Abstract
Purpose
Visualization of the cell cycle in living subjects has long been a big challenge. The present study aimed to noninvasively visualize mitotic arrest of the cell cycle with an optical reporter in living subjects.
Procedures
An N-terminal cyclin B1–luciferase fusion construct (cyclin B-Luc) controlled by the cyclin B promoter, as a mitosis reporter, was generated. HeLa or HCT116 cells stably expressing cyclin B-Luc reporter were used to evaluate its cell cycle-dependent regulation and ubiquitination-mediated degradation. We also evaluated its feasibility to monitor the mitotic arrest caused by Taxotere both in vitro and in vivo.
Results
We showed that the cyclin B-Luc fusion protein was regulated in a cell cycle-dependent manner and accumulated in the mitotic phase (M phase) in cellular assays. The regulation of cyclin B-Luc reporter was mediated by proteasome ubiquitination. In the present study, in vitro imaging showed that antimitotic reagents like Taxotere upregulated the reporter through cell cycle arrest in the M phase. Noninvasive longitudinal bioluminescence imaging further demonstrated an upregulation of the reporter consistent with mitotic arrest induced in tumor xenograft models. Induction of this reporter was also observed with a kinesin spindle protein inhibitor, which causes cell cycle blockage in the M phase.
Conclusions
Our results demonstrate that the cyclin B-Luc reporter can be used to image whether compounds are capable, in vivo, of causing an M phase arrest and/or altering cyclin B turnover. This reporter can also be potentially used in high-throughput screening efforts aimed at discovering novel molecules that will cause cell cycle arrest at the M phase in cultivated cell lines and animal models.
Key words: Optical imaging, Mitotic arrest, Cell cycle, Cyclin B1, Bioluminescence
Introduction
Cell cycle progression is tightly controlled by cyclin and cyclin-dependent kinase (Cdk) families, which interact with, and are in turn activated by, the transcription factor E2F [1]. In the mitotic phase (M phase) of the cell cycle, the cell divides to produce two daughter cells, each inheriting a copy of the entire genome [2]. Dysfunctional cell cycle regulation, especially abnormal cell proliferation, leads to the development and progression of cancer. Aberrant mitosis, such as mitotic catastrophe, can lead to cell death, thus antimitotic drugs are being developed as novel targeted therapeutics [3, 4].
Antimitotic therapies that inhibit targets with specific functions in mitosis have now been identified and show promising antitumor activity in preclinical model systems [3]. These targets include polo kinases, Akt, Chk1, and kinesin spindle proteins (KSPs), and the pharmacodynamic activities of new compounds affecting these new targets have been demonstrated in cancer patients [3]. Cellular response to these new mitotic inhibitors is quite varied, depending on different cell lines and/or inhibitors, which includes apoptosis, mitotic catastrophe, mitotic slippage, senescence, and reversible mitotic arrest [3, 4]. At present, no biomarkers have been identified to define the particular responses of different tumor cells to mitotic injuries; however, it will be helpful to identify patients who will respond to the antimitotic therapy [5].
Anticancer drug discovery and development involves many processes, including target identification and validation, high-throughput screening, lead optimization, preclinical development/evaluation, and further clinical trials [6]. One of the key objectives of drug discovery is to evaluate whether a compound is able to hit the target, if it alters downstream molecular pathways in vitro (in cultured cells), and if it can also do so in living animals. Traditionally, in animal studies, target validation is performed by immunohistochemistry or molecular profiling after dissection of targeted organs/tissues [7]. Those studies are invasive, requiring termination of large numbers of animals [7]. A noninvasive imaging reporter approach not only provides a longitudinal and temporal pharmacodynamic readout in the same group of animals but also measures real-time dynamic changes in drug targets [8]. Thus, development of a reporter to noninvasively monitor mitotic arrest providing an optical readout for cell cycle distribution in living animals would be useful to identify/validate any agents for their potential in arresting the cell cycle in the M phase.
Cyclins are a family of proteins that bind to and activate Cdks. Cyclins are produced at specific times during the cell cycle, and their expression levels and locations are tightly controlled. Cell cycle-dependent kinase p34cdc2 (cdk1) activity is absent in G1 and increases through the S, G2, and M phases in a manner that correlates with its association to cyclin B1, the first human cyclin identified [1]. Cyclin B1 is synthesized during the late S and G2 phases and complexes with cdk1 [9]. As mitosis proceeds, cyclin B1 is specifically degraded so that, once the cells have reentered the G1 phase, very little cyclin B1 is present [9, 10]. The activity of cdk1 kinase has been shown to vary through the cell cycle even though the level of the protein itself does not change.
