remains distinct from the host genome and is expressed episomally.
Figure 1.
Schematic illustration of adenovirus genome, mechanism of entry, and therapeutic gene production in host cell.
Alterations to improve the safety and efficiency of the adenovirus have led to several iterations of improvements and resulted in the current adenoviral vector.2 (See also articles cited in reference 2.) Current adenoviral vectors have viral genome within the packaged payload, but require an E1-deficient helper adenovirus and E1-expressing cell line to replicate. It can also carry a larger payload and shows reduced immunogenicity. Minimization of helper virus contamination was further accomplished using a Cre/Lox-P system and HEK293 cells expressing cre-recombinase. Research revealed that even <0.01% helper virus contamination cause serious adverse effects in hosts also, particularly when given in large doses.
Adenovirus was the one of the first vectors investigated to deliver genes in corneal tissue. These vectors successfully delivered genes in the corneal stroma and endothelium in mice, sheep, and rats in vitro and in vivo, and in humans ex vivo.2-12 Adenovirus-delivered transgene expression was short-lived and associated with strong inflammatory immune reactions. Short-term expression necessitates repeated treatment, yet repeated applications have been shown to be more toxic than the original exposure.9
In summary, currently available adenoviral vectors are good for corneal disorders that require only short-term therapeutic gene expression; however, additional research is needed to improve their safety and utility for wider application to treat ocular surface diseases.
2. Retrovirus Vectors
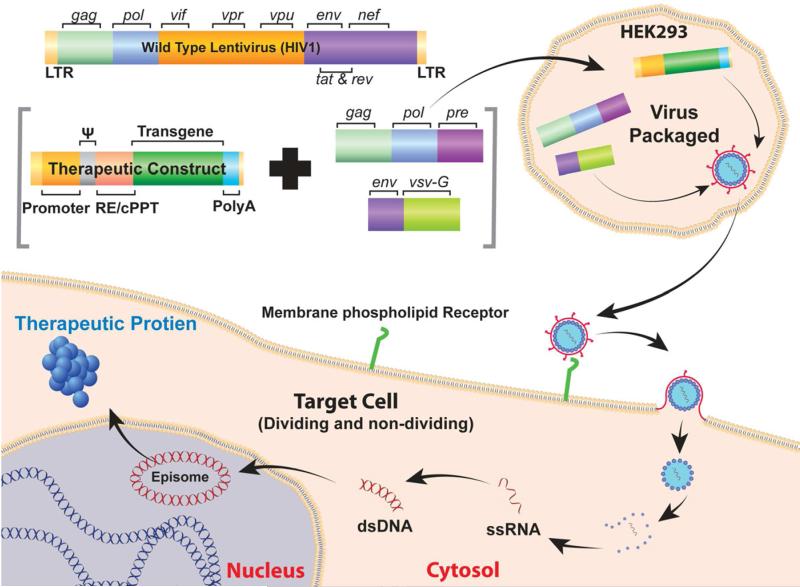
The retrovirus family includes oncovirinae, lentivirinae, and spumavirinae. The oncoretroviruses were the first viruses used as gene therapy vectors. Research into retroviral vectors resulted in many important technical and conceptual advancements in viral vectors. Oncoretroviruses are composed of a cylindrical nuclear core containing two identical copies of a linear single-stranded RNA genome of 7–11 kb. It contains three essential genes: gag encodes viral structural proteins, pol encodes reverse transcriptase/integrase, and env encodes viral envelope glycoprotein flanked by long terminal repeats (LTRs). The retroviral envelope proteins bind to surface receptors of the host cell, and the viral core enters the cell through membrane fusion. The viral genome is then released and converted into a double-stranded proviral DNA by viral reverse transcriptase. The newly synthesized genome is then transported into the nucleus of dividing cells, and a viral integrase mediates its integration into the host cell genome. Transcription factors within the LTR then initiate viral genome transcription to form new viral proteins. The mode of gene delivery by retroviral and lentiviral vectors is essentially similar (Figure 2), except that the former use host's genome and the latter express episomally.
Figure 2.
Schematic illustration of lentivirus genome, mechanism of entry, and therapeutic gene production in host cell.
Retroviral vectors are highly valuable for in vitro application, as they have been frequently used to immortalize ocular cells, including human corneal epithelial and endothelial cells, which typically do not grow in cultures.13-16 Topical application of replication-deficient retroviral vector on the rabbit cornea following superficial keratectomy transduced 25–40% of keratocytes in rabbit corneas in vivo.17 The use of different polycations, such as polybrene and protamine sulfate, has been shown to significantly improve transduction efficiency of retroviral vector for delivering genes into human keratocytes in vitro.18 Retroviral vectors can ferry large genes (8kb) and may provide permanent expression of therapeutic genes. Nonetheless, the inability of oncoretrovirus vectors to transduce nondividing cells and the risk for insertional oncogenesis largely limits their application in treating corneal diseases in human patients.
3. Lentivirus Vectors
Vectors for gene therapy from HIV1 and other lentiviruses have also been developed. Figure 2 illustrates lentivirus genome, production, and mode of entry in host cell. Lentivirus infects cells through association with a surface receptor specific for the virion type. After binding the receptor, the viral envelope fuses with the cell membrane and ejects the cylindrical core into the cell. Viral reverse transcriptase then generates DNA from the viral mRNA, and the DNA moves into the nucleus of the cell to begin generating viral proteins. Removal of most of the viral genome from the packaged genome has resulted in a vector without replication and infectious capabilities. The replacement of LTRs with CMV promoters and self-inactivating LTR hybrids removed both integration capabilities and the need for several viral genes. Current HIV-derived lentiviral vectors require less than 5% of the viral genome on the vector plasmid and less than 25% of the viral genome for production of the therapeutic plasmid.19
The final hurdle to creating a successful HIV1 vector was the expansion of its receptor specificity. Replacement of the HIV1 env proteins, fairly specific to immune cells, with the G protein of the vesicular stomatitis virus expanded transduction capacity to most cell types, as it seems to bind phospholipids present in all cell membranes. The resulting construct contains only a fraction of the original genome, has little chance of integration in the host cell genome, and can transduce most cell types. Similar methods have been used to generate vectors from other lentiviruses.
Lentiviruses have been reported to efficiently transduce corneal epithelium, endothelium, and keratocytes with high levels of transgene expression in vitro, in the mouse cornea in vivo, and in the human cornea ex vivo.20-26 Injection of HIV-based lentivirus in the anterior chamber transduced endothelial cells in a rodent model in vivo.21,22 Lentivirus vector derived from bovine immunodeficiency virus demonstrated post-delivery gene expression for 2-20 weeks in rodent corneal endothelium in vivo.23 Likewise, self-inactivating HIV1 and Equine Infectious Anemia Virus showed efficient transgene delivery in murine, rabbit, and human corneas.24 These vectors showed fairly high (80-90%) in vivo transduction efficiency.25 The HIV1-derived lentiviral vector introduced into the mouse cornea in vivo via two custom delivery techniques showed efficient transduction of targeted keratocytes.26 A single 2 μl topical application of lentivirus (1× 105 titer) on the de-epithelialized cornea for 2 min provided transduced anterior keratocytes, whereas microinjection of the same quantity and titer in the stroma with a glass needle transduced keratocytes around the site of injection (Figure 3). Integration-deficient lentivirus vectors show high promise for corneal gene therapy, although the risk of integration into the patient genome remains a major obstacle for transition to clinical therapy.
Figure 3.
Representative montage of the reconstructed mouse corneas showing actual surface area, quantity, and location of transgene in the entire cornea detected at two weeks. The transgene was delivered in vivo with lentivirus vector encoding for green fluorescent protein (GFP) gene. 2 μl of lentivirus titer (1×105) was topically applied on de-epithelialized cornea for 2 min (A) or microinjected with glass needle using Hamilton microinjection syringe system (B). A significant transduction of keratocytes in the anterior stroma of the mouse cornea in vivo was detected. The delivered-GFP in tissues is detected as red as sections were immunostained with Alexa594-conjugated antibody.
