Abstract
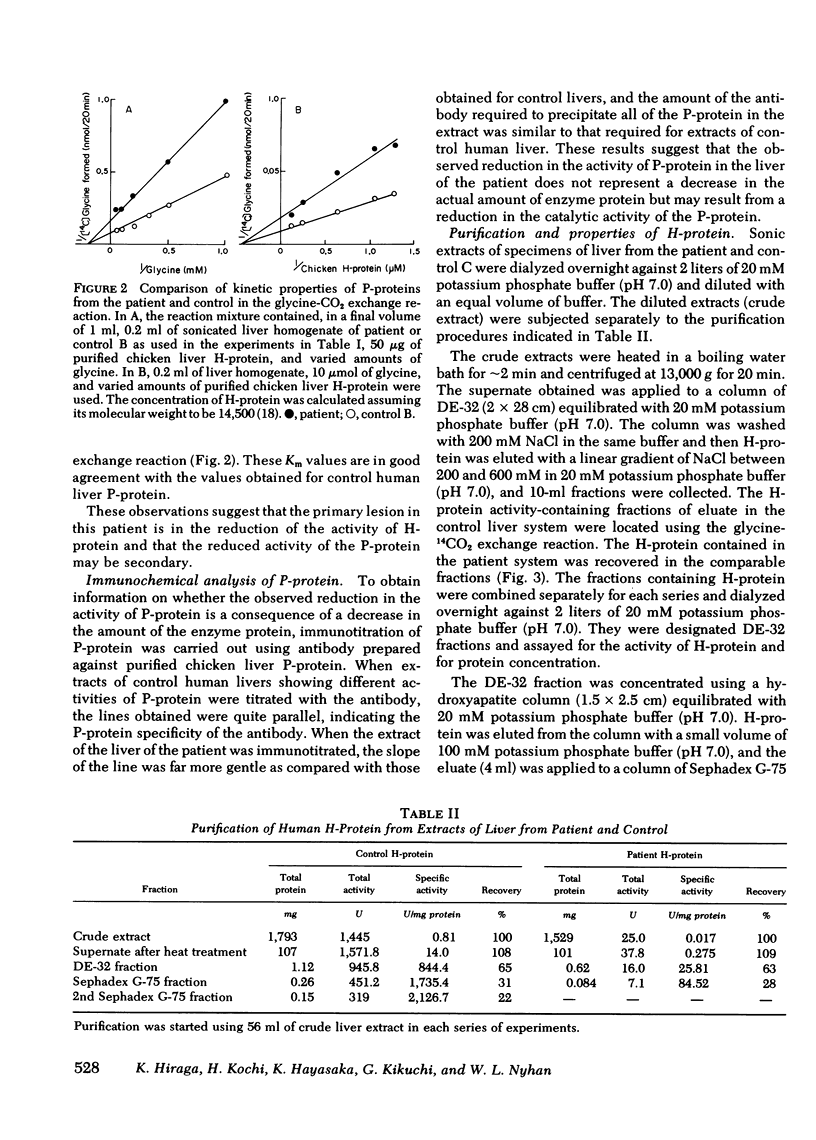
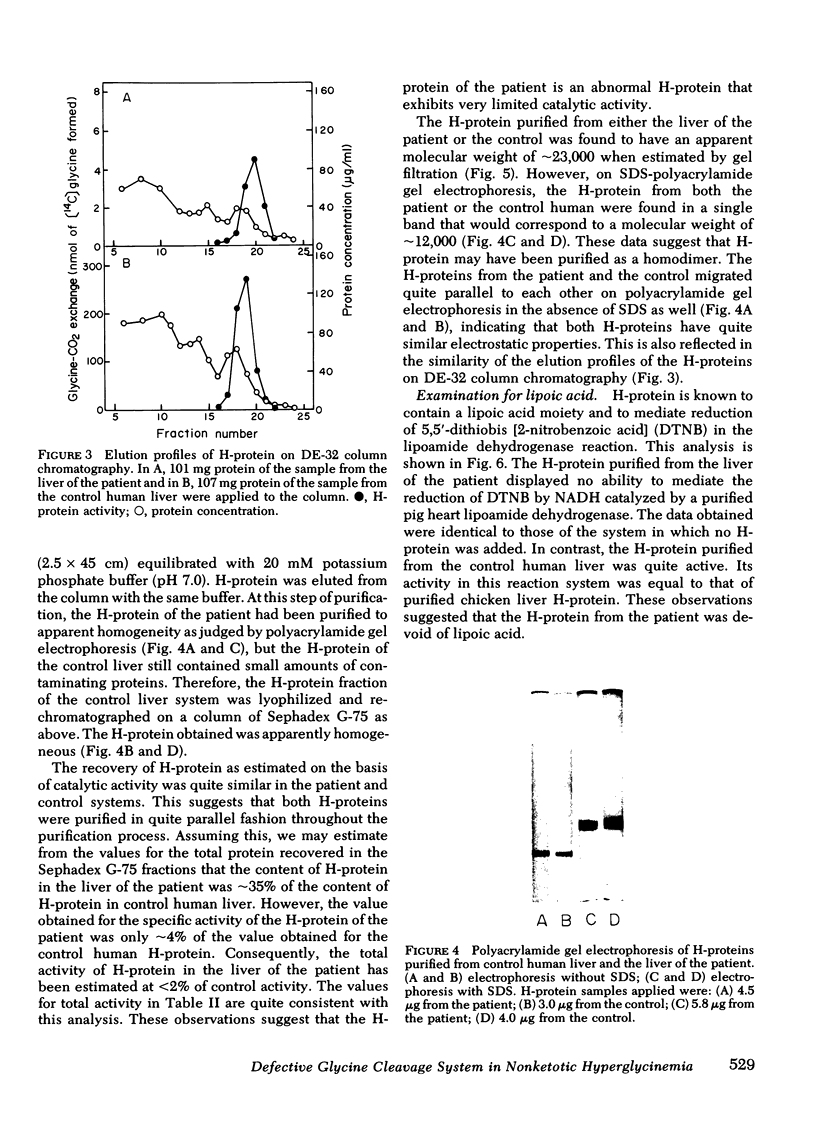
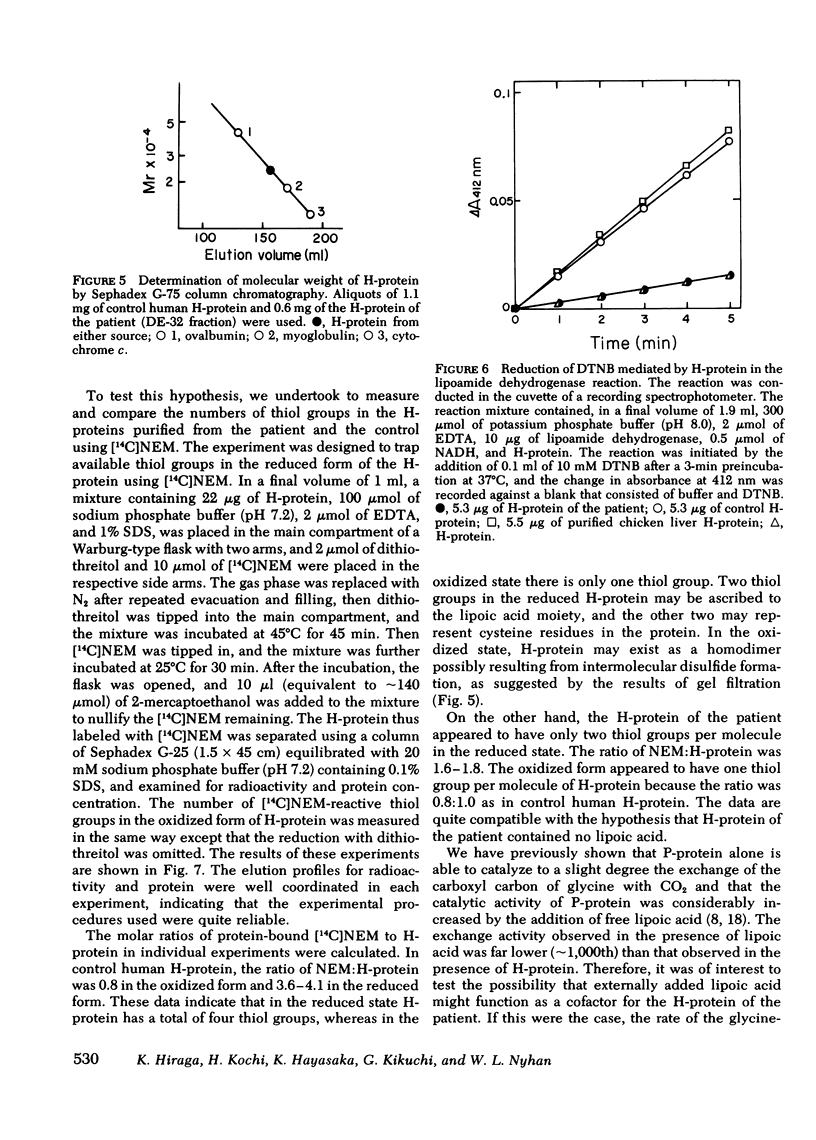
The activities of then glycine cleavage system in the liver and brain of patient with nonketotic hyperglycinemia was extremely low as compared with those of control human liver and brain. The activities of glycine decarboxylase (P-protein) and the aminomethyl carrier protein (H-protein), two of the four protein components of the glycine cleavage system, were considerably reduced in both the liver and brain; the extent of reduction was greater in the H-protein. The activity of the T-protein was normal. Purified H-protein from the patient did not react with lipoamide dehydrogenase, and titration of thiol groups with [2,3-14C]N-ethylmaleimide suggested that this H-protein is devoid of lipoic acid. This structural abnormality in the H-protein is considered to constitute the primary molecular lesion in this patient with non-ketotic hyperglycinemia. Immunochemical studies using an antibody specific for P-protein showed that the patient was due to reduction of the catalytic activity of the protein rather than a decrease in the actual amount of the P-protein. Partial inactivation of P-protein could result secondarily from impaired metabolism of glycine resulting from deficiency in the activity of H-protein. However, the H-protein from the patient could stimulate the P-protein catalyzed exchange of the carboxyl carbon of glycine with 14CO2, although the specific activity of the purified H-protein from the patient was only 4% of that of control human H-protein. The content of H-protein in the liver of the patient was approximately 35% of that of control human liver.
Full text
PDF