In the present study, we report a cyclin B–luciferase fusion protein used as an indicator of mitotic arrest and demonstrate that this indicator can serve as an optical reporter to visualize cell cycle changes in vivo. Through imaging cell proliferation as well as the change of cell cycle distribution, one can noninvasively monitor the tumorigenesis, development, and progression of cancer and therapeutic responses to anticancer drugs.
Materials and Methods
Antibodies and Chemicals
Rabbit polyclonal anti-luciferase was purchased from Sigma, and rabbit polyclonal anti-cdk1 (C-19) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit monoclonal anti-cyclin B1 [Y106] was purchased from Abcam Inc. (Cambridge, MA, USA) and rabbit polyclonal anti-phospho-histone H3 (Ser10) was purchased from Millipore Corp. (Billerica, MA, USA). Nocodazole and mimosine were purchased from Sigma-Aldrich (Atlanta, GA, USA). ALLN and proteasome inhibitor I were purchased from EMD Bioscience/Calbiochem (La Jolla, CA, USA). Small interfering RNAs (siRNAs) were synthesized by Invitrogen (Carlsbad, CA, USA) based on previously published sequences (sense strand), i.e., CDH1, UGAGAAGUCUCCCAGUCAG [11]; scramble as a control, UUCCGUCGCGGGCAGGUUG. Antimitotic drugs used in this study are Taxotere from Sanofi-Aventis (Bridgewater, NJ, USA) and Taxol from Sigma. The KSP inhibitor (KSPi) was synthesized as previously published [12].
Generation of Cyclin B1 Reporter, pGL3-cyclin B-Luc
The following primers (forward primer: 5′-GCGCAAGCTTGCCACCATGGCGCTCCGAGTCACCAGGAA, reverse primer: 5′-GCGCCCATGGTCACATATTCACTACAAAGGTTTGG) were used for polymerase chain reaction (PCR) amplification of cDNA encoding the N terminus of cyclin B1 (173 amino acids). The PCR product was digested with HindIII/NcoI and ligated into the pGL3 control vector (Promega) already cut with the same restriction enzymes. The resulting plasmid was named pGL3-NB-Luc. Then, the cyclin B promoter was amplified with PCR and used to replace the SV40 promoter in the pGL3-NB-Luc vector to make pGL3-cyclin B-Luc.
Tissue Culture and Transfection
Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; for HeLa cells) or McCoy’s 5A (for HCT116 cells) supplemented with 10 % fetal bovine serum (FBS). To make stable cell lines, HeLa or HCT116 cells were co-transfected with 5 μg of pGL3-cyclin B-Luc and 0.5 μg of empty pcDNA3 (Invitrogen). Twenty-four hours later, transfected cells were selected and maintained by growth in media containing G418 (1 mg/ml). Monoclonal cell lines were established with single-cell deposition into each well of the 96-well plate using the fluorescence-activated cell sorting (FACS) sorter. RNA transfection was carried out using Oligofectamine (Invitrogen) according to the manufacturer’s instructions. Forty-eight to 72 h after transfection, cells were analyzed by in vitro imaging, flow cytometry (FACS), or Western blot.
Cell Cycle Analysis
Subconfluent HeLa-cyclin B-Luc cells were blocked in late G1 or M phase by growth in media containing mimosine or nocodazole for 18 h and then lysed for luciferase assay or fixed with ice-cold 70 % ethanol for FACS analysis. Fixed cells were incubated in phosphate-buffered saline (PBS) containing 69 μM propidium iodide and 20 μg/ml RNAse A for 30 min at 37 °C. DNA content per nucleus was analyzed using a FACScan flow cytometer.
Luciferase Assay
Luciferase assay system (Promega) was used according to the manufacturer’s instructions. Cells were lysed by rocking in passive lysis buffer (Promega) for 15 min at room temperature. Ten microliters of cell extract was assayed using a Lumat LB9507 luminometer (Berthold Technologies). Luciferase values for stable cell lines were normalized to total protein concentration.
Hollow Fiber Assay and Tumor Xenograft
Cells were grown in hollow fibers, essentially as described previously. Briefly, a semipermeable hollow fiber was filled with cells (5 × 106 cells/ml), heat sealed at 1.5 cm intervals, and cut into pieces that were sealed at both ends. For in vitro studies, hollow fibers were placed in six-well culture dishes containing DMEM with 10 % FBS before adding anticancer drugs. For in vivo studies, Crl:Nu/Nu mice (Charles River, Wilmington, MA, USA) were anesthetized (ketamine 140 mg/kg and xylazine 12 mg/kg given by intraperitoneal (i.p.) injection), and hollow fibers were implanted subcutaneously using an 11-gauge trocar inserted through a neck incision. For tumor xenograft studies, approximately 1 × 106 cells in 100 μl PBS were injected subcutaneously per site into the flanks of anesthetized Nu/Nu mice. All the animal experiments described in this paper were approved by the Merck Institutional Animal Care and Use Committee.