4. Adeno-Associated Virus (AAV) Vectors
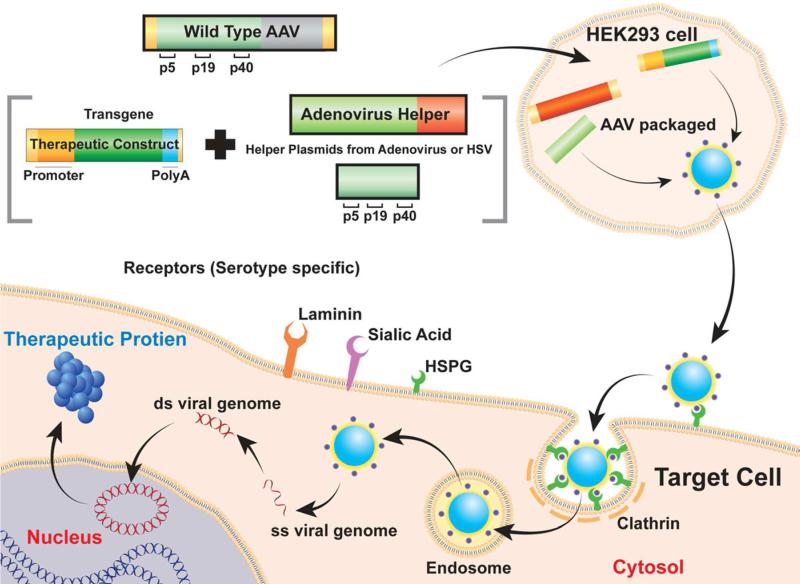
AAV is a small, nonpathogenic, single-stranded DNA virus with an icosahedral capsid of the parvovirus family that has demonstrated itself to be an efficient vector for gene therapy. About 110 serotypes of AAV are known, but only serotypes 1 through 10 have been tested for gene therapy. Figure 4 presents the components of AAV construction and the steps involved in production, gene delivery, and gene expression in target cells. The AAV genome is 4.7 kb long and contains two open reading frames (ORFs) that encode for rep and cap between 145 base-long inverted terminal repeats at both the 5’ and 3’ end of the genome. The rep ORF encodes for four distinct proteins labeled rep40, rep52, rep68, and rep78. Rep68 and rep78 are required for the replication and translation of the viral genome, while rep40 and rep52 are required to package the genome within the capsid. This combination of site-specificity and the requirement of two distinct gene products allow the generation of an integration-deficient vector with little chance of oncogenesis.
Figure 4.
Schematic representation of AAV genome, mechanism of entry, and therapeutic gene production in host cell.
The cap genes encode for the various capsid proteins: VP1, VP2, and VP3. Once assembled and ready for infection, the virus must first bind the appropriate cell surface receptor. The binding of virus to cell surface receptor is serotype-specific, is determined by the sequence of the viral capsid, and is responsible for variance in tissue trophism between serotypes. AAV serotypes 1-3 bind to heparin sulphate proteoglycans, serotypes 4-6 bind to 2,3-linked Sialic acid, and serotypes 8 and 9 bind to laminin. Similar receptor binding may result in similar tissue tropism across the serotypes. Once the viral capsid has associated with its primary receptor, and either integrin αvβ5 or the basic fibroblast growth factor co-receptors, the viral particle is endocytosed by receptor-mediated formation of a clathrin-coated pit. The AAV genome is then released into the cytosol through endosomal lysis and anneal to a complementary strand provided by another infecting virus or through endogenous host machinery (Figure 4). The wild-type AAV genome is either integrated into chromosome 19 via rep68 and rep78 or it remains episomal. After reaching the nucleus AAV is translated by the genetic machinery of the host cells.
The first generation recombinant AAV vectors were produced by replacing the rep and cap ORFs from the viral genome with the gene of interest. This construct required E-1 expressing HEK293 cells that contain E1-deficient adenovirus and a helper plasmid having rep and cap. In these vectors, helper virus contamination was a problem during their production. To address this, second-generation rAAV vectors were produced that eliminated the need for helper virus. This system replaced the adenovirus with dual transfection of an adenovirus-helper-plasmid that expresses E4 and E2A and a helper-plasmid expressing rep and cap into HEK293 cells that provide E1. Further modification of this method involved combination of AAV-helper and adenovirus-helper genes into a single plasmid to alleviate dual transfections.27
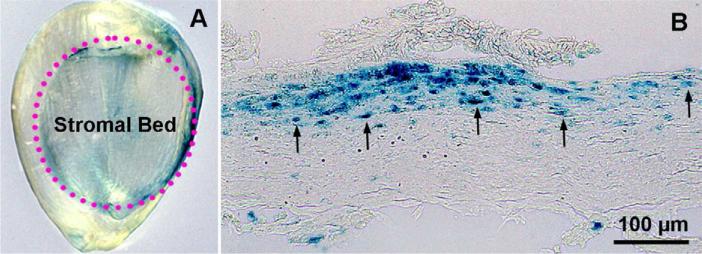
AAV gene transfer efficiency and delivered transgene duration depend on serotype and capsid.28-30 AAV2 was the first tested serotype that demonstrated significant transgene delivery in the rabbit cornea in vivo.31 This vector showed substantial gene transfer only when topically applied onto stromal bed after producing a lamellar flap with a microkeratome. This study showed that circumventing the epithelial barrier is critical to deliver genes into keratocytes in vivo (Figure 5).
Figure 5.
Representative images of whole-mount rabbit cornea (A) and corneal tissue section (B) showing AAV2 delivered expression of β-galactosidase marker gene (Blue) detected at day 7. The AAV2 vector (1X1011 titer) was topically applied onto the rabbit corneal stromal bed after making a lamellar flap with a microkeratome. The transgene is expressed under the control of CMV promoter. Scale bar denotes 100 μm.
The AAV2 occurs naturally in humans, and existent humoral immune responses may diminish its efficacy or induce inflammation. This prompted the development of hybrid AAV vectors using the AAV2 genome and the capsid of different serotypes. The hybrid vectors were found to be highly efficient for many ocular tissues, including the cornea, in several species. They showed a wide range of tissue preference and variation in transduction efficiency, as well as vastly different first transgene appearance timing and delivered-transgene expression levels and duration.32-37 AAV2/5 administered in the rabbit or rodent cornea provided significant tissue-targeted gene transfer in the stroma in vivo after the administration of the vector via two different custom delivery techniques (Figure 6). The AAV2/5 has shown delivered transgene expression for up to 12 months.2,36
Figure 6.
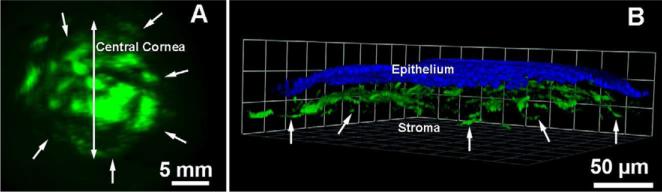
Representative stereomicroscopy (A) and confocal microscopy (B) images showing targeted green fluorescent marker gene delivery with hybrid AAV2/5 vector in the rabbit stroma in vivo observed 2 weeks after a single topical application of 100 μl vector (1× 1010 titer) on de-epithelialized cornea for 2 min. Vector expresses enhanced green fluorescent protein gene under control of hybrid CMV+chicken-β-actin promoter. Nuclei are stained blue with DAPI.
In addition to serotype 2/5, hybrid AAV2/6, AAV2/8, and AAV2/9 vectors have also been found to be efficient for delivering genes in human corneal fibroblasts in vitro, in mouse corneas in vivo, and in human corneas ex vivo.36-38 The levels of gene transfer by these hybrid AAV vectors for human corneal fibroblasts depended on the titer; however, AAV2/6 showed 30-50-fold higher transduction at all tested doses compared to AAV2/8 or AAV2/9 in vitro (Figure 7; P<.001), with all showing similar safety profiles.37 Interestingly, subsequent in vivo testing of these three hybrid vectors in mouse corneas demonstrated distinctly different transduction profiles than were seen in vitro studies: the hybrid AAV 2/6 showed lowest gene transfer, and AAV 2/9 showed greatest keratocyte transduction in the mouse cornea in vivo at day-30 (Figure 8). The first transgene appearance and duration of transgene expression were similar for all three AAV hybrid vectors for the mouse corneas in vivo.38 The levels of delivered-transgene by these vectors in the human corneas ex vivo followed the in vivo expression pattern: AAV 2/6 > AAV 2/8 > AAV 2/9, as shown in Figure 9.