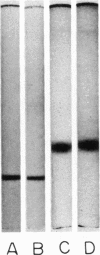
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAUFAY H., BENDALL D. S., BAUDHUIN P., DE DUVE C. Tissue fractionation studies. 12. Intracellular distribution of some dehydrogenases, alkaline deoxyribonuclease and iron in rat-liver tissue. Biochem J. 1959 Dec;73:623–628. doi: 10.1042/bj0730623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum S. D., Blass J. P., Minkoff N., Brown W. J., Cotton M. E., Harris S. H. Sensitivity to carbohydrate in a patient with familial intermittent lactic acidosis and pyruvate dehydrogenase deficiency. Pediatr Res. 1976 Aug;10(8):713–720. doi: 10.1203/00006450-197608000-00002. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Keating J. P. Beta-ketothiolase deficiency as a cause of the "ketotic hyperglycinemia syndrome". Pediatrics. 1974 Feb;53(2):221–225. [PubMed] [Google Scholar]
- Hiraga K., Kikuchi G. The mitochondrial glycine cleavage system. Functional association of glycine decarboxylase and aminomethyl carrier protein. J Biol Chem. 1980 Dec 25;255(24):11671–11676. [PubMed] [Google Scholar]
- Hiraga K., Kikuchi G. The mitochondrial glycine cleavage system. Purification and properties of glycine decarboxylase from chicken liver mitochondria. J Biol Chem. 1980 Dec 25;255(24):11664–11670. [PubMed] [Google Scholar]
- Hsia Y. E., Scully K. J., Rosenberg L. E. Defective propionate carboxylation in ketotic hyperglycinaemia. Lancet. 1969 Apr 12;1(7598):757–758. doi: 10.1016/s0140-6736(69)91757-7. [DOI] [PubMed] [Google Scholar]
- Ide S., Hayakawa T., Okabe K., Koike M. Lipoamide dehydrogenase from human liver. J Biol Chem. 1967 Jan 10;242(1):54–60. [PubMed] [Google Scholar]
