Abstract
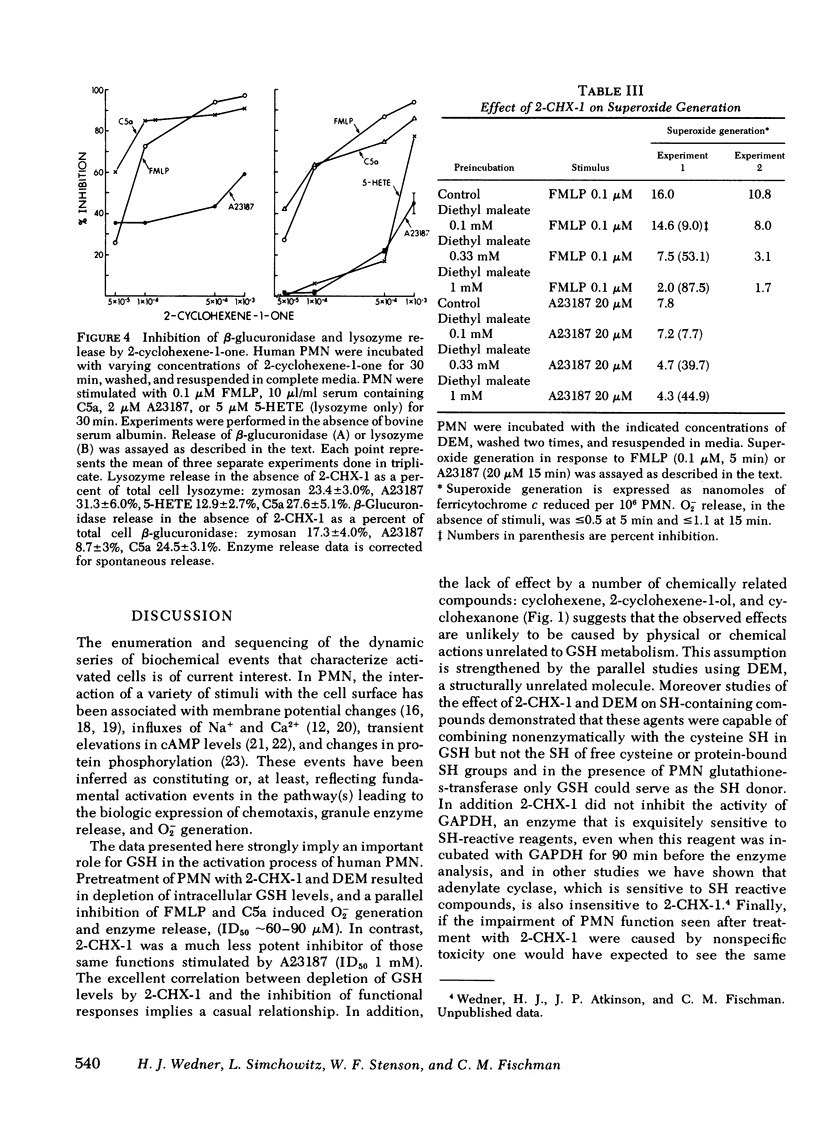
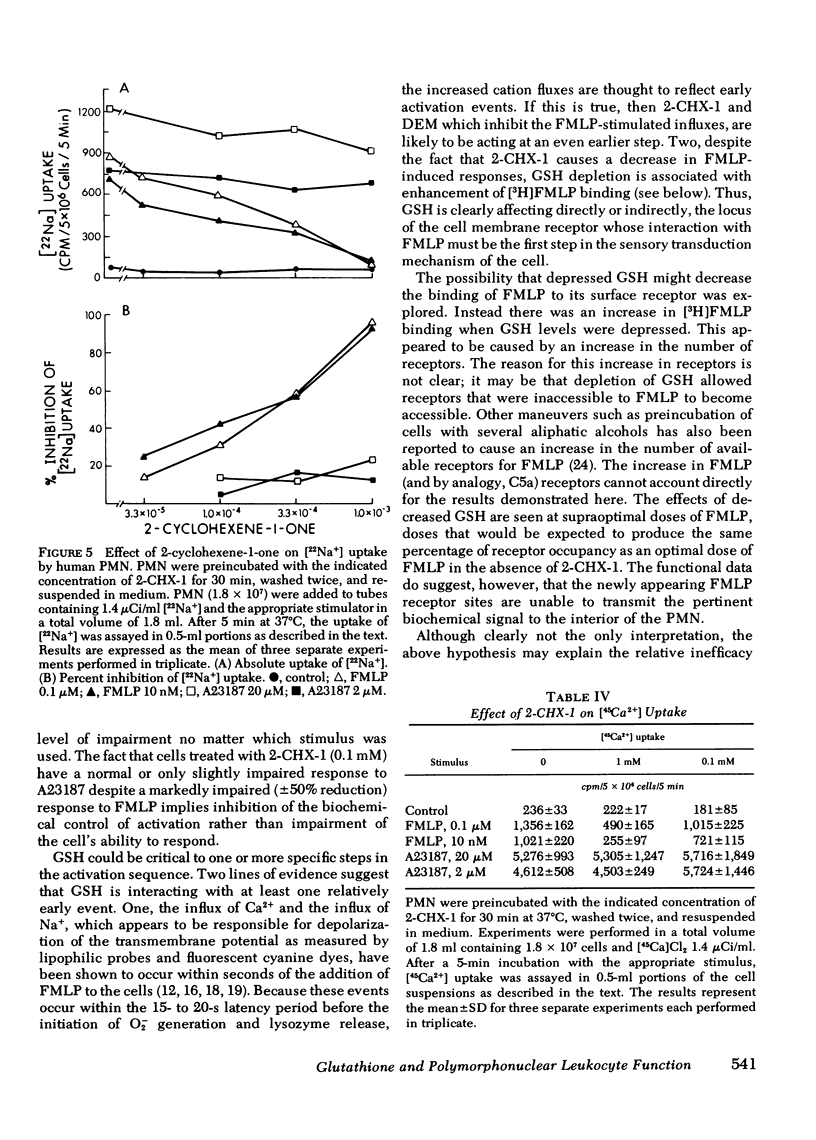
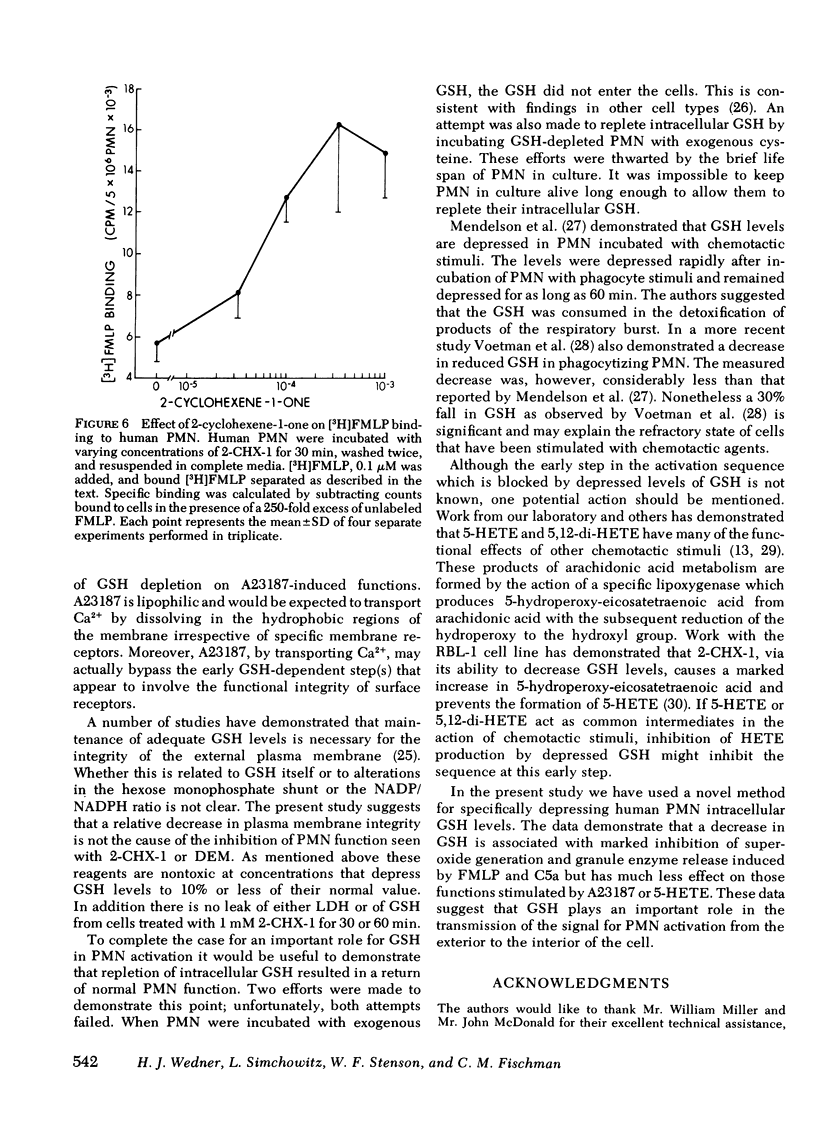
2-cyclohexene-1-one and diethyl maleate specifically decrease reduced glutathione (GSH) levels in human polymorphonuclear leukocytes (PMN) by direct conjugation, and by interaction with the glutathione-s-transferase system. Using these two nontoxic reagents we have examined the effect of decreased GSH levels on five parameters of PMN activation: superoxide generation, release of the lysosomal enzymes lysozyme and beta-glucuronidase, and increases in the influx of Na+ and Ca2+. When PMN pretreated with 2-cyclohexene-1-one or diethyl maleate were incubated with formyl-methionyl-leucyl-phenylalanine (FMLP) or the proteolytic fragment of the fifth component membrane of complement, C5a, agents that interact with surface membrane receptors, increases in all five parameters were inhibited in a dose-dependent manner. For O-2 generation and lysosomal enzyme release the ID50 for 2-CHX-1 was 40--90 micrometers corresponding with a 30--50% decrease in intracellular GHS. In contrast stimulation of treated PMN by the divalent cation ionophore A23187 or 5-hydroxyeicosatetraenoic acid was much less sensitive to depressed GSH; the ID50 for 2-cyclohexene-1-one was 1 mM or greater, corresponding with an 80--90% decrease in GSH. The effect of lowered GSH was not the result of decreased binding of FMLP to surface receptors because [3H]-FMLP binding studies demonstrated a two- to three-fold increase in the number of available binding sites. These data indicate that normal GSH levels are necessary for the transduction of the activation signal from the exterior to the interior of the PMN, but once initiated the activation sequence proceeds normally despite markedly lowered intracellular GSH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannai S., Tsukeda H. The export of glutathione from human diploid cells in culture. J Biol Chem. 1979 May 10;254(9):3444–3450. [PubMed] [Google Scholar]