Figure 7.
Quantification of alkaline phosphatase reporter gene delivery detected 36 hrs after AAV2/6, AAV2/8 or AAV2/9 vector transduction in human corneal fibroblasts in vitro at two different concentrations. AAV2/6 vector demonstrated significantly higher transduction (30-50-fold; *P<.01 #p < .001) compared to the AAV2/8 or AAV2/9 vectors for human corneal fibroblasts in culture. The used AAV vectors express alkaline phosphatase gene under control of RSV promoter.
Figure 8.
Quantification of delivered alkaline phosphatase gene into the mouse corneas in vivo with AAV6, AAV8 or AAV9 vector at day 4 and day 30. AAV 2/9 and AAV 2/8 showed 30-45% higher transgene delivery in mouse stroma compared to AAV 2/6 at day 30 (*P<.01 or ψP< .05).
Figure 9.
Representative human corneal sections images showing delivered levels of transgene with AAV2/6 (B), AAV2/8 (C) or AAV2/9 (D) vector detected on dat-5 after transduction in human corneas ex vivo. The AAV 2/9 showed high, AAV 2/8 showed moderate, and AAV 2/6 showed low transgene delivery into human corneas.
These studies suggested that 1) the AAV 2/6 is an efficient vector for delivering genes in the cornea in vitro, 2) AAV 2/5, 2/8 and 2/9 are vectors of choice for in vivo corneal gene therapy, and 3) hybrid AAV 2/5, 2/6, 2/8 and 2/9 are safe vectors for the cornea, as none of these serotypes caused significant adverse effects, such as unusual cell death, loss of cellular viability, inflammation, and/or severe immune reaction.35-38
A crucial step in the production of AAV-mediated transgene expression requires the single-stranded viral genome conversion into a double-stranded DNA, either spontaneous annealing with a complementary strand from another viral particle or the generation of a complementary strand by host machinery. This process can take some time and results in delayed initial and slow peak transgene expression (up to 72 hrs and 7 days, respectively, in the cornea). Alterations to the viral genome have been shown to circumvent this limitation and result in the development of double-stranded self-complimentary AAV (scAAV) vector. Because AAV replication and packaging relies on hairpin-forming terminal repeat segments, it is possible to encourage the formation of dimeric inverted repeat genomes. If these dimers are small enough, they are capable of being spontaneously packaged into the viral envelope, and once released into the host cell, they will spontaneously self-anneal to form a double-stranded DNA segment available for rapid gene expression. This advance circumvents the requirement in conventional AAV for complementary strand generation and has shown accelerated gene expression in several tissues. There is also evidence that efficiency may improve at lower viral titers when with use of scAAV in a tissue- and serotype-dependant manner.39-41
While these are marked benefits, dimerization of the genome magnifies the limitation of genome packaging size present in viral vectors and is functionally limited to genes of 2.2kb or smaller. As such, scAAV may be most appropriate for developing therapies utilizing RNA-constructs, such as short hairpin RNA, microRNA, and ribozymes.
The intracellular trafficking of the viral capsid plays a major role in defining vector entry into cells and transduction efficiency.42 This led to the creation of recombinant tyrosine mutant AAV vectors by modifying tyrosine residues on the surface of the capsid. The tyrosine residues appear to play a significant role in virus function, as within the cell tyrosine residues along the exterior of the capsid are available for tyrosine kinases within the cell to phosphorylate them. This marks the AAV for ubiquitination and subsequent proteasome-mediated degradation prior to its ejection of DNA and transduction of the cell. These residues seem to be the primary site for ubiquitination, as phosphorylation of these residues by epithelial growth factor receptor protein tyrosine kinase reduced transduction by 74% for single-stranded viruses. There are seven of these tyrosine residues within the AAV capsid (Y252, Y272, Y444, Y500, Y700, Y704, and Y730) and single/multiple tyrosine-phenylalanine mutations increased multiplicity of infections ranging from 100 to 10,000.43-45 Tyrosine mutant AAV2, AAV8, and AAV9 vectors were found to be efficient for delivering genes into keratocytes and endothelial cells of the mouse corneas in vivo and human corneal endothelial cells in vitro.46 and Mohan et al (unpublished data) The increased transduction efficiency of tyrosine mutant vectors renders lower virus dosage, reduces side effects, and enhances safety of AAV-based gene therapy treatments.
Although AAV vectors offer several advantages over other viral vectors, AAV does have limitations: therapeutic genes larger than 1.8 kb are difficult to package in AAV; production of high viral titers is technically demanding and time-consuming; and earlier exposure of humans to AAV serotypes 1-6 raises immunological concerns.
B. Nonviral Vectors
1. Physical and Chemical Methods
The success of gene transfer with chemicals led to the development of many plasmids, lipids, polymers, and physical methods. The nonviral vectors can transport any size gene without major toxicity, but they generally show poor transgene delivery. Nonviral vectors mainly use endocytosis to enter cells. Lipids are routinely used in laboratory settings for studying functions of genes in cells. Successful gene transfer into corneal cells in vitro and in vivo with lipids has been reported.31,47-52 Lipid formulations made from dioleoylphosphatidyl-ethanolamine and dimethyl dioctadecyl ammonium bromide have shown significant transgene delivery in the mouse and rabbit stroma in vivo (7-8%; P<.01) after topical application on de-epithelized cornea via custom delivery techniques (Figure 10). Recently, a mixture of five lipids yielded 7-17% gene transfer in the corneal endothelium in vivo, the most efficient gene transfer reported so far with lipids in vivo.53
Figure 10.
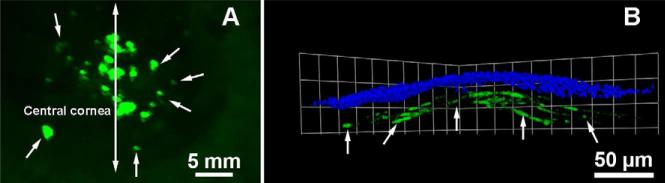
Representative stereomicroscopy (A) and confocal microscopy (B) images showing transgene delivery in the rabbit stroma in vivo noted 2 days after topical application of transfection solution (1 μg/μl plasmid in 50 nmol DDAB and 50 nmol DOPE in 100 μl lactated Ringer's solution) onto the rabbit cornea via custom delivery technique. The plasmid expresses transgene under control of CMV+chicken-β-actin promoter. Nuclei are stained blue with DAPI.
Physical methods for delivering genes include surgical techniques, ultrasonic waves, iontophoresis, or bombardment of plasmid across the cellular membrane. Methods such as electroporation are commonly used for delivering genes in bacteria for plasmid replication. Using custom devices and controlled electrical current, genetic material has been successfully introduced into corneal endothelial and stromal cells in vivo and human cornea ex vivo.54-57 The potentials of ultrasound and shock wave have also been investigated for delivering therapeutic genes in the cornea.57-59
2. Nanoparticle Vectors
Many particles ranging from 1-100 nm in size, referred to as “nanoparticles,” have been developed and tested for delivering therapeutics in ocular tissues, including the cornea. Nanoparticles are exceedingly suitable for gene therapy because of their small size, ability to access the intracellular compartment, incredible surface-area-to-volume ratio, capacity to carry large payload, and minimal damage to cell membranes and cellular environment. Multiplexing properties allow transport of multiple ligands, including DNA, antibodies, peptides, molecular sensors, therapeutic molecules, and probes into target cells with high precision and specificity. Nanoparticles use a variety of mechanisms, including phagocytosis, macro-pinocytosis, and clathrin- or caveolae-dependent or -independent pathways to enter the cells. Nanoparticles are classified in three groups: metallic, polymeric, and hybrid (metallic core with a polymeric coat).