- Jaeken J., Corbeel L., Casaer P., Carchon H., Eggermont E., Eeckels R. Dipropylacetate (valproate) and glycine metabolism. Lancet. 1977 Sep 17;2(8038):617–617. doi: 10.1016/s0140-6736(77)91475-1. [DOI] [PubMed] [Google Scholar]
- KISLIUK R. L. Studies on the mechanism of formaldehyde incorporation into serine. J Biol Chem. 1957 Aug;227(2):805–814. [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Kochi H., Hayasaka K., Hiraga K., Kikuchi G. Reduction of the level of the glycine cleavage system in the rat liver resulting from administration of dipropylacetic acid: an experimental approach to hyperglycinemia. Arch Biochem Biophys. 1979 Dec;198(2):589–597. doi: 10.1016/0003-9861(79)90535-6. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Glycine metabolism in rat liver mitochondria. V. Intramitochondrial localization of the reversible glycine cleavage system and serine hydroxymethyltransferase. Arch Biochem Biophys. 1971 Oct;146(2):461–464. doi: 10.1016/0003-9861(71)90149-4. [DOI] [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G., Narisawa K., Arakawa T. Reduced level of glycine cleavage system in the liver of hyperglycinemia patients. Clin Chim Acta. 1977 Aug 15;79(1):173–181. doi: 10.1016/0009-8981(77)90475-2. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Urquhart N., Hansen S. Studies of the glycine cleavage enzyme system in brain from infants with glycine encephalopathy. Pediatr Res. 1977 Dec;11(12):1192–1197. doi: 10.1203/00006450-197712000-00005. [DOI] [PubMed] [Google Scholar]
- Revsin B., Lebowitz J., Morrow G., 3rd Effect of valine on propionate metabolism in control and hyperglycinemic fibroblasts and in rat liver. Pediatr Res. 1977 Jun;11(6):749–753. doi: 10.1203/00006450-197706000-00011. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. E., Lilljeqvist A. C., Hsia Y. E. Methylmalonic aciduria. An inborn error leading to metabolic acidosis, long-chain ketonuria and intermittent hyperglycinemia. N Engl J Med. 1968 Jun 13;278(24):1319–1322. doi: 10.1056/NEJM196806132782404. [DOI] [PubMed] [Google Scholar]
- Sato T., Kochi H., Sato N., Kikuchi G. Glycine metabolism by rat liver mitochondria. 3. The glycine cleavage and the exchange of carboxyl carbon of glycine with bicarbonate. J Biochem. 1969 Jan;65(1):77–83. [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. A., McAfee J., Parks J. K., Eguren L. Propionate inhibition of succinate:CoA ligase (GDP) and the citric acid cycle in mitochondria. Pediatr Res. 1980 Oct;14(10):1127–1131. doi: 10.1203/00006450-198010000-00008. [DOI] [PubMed] [Google Scholar]
- Tada K. A block in glycine cleavage reaction as a common mechanism in ketotic and nonketotic hyperglycinemia. Pediatr Res. 1974 Jul;8(7):721–723. doi: 10.1203/00006450-197407000-00007. [DOI] [PubMed] [Google Scholar]
- Trauner D. A., Page T., Greco C., Sweetman L., Kulovich S., Nyhan W. L. Progressive neurodegenerative disorder in a patient with nonketotic hyperglycinemia. J Pediatr. 1981 Feb;98(2):272–275. doi: 10.1016/s0022-3476(81)80659-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Comparative study on major pathways of glycine and serine catabolism in vertebrate livers. J Biochem. 1972 Dec;72(6):1503–1516. doi: 10.1093/oxfordjournals.jbchem.a130042. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Physiological significance of glycine cleavage system in human liver as revealed by the study of a case of hyperglycinemia. Biochem Biophys Res Commun. 1969 May 22;35(4):577–583. doi: 10.1016/0006-291x(69)90387-8. [DOI] [PubMed] [Google Scholar]