- Brovelli A., Suhail M., Pallavicini G., Sinigaglia F., Balduini C. Self-digestion of human erythrocyte membranes. Role of adenosine triphosphate and glutathione. Biochem J. 1977 May 15;164(2):469–472. doi: 10.1042/bj1640469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fantone J., Senior R. M., Kreutzer D. L., Jones M., Ward P. A. Biochemical quantitation of the chemotactic factor inactivator activity in human serum. J Lab Clin Med. 1979 Jan;93(1):17–24. [PubMed] [Google Scholar]
- Goetzl E. J., Sun F. F. Generation of unique mono-hydroxy-eicosatetraenoic acids from arachidonic acid by human neutrophils. J Exp Med. 1979 Aug 1;150(2):406–411. doi: 10.1084/jem.150.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. S., Freer R. J. Cryptic receptors for chemotactic peptides in rabbit neutrophils. Biochem Biophys Res Commun. 1980 Mar 28;93(2):566–571. doi: 10.1016/0006-291x(80)91114-6. [DOI] [PubMed] [Google Scholar]
- Mendelson D. S., Metz E. N., Sagone A. L., Jr Effect of phagocytosis on the reduced soluble sulfhydryl content of human granulocytes. Blood. 1977 Dec;50(6):1023–1030. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Albertini D. F., Berlin R. D. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976 Dec;71(3):921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. W., Fischman C. M., Wedner H. J. Relationship of biosynthesis of slow reacting substance to intracellular glutathione concentrations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6870–6873. doi: 10.1073/pnas.77.11.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., Mills E. L., Holmes B. Molecular events during phagocytosis by human neutrophils. Prog Hematol. 