Several polymeric and hybrid nanoparticles have been investigated for gene delivery in the eye. The polymeric nanoparticles prepared from polyethyleneimine, albumin, chitosan, polyethylene glycol, and hybrid nanoparticles such as gold-polyethyleneimine have been reported to efficiently deliver genes in rodent corneas in vivo without significant side effects.60-65 Delivery of transgene into human corneal epithelial and endothelial cells in vitro with these nanoparticles has also been reported.60,61 Poly (D,L-lactide-co-glycolide) nanoparticles were not found to be efficacious for the cornea but can deliver genes in rabbit conjunctival epithelium.66
C. Cell and Tissue Targeting
One key to reducing toxicity and enhancing the potency of gene therapy is selective gene delivery into the desired cells. One method by which this can be accomplished is the utilization of cell-specific promoters. The cell-specific promoters for corneal epithelial cells and keratocytes have been identified, but no promoter is yet available for the corneal endothelial cells.11,67,68 An alternative approach using an inducible vector system with a switch-on and -off mechanism and steroid-inducible promoter has been tested for securing targeted gene therapy in the cornea.69 Unfortunately, all currently available corneal cell promoters, such as keratin 12 (an epithelial-specific promoter) and keratocan (keratocyte-specific promoter), are leaky, and an inducible vector system has many challenges. The best possible alternative in the absence of ideal cornea-specific promoter is to use simple surgical techniques, such as intracameral, intrastromal, subconjunctival injection, topical application, etc. Several minimally invasive techniques have been recently developed and tested for achieving tissue-selective gene transfer in vivo using rabbit and rodent models.2,35 The risk of damaging the integrity of some ocular cells and/or extracellular components within the cornea remains a concern with administration of vector with such techniques. Further research is needed to achieve cell/tissue-specific gene delivery in the cornea.
III. Overview of Corneal Gene Therapy Studies
Although corneal blindness is the third leading cause of blindness worldwide, no gene-based therapy is yet available for treating corneal diseases and disorders. Many recent preclinical studies have paved the way for gene therapy to advance into the clinic. The effects of genes tested for corneal disorders are discussed below and summarized in Table 2.
Table-2.
Therapeutic genes, vectors and delivery techniques used in gene therapy for ocular surface disorders and diseases
| Corneal Graft Rejection | ||||
|---|---|---|---|---|
| Gene | Vector | Model/Delivery | Species | Outcome |
| Bcl-xL | Lentivirus | Ex vivo | Mice | 90% survival rate after gene therapy versus 30% in controls |
| Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) | Adenovirus | Ex vivo, IV | Rat |
Ex vivo: Marginal prolongation in graft survival (1 day). i.v. Prolongation in graft survival by 9 days |
| Inducible T cell co-stimulator (ICOS) | Adenovirus | Ex vivo, IP | Rat | No significant prolongation in graft survival |
| IL-10 | Adenovirus | Ex vivo, IP | Rat | Ex vivo: No prolongation in graft survival i.p.: Marginal prolongation in graft survival (4 days) |
| IL-10 | Adenovirus | Ex vivo | Sheep | Remarkable prolongation in graft survival (35 days) |
| IL-12p40 gene | Adenovirus | Ex vivo, IP | Rat | No detectable change in graft survival |
| IL-12p40 gene | Adenovirus | Ex vivo | Sheep | Prolongation in graft survival from 18 days to 45 days |
| IL-4 | Adenovirus | Ex vivo | Rat | Rejection rate 85% in treated corneas and 63% in controls |
| IL-4 + CTLA4-Ig | MIDGE | Gene gun | Rat | Remarkable prolongation in graft survival |
| NGF | Adenovirus | Ex vivo and IP | Rat | Ex vivo: Median 3 days increase in graft survival No notable prolongation in graft survival |
| Corneal Wound Healing and Corneal Scarring / Haze | ||||
| Soluble TGFβR2 | Adenovirus. AAV, Nanoparticles |
IM, Topical application In vitro | Mice Rabbit Human |
Reduction in corneal edema, angiogenesis, inflammatory cell infiltration and deposition of extra-cellular matrix myofibroblast reduction |
| Tissue plasminogen activator | Plasmid | Intracameral electroporation | Rat | Decrease in fibrin deposition and opacity |
| Decorin | AAV | Topical | Rabbit Mice |
Decreased myofibroblasts, abnormal healing and opacity |
| Thymidine kinase | Retrovirus | Topical | Rabbit | Remarkable decrease in corneal haze in treated corneas |
| CyclinG1 | Retrovirus | Topical | Rabbit | Reduction in haze, a dramatic reduction in abnormal extracellular matrix production |
| Decorin | AAV | Topical, In vitro | Rabbit Human |
Remarkable reduction in corneal haze |
| Smad7 | Nanoparticles AAV |
Topical, In vitro | Rabbit | Reduction in corneal haze |
| BMP7 | Nanoparticles | Topical | Rabbit | Reduction in corneal haze |
| Alkali Burn Injury | ||||
| Smad7 | Adenovirus | Topical | Mice | Suppression of MCP-1, TGF-beta1, TGF-beta2, VEGF, MMP -9 and prevention of myofibroblast formation |
| BMP7 | Adenovirus, AAV | Topical, In vitro | Mice | Decrease in inflammatory cytokines, cell infiltration and myofibroblast formation. No reduction in neovascularization |
| PPARγ | Adenovirus | Topical | Mice | Inhibition of growth factors, MMP upregulation, monocytes/macrophages infiltration and myofibroblasts formation |
| Corneal Neovascularization | ||||
| sFlt-1 | Plasmid | Intrastromal | Mice | Complete inhibition of VEGF induced neovascularization |
| sFlt-1 | Adenovirus | Intracameral | Rat | 18% treated animals showed neovascularization compared to 82% controls |
| sFlt-1 | Adeno-associated virus | Intracameral | Mice Monkey |
Long term (8 months) regression of neovascularization in 85% of treated eyes |
| Flt23K, Flt24K | Plasmid, albumin polyplexes | Intrastromal | Mice | Intrastromal delivery of plasmid Flt23K suppressed VEGF (40%), leukocytes (49%) and neovascularization (66%). Flt24K suppressed VEGF expression (30%), leukocytes (25%) and neovascularization (49%) |
| Kringle 5 domain of plasminogen | Plasmid | Electroporation | Rat | Corneal neovascularization was significantly suppressed by K5 gene transfer in rats’ eyes |
| Endostatin- Kringle 5 | Lentivirus | Ex vivo | Rabbit | None of the 10 treated corneas developed neovascularization |
| IL10, IL12 | Plasmid | Topical | Mice | Significant suppression of neovascularization |
| Decorin | AAV | Rabbit | Significant suppression of neovascularization | |
| PEDF | AAV, GNP | Rabbit, Human cornea in vitro | Significant suppression of neovascularization | |
| Angiostatin | AAV | Subconjunctival injection | Rat, Organ culture | 50% reduction in area of neovascularization in treated animals |
| Vasohibin-1 | Adenovirus | Subconjunctival injection | Mice | 20% reduction in area of neovascularization in treated animals |
| Corneal Dystrophies | ||||
| β-glucuronidase | Adenovirus | Intrastromal, Intracameral | Mice | Correction of mucopolysaccharidosis phenotype |
| Herpes simplex Virus (HSV) Keratitis | ||||
| HSV glycoproteins (gB, gC, gD, gE, gI) | Plasmid | Multipe (IP, IM, sub-conjunctival) | Mice, Rabbit | Subconjunctival: Prevention of stromal keratitis IP, IM: Prevention of stromal as well as epithelial keratitis |
| Cytokines (IL-2 IL-10, TNFα, IFNα1) | Plasmid | Topical | Mice | Limited beneficial effect |
| Hepatocyte growth factor c-met | Adenovirus | In vitro | Human | Improved HGF signaling and restoration of normal protein patterns |
| Lacrimal gland, Dry Eye, Sjogren syndrome | ||||
| TNFα inhibitor | Adenovirus | Lacrimal gland injection | Rabbit | Enhanced tear production and suppression of immunohistopathology |
| IL-10 | Adenovirus | Lacrimal gland injection | Rabbit | Partially suppressed appearance of Sjögren-syndrome- features of reduced tear production, accelerated tear breakup, ocular surface disease and immunopathologic features |
| Conjunctival Scarring | ||||
| Smad7 | Adenovirus | Topical | Mice | Suppression of alpha-smooth muscle actin and VEGF in fibroblasts and invasion of macrophages |
| PPARγ | Adenovirus | Topical | Mice | Inhibition of monocyte/macrophage invasion, generation of myofibroblasts, and mRNA upregulation of cytokines/growth factors and collagen I alpha2 chain in healing conjunctiva. |
| Dominant negative p38MAPkinase | Adenovirus | Topical | Mice | Reduction of myofibroblast generation and suppression of mRNA expression of connective tissue growth factor and MCP-1 |
IM: Intra muscular, IP: Intra peritoneal, BMP: Bone morphogenic protein, CTLA-4 Ig: Cytotoxic T-lymphocyte-associated antigen-4 conjugated to human IgG heavy chain, Flt-1: A soluble form of the VEGF receptors, ICOS: Inducible co-stimulatory receptor expressed by activated T cells, IFN-γ: Interferon-gamma, IL: Interleukin, MCP-1: Macrophage chemoattractant protein-1, MMP: Matrix metalloproteinase, NGF: Nerve growth factor, PPARγ: Peroxisome proliferator-activated receptor-gamma, TGFβ: Transforming growth factor beta, TNFα: Tumor necrosis factor, VEGF: Vascular endothelium derived growth factor, GNP: Gold Nanoparticles
A. Corneal Grafts
Attempts to improve allograft survival using gene transfer technology have focused on the modulation of genes regulating cellular transport, apoptosis, angiogenesis, and/or wound healing using various models. A gene transfer approach to halt activated T cells within and around the graft to reduce cytotoxicity and immune rejection of allographs has been tested. The presence of Indoleamine 2,3-dioxygenase (IDO) is believed to arrest activated T cells in the G1 phase of cell division and results in prevention of proliferation and promotion of immune tolerance in the exposed tissue. The delivery of IDO in murine corneal endothelium and full-thickness corneal grafts via lentivirus has been demonstrated to significantly prolong corneal allograft survival.70
A strategy to modulate cell survival-death mechanism to prolong graft survival demonstrated 90% graft survival rate 8 weeks post-transplantation after lentivirus-mediated delivery of four anti-apoptotic genes (bcl-xL, bcl-2, survivin and p35) in a rodent model, while all control grafts failed.71 Recently, lentivirus-mediated delivery of bcl-xL and p35 genes in primary human endothelial cells and human corneas has been shown to enhance endothelial survival.72 The potential of IL4, IL10, CTLA4-Ig, p40-IL12, viral MIPII, and nerve growth factor gene therapy in enhancing graft survival has also been reported.2,73 An alternative approach utilizing adenovirus-mediated delivery of transcription factor E2F2 in rabbit and human corneas ex vivo has been shown to extend endothelial cell survival and increase cell count, presumably through the modulation of G1 to S phase of cell cycle.74,75 Although E2F2 expression was somewhat transient, this work highlights the promise of gene therapy in promoting cell survival in corneal tissue and corneal endotheliopathies.
B. Corneal Scarring and Wound Healing
Abnormal healing in the cornea following injury is known to cause opacification and loss of visual acuity. Increased release and hyperactivity of transforming growth factor beta (TGFβ) due to injury appear to play a major role in myofibroblast production and fibrosis development in the cornea. An approach has been developed to inhibit the pathological function of TGFβ utilizing a natural inhibitor of TGFβ, decorin. Decorin gene delivery into human corneal fibroblasts significantly inhibited myofibroblast formation without compromising cellular viability or phenotype.76,77 Subsequently, it was reported that tissue-selected targeted delivery of deocorin into rabbit eye with AAV 2/5 vector significantly decreased corneal scarring in rabbits in vivo (59-73%; P<.001) without noticeable acute adverse effects.78
Using a gene transfer approach, it was demonstrated that TGFβ relays its signal via the Smad pathway; as such, overexpression of Smad7 or knockdown of Smad2 or 3 has the potential to treat corneal scarring.79 and Mohan R (unpublished data) Another approach that could be applied to controlling corneal scarring is to limit keratocyte proliferation and fibrotic cell populations. Reduction in corneal haze was noted with retrovirus-mediated delivery of herpes simplex virus (HSV) thymidine kinase gene after keratectomy and subsequent topical application of ganciclovir in rabbit laser-induced corneal haze model.17 Blockade of cyclins and cyclin-dependent kinases that control cell division were also tested by delivering a dominant-negative mutant construct for cyclin G1 via a retroviral vector into the cornea using rabbit fibrosis model. Rabbit corneas expressing retrovirus-delivered dominant-negative cyclin G1 showed decreased production of extracellular matrix and development of corneal haze.17 The beneficial effects were likely due to apoptosis of activated keratocytes by the dominant-negative cyclin expression. All of these approaches show promise for developing gene-based molecular therapy for corneal scarring.
C. Corneal Alkali Burn
An alkali injury to the eye in humans triggers corneal opacification, ulceration, scarring, and/or neovascularization. The potential of gene therapy to treat complications of corneal alkali injury has been tested by modifying functions of the TGFβ superfamily. Topical administration of Cre-adenovirus expressing Smad7 effectively reduced alkali-induced corneal scaring. Similarly, delivery of bone morphogenic protein-7 (BMP7) was found to antagonize the pathologic effects of TGFβ in the cornea. Adenovirus-mediated BMP7 gene transfer in the cornea has been shown to accelerate re-epithelialization of the corneal surface and suppress myofibroblast formation, monocyte/macrophage infiltration, and expression of macrophage chemoattractant protein-1, TGFβ, and collagens in alkali-injured mouse corneas.80 BMP7 gene therapy delivered with hybrid gold nanoparticles showed significant reduction in an alkali- and laser-induced corneal haze in rabbits in vivo.81 It has also been reported that peroxisome proliferator-activated receptor-gamma delivered in the rodent cornea with adenovirus reduces inflammatory and fibrogenic responses in the alkali-burn mouse cornea by inhibiting growth factor release and modulating matrix metalloproteinase production and infiltration of monocytes and macrophages in the healing cornea in vivo.
D. Corneal Neovascularization
Development of corneal neovasculature (CNV) in the eye results in a loss of transparency, increased immune exposure, and increased risk of an inflammatory response. Direct targeting of the vascular endothelial growth factor (VEGF) pathway has shown great promise in vivo. Soluble VEGF receptors Flt-1 and Flk-1 delivered with adenovirus or nanoparticle vectors inhibited CNV in the mouse and rat eye, respectively. 65,82,83 Intracellular sequestration of VEGF using Flt23K or Flt24K peptide (the VEGF-binding domains of sFlt-1) coupled with an endoplasmic reticulum-retaining peptide via intrastromal injection in mouse eye showed notable inhibition of CNV.84,85
The potential of endostatin, kringle-5 domain of plasminogen and collagen type XVIII genes have also been tested. These genes regulate vascular endothelial cell adhesion, migration, proliferation, and apoptosis, and have been shown to reduce CNV in mice and rats.86-88 Many other genes, such as CD36, IL18, pigment epithelium derived factor, GA-binding protein, and decorin, have shown some promise in treating CNV. Decorin gene therapy delivered with AAV5 vector showed significant CNV reduction in rabbit eyes in vivo without apparent side effects.89 Most of these studies have demonstrated 50% or more CNV inhibition.