1977;10:193–210. [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980 Sep;66(3):493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Fischbein L. C., Spilberg I., Atkinson J. P. Induction of a transient elevation in intracellular levels of adenosine-3',5'-cyclic monophosphate by chemotactic factors: an early event in human neutrophil activation. J Immunol. 1980 Mar;124(3):1482–1491. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I., Atkinson J. P. Superoxide generation and granule enzyme release induced by ionophore A23187. Studies on the early events of neutrophil activation. J Lab Clin Med. 1980 Sep;96(3):408–424. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. Increased levels of cyclic adenosine-3',5'-monophosphate in human polymorphonuclear leukocytes after surface stimulation. J Clin Invest. 1980 May;65(5):1077–1085. doi: 10.1172/JCI109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilberg I., Mehta J. Demonstration of a specific neutrophil receptor for a cell-derived chemotactic factor. J Clin Invest. 1979 Jan;63(1):85–88. doi: 10.1172/JCI109282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979 Nov;64(5):1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Monohydroxyeicosatetraenoic acids (HETEs) induce degranulation of human neutrophils. J Immunol. 1980 May;124(5):2100–2104. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Voetman A. A., Loos J. A., Roos D. Changes in the levels of glutathione in phagocytosing human neutrophils. Blood. 1980 May;55(5):741–747. [PubMed] [Google Scholar]
- Wedner H. J. The effect of diamide on cyclic AMP levels and cyclic nucleotide phosphodiesterase in human peripheral blood lymphocytes. Biochim Biophys Acta. 1980 Apr 3;628(4):407–418. doi: 10.1016/0304-4165(80)90390-6. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Seegmiller J. E. The inhibition by 6-diazo-5-oxo-l-norleucine of glutamine catabolism of the cultured human lymphoblast. J Cell Physiol. 1977 Dec;93(3):375–382. doi: 10.1002/jcp.1040930308. [DOI] [PubMed] [Google Scholar]