E. Corneal Dystrophies
Gene therapy can be used to treat corneal dystrophies like mucopolysaccharidosis, a group of metabolic disorders characterized by deficiency of lysosomal enzymes and congenital stromal corneal dystrophies characterized by accumulation of mutant decorin in the stroma. The former can be treated by delivering genes such as beta-glucuronidase, and the latter can be treated employing siRNA-based gene therapy by placing a mechanism that prevents mutant decorin formation in the cornea (Mohan et al, unpublished data). Nonetheless, a major hindrance to the development of gene therapy for corneal dystrophies has been the lack of experimental models. Recently, major progress is made in this regard with the development of a mouse model that recapitulates human congenital stromal corneal dystrophy phenotype and pathophysiology.90
F. Other Common Ocular Surface Disorders
The potential of gene therapy for HSV keratitis has been investigated. A plasmid expressing HSV-1 glycoproteins such as glycoprotein (g) D, gB1 intramuscularly demonstrated marked protection against keratitis. Varied success in controlling keratitis with a topical application of naked plasmid vector encoding for cytokines such as interleukin (IL)2, IL4, IL10, interferon, or tumor necrosis factor alpha has been observed. These studies revealed that gene therapy is capable of preventing or reversing HSV keratitis and prevent scarring. The effects of hepatocyte growth factor receptor c-met proto-oncogene were studied to control corneal neuropathy and epitheliopathy caused by type-I and -II diabetes. The adenovirus mediated cmet gene transfer into human endothelial cells in vitro improved reestablishment of hepatocyte growth factor (HGF) signaling, restored normal protein patterns, and enhanced wound healing.91
Some research has also been done on gene-based treatments for defects in conjunctiva or lacrimal gland function, such as occurs in Sjorgen syndrome, dry eye, etc.92-96 The effects of IL-10, IL-4, and anti-tumor necrosis factor (TNF) alpha genes on lacrimal gland function have been investigated. Virus-mediated IL-10 gene delivery in rabbit lacrimal gland resulted in protection against lacrimal gland immunopathology and decrease in tear production, whereas delivery of TNF inhibitor showed increased basal tear production and reduced immune cell infiltration in the lacrimal gland in vivo.92,93 Inhibition of scarring in the conjunctiva has been a primary focus of conjunctival gene therapy studies. Adenovirus-mediated delivery of p38 mitogen activated protein kinase, Smad7, or peroxisome proliferator-activated receptor gamma (PPARγ) gene transfer have been shown to suppress the fibrogenic reaction in cultured human subconjunctival fibroblasts in vitro and conjunctival scarring in vivo.94-96
IV. Conclusions and Future Directions
Gene therapy holds great potential for the treatment of corneal diseases and the prevention of blindness. The development of novel methods of introducing genetic material into cells, the identification of new therapeutic targets, and improved tissue selectivity may revolutionize our approach to current therapeutic challenges and treat currently incurable illness and disease. Nanoparticle vectors offer great promise for the development of efficient and safe patient-specific gene therapy. Likewise, the ability of next generation AAV vectors to safely induce long-term gene delivery offer tremendous potential. The recent development of RNA interference (RNAi) technology is particularly attractive for developing gene therapy treatments to cure corneal genetic dystrophies like congenital stromal corneal dystrophy. RNAi gene therapy offers gene silencing in a sequence-specific manner, and will be functionally similar to posttranslational gene silencing. Several obstacles still must be addressed, including patient safety, improvement of current vectors, and investigation into the potential for unintended biological consequences of vector exposure and transgene expression. Many of these hurdles have been partially overcome, and revolutionary treatments for corneal diseases and dystrophies are expected to emerge in the near future.
Acknowledgments
This work was supported by the RO1EY17294 National Eye Institute, NIH, Bethesda, Maryland, USA, and 1I01BX00035701 Veteran Health Affairs, Washington DC, USA grants to RRM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no commercial or proprietary interest in any concept or product discussed in this article.
References
- 1.Volpers C, Kochanek S. Adenoviral vectors for gene transfer and therapy. J Gene Med. 2004;6:S164–71. doi: 10.1002/jgm.496. [DOI] [PubMed] [Google Scholar]
- 2.Fehervari Z, Rayner SA, Oral HB, et al. Gene transfer to ex vivo stored corneas. Cornea. 1997;16:459–64. [PubMed] [Google Scholar]
- 3.Mohan RR, Tovey J, Sharma A, Tandon A. Gene therapy in the cornea: 2005-present. Prog Retin Eye Res. 2012;31:43–64. doi: 10.1016/j.preteyeres.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan RR, Sharma A, Netto MV, et al. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24:537–59. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Mashhour B, Couton D, Perricaudet M, Briand P. In vivo adenovirus-mediated gene transfer into ocular tissues. Gene Ther. 1994;1:122–6. [PubMed] [Google Scholar]
- 6.Larkin DF, Oral HB, Ring CJ, et al. Adenovirus-mediated gene delivery to the corneal endothelium. Transplantation. 1996;61:363–70. doi: 10.1097/00007890-199602150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Klebe S, Sykes PJ, Coster DJ, et al. Prolongation of sheep corneal allograft survival by ex vivo transfer of the gene encoding interleukin-10. Transplantation. 2001;71:1214–20. doi: 10.1097/00007890-200105150-00006. [DOI] [PubMed] [Google Scholar]
- 8.Borras T, Tamm ER, Zigler JS., Jr Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol Vis Sci. 1996;37:1282–13. [PubMed] [Google Scholar]
- 9.Borras T, Gabelt BT, Klintworth GK, et al. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med. 2001;3:437–49. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- 10.Budenz DL, Bennett J, Alonso L. In vivo gene transfer into murine corneal endothelial and trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1995;36:2211–5. [PubMed] [Google Scholar]
- 11.Carlson EC, Liu CY, Yang X. In vivo gene delivery and visualization of corneal stromal cells using an adenoviral vector and keratocyte-specific promoter. Invest Ophthalmol Vis Sci. 2004;45:2194–200. doi: 10.1167/iovs.03-1224. [DOI] [PubMed] [Google Scholar]
- 12.Tsubota K, Inoue H, Ando K, et al. Adenovirus-mediated gene transfer to the ocular surface epithelium. Exp Eye Res. 1998;67:531–38. doi: 10.1006/exer.1998.0557. [DOI] [PubMed] [Google Scholar]
- 13.Mohan RR, Possin DE, Mohan RR, et al. Development of genetically engineered tet HPV16-E6/E7 transduced human corneal epithelial clones having tight regulation of proliferation and normal differentiation. Exp Eye Res. 2003;76:373–407. doi: 10.1016/s0014-4835(03)00175-1. [DOI] [PubMed] [Google Scholar]
- 14.Jester JV, Huang J, Fischer S, et al. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:1850–8. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- 15.Wilson SE, Weng J, Blair S, et al. Expression of E6/E7 or SV40 large T antigen-coding oncogenes in human corneal endothelial cells indicates regulated high-proliferative capacity. Invest Ophthalmol Vis Sci. 1995;36:32–40. [PubMed] [Google Scholar]
- 16.He Y, Weng J, Li Q, et al. Fuchs’ corneal endothelial cells transduced with the human papilloma virus E6/E7 oncogenes. Exp Eye Res. 1997;65:135–42. doi: 10.1006/exer.1997.0320. [DOI] [PubMed] [Google Scholar]
- 17.Seitz B, Moreira L, Baktanian E, et al. Retroviral vector-mediated gene transfer into keratocytes in vitro and in vivo. Am J Ophthalmo. 1998;126:630–9. doi: 10.1016/s0002-9394(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 18.Seitz B, Baktanian E, Gordon EM, et al. Retroviral vector-mediated gene transfer into keratocytes: in vitro effects of polybrene and protamine sulfate. Graefes Arch Clin Exp Ophthalmol. 1998;236:602–12. doi: 10.1007/s004170050129. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi H, Blomer U, Takahasi M, et al. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–7. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Appukuttan B, Ott S, et al. Efficient and sustained transgene expression in human corneal cells mediated by a lentiviral vectors. Gene Ther. 2000;7:196–200. doi: 10.1038/sj.gt.3301075. [DOI] [PubMed] [Google Scholar]
- 21.Bainbridge JW, Stephens C, Parsley K, et al. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 2001;8:1665–8. doi: 10.1038/sj.gt.3301574. [DOI] [PubMed] [Google Scholar]
- 22.Challa P, Luna C, Liton PB, et al. Lentiviral mediated gene delivery to the anterior chamber of rodent eyes. Mol Vis. 2005;11:425–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi K, Luo T, Saishin Y, et al. Sustained transduction of ocular cells with a bovine immunodeficiency viral vector. Hum Gene Ther. 2002;13:1305–16. doi: 10.1089/104303402760128531. [DOI] [PubMed] [Google Scholar]
- 24.Beutelspacher SC, Ardjomand N, Tan PH, et al. Comparison of HIV-1 and EIAV-based lentiviral vectors in corneal transduction. Exp Eye Res. 2005;80:787–94. doi: 10.1016/j.exer.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Parker DG, Kaufmann C, Brereton HM, et al. Lentivirus-mediated gene transfer to the rat, ovine and human cornea. Gene Ther. 2007;14:760–7. doi: 10.1038/sj.gt.3302921. [DOI] [PubMed] [Google Scholar]
- 26.Mohan RR, Schultz GS, Sinha S, et al. Controlled and site-selective gene delivery into mouse keratocytes by adeno-associated viral, lentiviral and plasmid vectors. Inves Ophthalmol Vis Sci. 2005 ARVO abstract 46:2163. [Google Scholar]
- 27.Grimm D, Kay MA, Kleinschmidt JA. Helper virus-free, optically controllable, and twoplasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol Ther. 2003;7:839–50. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 28.Alexander JJ, Hauswirth WW. Adeno-associated viral vectors and the retina. Adv Exp Med Biol. 2008;613:121–8. doi: 10.1007/978-0-387-74904-4_13. [DOI] [PubMed] [Google Scholar]
- 29.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–97. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 30.Vandenberghe LH, Auricchio A. Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 2012;19:162–8. doi: 10.1038/gt.2011.151. [DOI] [PubMed] [Google Scholar]
- 31.Mohan RR, Schults GS, Hong JW, et al. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp Eye Res. 2003;76:373–83. doi: 10.1016/s0014-4835(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 32.Stieger K, Cronin T, Bennett J, Rolling F. Adeno-associated virus mediated gene therapy for retinal degenerative diseases. Methods Mol Biol. 2011;807:179–218. doi: 10.1007/978-1-61779-370-7_8. [DOI] [PubMed] [Google Scholar]
- 33.Buss DG, Giuliano E, Sharma A, Mohan RR. Gene delivery in the equine cornea: a novel therapeutic strategy. Vet Ophthalmol. 2010;13:301–6. doi: 10.1111/j.1463-5224.2010.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebherz C, Maguire A, Tang W, et al. Novel AAV serotypes for improved ocular gene transfer. J Gene Med. 2008;10:375–82. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohan RR, Sinha S, Tandon A, et al. Efficacious and safe tissue-selective controlled gene therapy approaches for the cornea. PLoS One. 2011;6:e18771. doi: 10.1371/journal.pone.0018771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma A, Ghosh A, Siddappa C, Mohan RR. Ocular surface: gene tTherapy. In: Besharse J, Dana R, Dartt DA, editors. Encyclopedia of the eye. Elsevier; 2010. pp. 185–94. [Google Scholar]
- 37.Sharma A, Ghosh A, Hansen ET, et al. Transduction efficiency of AAV 2/6, 2/8 and 2/9 vectors for delivering genes in human corneal fibroblasts. Brain Res Bull. 2010;81:273–78. doi: 10.1016/j.brainresbull.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma A, Tovey JC, Ghosh A, Mohan RR. AAV serotype influences gene transfer in corneal stroma in vivo. Exp Eye Res. 2010;91:440–8. doi: 10.1016/j.exer.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buie LK, Rasmussen CA, Porterfield EC, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010;51:236–48. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong F, Li W, Li X, Zheng Q, et al. Self-complementary AAV5 vector facilitates quicker transgene expression in photoreceptor and retinal pigment epithelial cells of normal mouse. Exp Eye Res. 2010;90:546–54. doi: 10.1016/j.exer.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoi K, Kachi S, Zhang HS, et al. Ocular gene transfer with self-complementary AAV vectors. Invest Ophthalmol Vis Sci. 2007;48:3324–8. doi: 10.1167/iovs.06-1306. [DOI] [PubMed] [Google Scholar]
- 42.Van Vliet KM, Blouin V, Brument N, et al. The role of the adeno-associated virus capsid in gene transfer. Methods Mol Biol. 2008;437:51–91. doi: 10.1007/978-1-59745-210-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrs-Silva H, Dinculescu A, Li Q, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–71. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong L, Li B, Jayandharan G, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryals RC, Boye SL, Dinculescu A, et al. Quantifying transduction efficiencies of unmodified and tyrosine capsid mutant AAV vectors in vitro using two ocular cell lines. Mol Vis. 2011;17:1090–102. [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma A, Tovey JCK, Hauswirth WW, et al. Remarkably high and selective transgene delivery into mouse corneal endothelium in vivo with tyrosine-mutant AAV8. Invest Ophthalmol Vis Sci. 2010 ARVO 51:abstract 2839. [Google Scholar]
- 47.Hart SL, Arancibia-Carcamo CV, Wolfert MA, et al. Lipid-mediated enhancement of transfection by a nonviral integrin-targeting vector. Hum Gene Ther. 1998;9:575–85. doi: 10.1089/hum.1998.9.4-575. [DOI] [PubMed] [Google Scholar]
- 48.Angella GJ, Sherwood MB, Balasubramanian L, et al. Enhanced short-term plasmid transfection of filtration surgery tissues. Invest Ophthalmol Vis Sci. 2000;41:4158–62. [PubMed] [Google Scholar]
- 49.Yoon KC, Ahn KY, Lee JH, et al. Lipid-mediated delivery of brain-specific angiogenesis inhibitor 1 gene reduces corneal neovascularization in an in vivo rabbit model. Gene Ther. 2005;12:617–24. doi: 10.1038/sj.gt.3302442. [DOI] [PubMed] [Google Scholar]
- 50.Pleyer U, Dannowski H. Delivery of genes via liposomes to corneal endothelial cells. Drug News Perspect. 2002;15:283–9. doi: 10.1358/dnp.2002.15.5.840041. [DOI] [PubMed] [Google Scholar]
- 51.Toropainen E, Hornof M, Kaarniranta K, et al. Corneal epithelium as a platform for secretion of transgene products after transfection with liposomal gene eyedrops. J Gene Med. 2007;9:208–16. doi: 10.1002/jgm.1011. [DOI] [PubMed] [Google Scholar]
- 52.Dannowski H, Bednarz J, Reszka R, et al. Lipid-mediated gene transfer of acidic fibroblast growth factor into human corneal endothelial cells. Exp Eye Res. 2005;80:93–101. doi: 10.1016/j.exer.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Oshima Y, Sakamoto T, Tamanaka I, et al. Targeted gene transfer to corneal endothelium in vivo by electric pulse. Gene Ther. 1998;5:1347–54. doi: 10.1038/sj.gt.3300725. [DOI] [PubMed] [Google Scholar]
- 54.Oshima Y, Sakamoto T, Hisatomi T, et al. Targeted gene transfer to corneal stroma in vivo by electric pulses. Exp. Eye Res. 2002;74:191–8. doi: 10.1006/exer.2001.1117. [DOI] [PubMed] [Google Scholar]
- 55.Zhou R, Dean DA. Gene transfer of interleukin 10 to the murine cornea using electroporation. Exp Biol Med (Maywood) 2007;232:362–9. [PMC free article] [PubMed] [Google Scholar]
- 56.He Z, Pipparelli A, Manissolle C, et al. Ex vivo gene electrotransfer to the endothelium of organ cultured human corneas. Ophthalmic Res. 2010;43:43–55. doi: 10.1159/000246577. [DOI] [PubMed] [Google Scholar]
- 57.Korampally M, Apperson SJ, Staley CS, et al. Transient pressure mediated intranuclear delivery of FITC-Dextran into chicken cardiomyocytes by MEMS-based nanothermite reaction actuator. Sensors and Actuators B: Chemical. 2012;171-172:1292–6. http://dx.doi.org/10.1016/j.snb.2012.06.081. [Google Scholar]
- 58.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–60. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 59.Sonoda S, Tachibana K, Uchino E, et al. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest Ophthalmol Vis Sci. 2006:47. doi: 10.1167/iovs.05-0889. [DOI] [PubMed] [Google Scholar]
- 60.Gupta R, Tandon A, Hansen ET, et al. Rapid and substantial gene delivery into cornea in vivo and in vitro with linearized polyethyleneimine nanoparticles. Invest Ophthalmol Vis Sci. 2011;52:494. ARVO E-abstract. [Google Scholar]
- 61.de la Fuente M, Seijo B, Alonso MJ. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Invest Ophthalmol Vis Sci. 2008;49:2016–24. doi: 10.1167/iovs.07-1077. [DOI] [PubMed] [Google Scholar]
- 62.Sharma A, Rodier J, Tandon A, et al. Attenuation of corneal myofibroblast development through nanoparticle-mediated soluble transforming growth factor-β type II receptor (sTGFβRII) gene transfer. Mol Vis. 2012;18:2598–607. [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma A, Tandon A, Tovey JCK, et al. Polyethelenimine-conjugated gold nanoparticles: Gene transfer potential and low toxicity in the cornea. Nanomed-nanotechnol. 2011;7:505–13. doi: 10.1016/j.nano.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klausner EA, Zhang Z, Chapman RL, et al. Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials. 2010;31:1814–20. doi: 10.1016/j.biomaterials.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 65.Jani PD, Singh N, Jenkins C. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–6. doi: 10.1167/iovs.06-0853. [DOI] [PubMed] [Google Scholar]
- 66.Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res. 2008;48:319–24. doi: 10.1016/j.visres.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shiraishi A, Converse RL, Liu CY, et al. Identification of the cornea-specific keratin 12 promoter by in vivo particle-mediated gene transfer. Invest Ophthalmol Vis Sci. 1998;39:2554–61. [PubMed] [Google Scholar]
- 68.Lu H, Lu Q, Zheng Y, Li Q. Notch signaling promotes the corneal epithelium wound healing. Mol Vis. 2012;18:403–11. [PMC free article] [PubMed] [Google Scholar]
- 69.Parker DG, Brereton HM, Klebe S, et al. A steroid-inducible promoter for the cornea. Br J Ophthalmol. 2009;93:1255–9. doi: 10.1136/bjo.2009.159137. [DOI] [PubMed] [Google Scholar]
- 70.Beutelspacher SC, Pillai R, Watson MP. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36:690–700. doi: 10.1002/eji.200535238. [DOI] [PubMed] [Google Scholar]
- 71.Barcia RN, Dana MR, Kazlauskas A. Corneal graft rejection is accompanied by apoptosis of the endothelium and is prevented by gene therapy with bcl-xL. Am J Transplant. 2007;7:2082–9. doi: 10.1111/j.1600-6143.2007.01897.x. [DOI] [PubMed] [Google Scholar]
- 72.Fuchsluger TA, Jurkunas U, Kazlauskas A, Dana R. Corneal endothelial cells are protected from apoptosis by gene therapy. Hum Gene Ther. 2011;22:549–58. doi: 10.1089/hum.2010.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gong N, Pleyer U, Yang J, et al. Influence of local and systemic CTLA4Ig gene transfer on corneal allograft survival. J Gene Med. 2006;8:459–67. doi: 10.1002/jgm.876. [DOI] [PubMed] [Google Scholar]
- 74.Joyce NC, Harris DL, McAlister JC, et al. Overexpression of the transcription factor E2F2 induces cell cycle progression in rabbit corneal endothelial cells. Invest Ophthalmol Vis Sci. 2004;45:1340–8. doi: 10.1167/iovs.03-0335. [DOI] [PubMed] [Google Scholar]
- 75.McAlister JC, Joyce NC, Harris DL, et al. Induction of replication in human corneal endothelial cells by E2F2 transcription factor cDNA transfer. Invest Ophthalmol Vis Sci. 2005;46:3597–603. doi: 10.1167/iovs.04-0551. [DOI] [PubMed] [Google Scholar]
- 76.Mohan RR, Gupta R, Mehan MK, et al. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res. 2010;91:238–45. doi: 10.1016/j.exer.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohan RR, Tovey JC, Gupta R, et al. Decorin biology, expression, function and therapy in the cornea. Curr Mol Med. 2011;11:110–28. doi: 10.2174/156652411794859241. [DOI] [PubMed] [Google Scholar]
- 78.Mohan RR, Tandon A, Sharma A, et al. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest Ophthalmol Vis Sci. 2011;52:4833–41. doi: 10.1167/iovs.11-7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tandon A, Tovey JC, Sharma A, et al. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr Mol Med. 2010;10:565–78. doi: 10.2174/1566524011009060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saika S, Ikeda K, Yamanaka O, et al. Therapeutic effects of edenoviral gene transfer of bone morphogenic protein-7 on a corneal alkali injury model in mice. Lab Invest. 2005;85:474–86. doi: 10.1038/labinvest.3700247. [DOI] [PubMed] [Google Scholar]
- 81.Tandon A, Tovey JCT, Rodier JT, et al. Bone morphogenic protein 7 gene therapy inhibits fibrosis by upregulating Smad1/5/8 in rabbit cornea, in vivo. Invest Ophthalmol Vis Sci. 2012;53:1086. ARVO E-abstract. [Google Scholar]
- 82.Lai CM, Brankov M, Zaknich T, et al. Inhibition of angiogenesis by adenovirus-mediated sFlt-1 expression in a rat model of corneal neovascularization. Hum Gene Ther. 2001;12:1299–1310. doi: 10.1089/104303401750270959. [DOI] [PubMed] [Google Scholar]
- 83.Yu H, Wu J, Li H, et al. Inhibition of corneal neovascularization by recombinant adenovirus-mediated sFlk-1 expression. Biochem Biophys Res Commun. 2007;361:946–52. doi: 10.1016/j.bbrc.2007.07.114. [DOI] [PubMed] [Google Scholar]
- 84.Cho YK, Uehara H, Young JR, et al. Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Invest Ophthalmol Vis Sci. 2012;53:2328–36. doi: 10.1167/iovs.11-8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh N, Amin S, Richter E, et al. Flt-1 intraceptors inhibit hypoxia-induced VEGF expression in vitro and corneal neovascularization in vivo. Invest Ophthalmol Vis Sci. 2005;46:1647–52. doi: 10.1167/iovs.04-1172. [DOI] [PubMed] [Google Scholar]
- 86.Lai LJ, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14:313–22. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- 87.Cheng HC, Yeh SI, Tsao YP, Kuo PC. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol Vi. 2007;13:2344–52. [PubMed] [Google Scholar]
- 88.Zhou SY, Xie ZL, Xiao O, et al. Inhibition of mouse alkali burn induced-corneal neovascularization by recombinant adenovirus encoding human vasohibin-1. Mol Vi. 2010;16:1389–98. [PMC free article] [PubMed] [Google Scholar]
- 89.Mohan RR, Tovey JCK, Sharma A, et al. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS One. 2011;6:e26432. doi: 10.1371/journal.pone.0026432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen S, Sun M, Meng X, et al. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am J Pathol. 2011;179:2409–19. doi: 10.1016/j.ajpath.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saghizadeh M, Kramerov AA, Yu F, et al. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest Ophthalmol Vis Sci. 2010;51:1970–80. doi: 10.1167/iovs.09-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomas PB, Samant DM, Selvam S, et al. Adeno-associated virus-mediated IL-10 gene transfer suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2010;51:5137–44. doi: 10.1167/iovs.10-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Selvam S, Thomas PB, Hamm-Alvarez SF, et al. Current status of gene delivery and gene therapy in lacrimal gland using viral vectors. Adv Drug Deliv Rev. 2006;58:1243–57. doi: 10.1016/j.addr.2006.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamanaka O, Ikeda K, Saika S, et al. Gene transfer of Smad7 modulates injury-induced conjunctival wound healing in mice. Mol Vis. 2006;12:841–51. [PubMed] [Google Scholar]
- 95.Yamanaka O, Saika S, Ohnishi Y, et al. Inhibition of p38MAP kinase suppresses fibrogenic reaction in conjunctiva in mice. Mol Vis. 2007;13:1730–9. [PubMed] [Google Scholar]
- 96.Yamanaka O, Miyazaki K, Kitano A, et al. Suppression of injury-induced conjunctiva scarring by peroxisome proliferator-activated receptor gamma gene transfer in mice. Invest Ophthalmol Vis Sci. 2009;50:187–93. doi: 10.1167/iovs.08-2282. [DOI] [PubMed] [Google Scholar]