Bioluminescence Imaging
For in vitro studies, d-luciferin was added to the media bathing the reporter cell lines (final concentration, 50 μg/ml). Five minutes later, photons were counted using the IVIS™ Spectrum Imaging System (Xenogen/Caliper) according to the manufacturer’s instructions. Data were analyzed using the Living Image software (version 3.0, Xenogen). For in vivo studies, mice were administered d-luciferin (90 mg/kg) by i.p. injection [13]. Ten minutes later, photons were counted and analyzed as previously described.
Immunohistochemistry
Tissues were fixed in 10 % neutral buffered formalin, processed to paraffin blocks, and sectioned at 4 μm. Antigen retrieval was performed using Dako Target Retrieval Solution (pH 6.0; Dako Corp., Carpinteria, CA, USA) in a pressure cooker (Biocare Medical, Concord, CA, USA) for 30 min (cyclin B1) or in a microwave oven for 10 min (phospho-histone H3). Endogenous peroxidase activity was blocked by incubating sections with 3 % hydrogen peroxide. To prevent nonspecific binding, sections were blocked with 5 % goat serum containing 1 % bovine serum albumin, 0.1 % cold fish skin gelatin, 0.1 % Triton X-100, 0.05 % Tween 20, and 0.05 % sodium azide. Sections were incubated overnight at 4 °C with rabbit monoclonal antibody (MoAb) anti-cyclin B1 [Y106] (1:100) or rabbit polyclonal anti-phospho-histone H3 (1:3,000). Bound antibodies were detected by sequential incubation with antirabbit Envision+™ horseradish peroxidase-conjugated polymer (Dako) followed by 3,3′-diaminobenzidine tetrahydrochloride. Sections were counterstained with hematoxylin. For quantitation of the positive signal, image analysis was performed on each entire section using the Ariol SL-50 software (Genetix Corp., San Jose, CA, USA).
Statistical Analysis
For imaging data analysis, the intensity of the reporter protein posttreatment was compared to the intensity of the protein pretreatment by calculating a ratio. Statistical significance was assessed using the Student’s t test, under the assumption of a normal distribution of the normalized ratios with an estimate of variance; the Wilcoxon signed rank test was also performed to provide an additional distribution-free assessment. All statistical tests were two-tailed.
Results
Cyclin B-Luc Protein is Degraded in a Cell Cycle-Dependent Manner
Cyclin B1, a tightly regulated cyclin that is expressed in the late S phase and is subsequently degraded during mitotic exit, was used as the basis for the generation of a cell cycle bioluminescence reporter. A cDNA encoding a fusion protein of the N terminus of cyclin B1 linked to firefly luciferase was generated and placed under the control of the cyclin B1 promoter. We hoped to harness the important elements that are responsible for the cell cycle-dependent mRNA expression, localization, and turnover of cyclin B1 (see the scheme shown in Fig. 1a). In particular, the fusion protein is expected to behave similarly to cyclin B1 with respect to ubiquitination-dependent degradation.
Fig. 1.
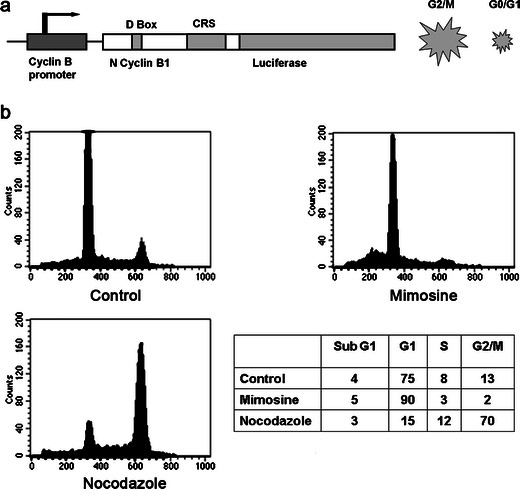
The mechanism of the fusion protein of cyclin B-Luc, regulated by the cyclin B promoter. a A schematic drawing of the mitotic reporter. A fusion protein of the N terminus of cyclin B fused to luciferase is driven by a cyclin B promoter. The N-terminal domain of cyclin B1 contains a conserved nine-amino-acid motif (RTALGDIGN) called the D-box that is necessary for cyclin B1 ubiquitination and subsequent degradation. The CRS region is responsible for nuclear/cytoplasmic shuttling of cyclin B. In the G2/M phase of the cell cycle, the reporter is stabilized and is degraded during the G0/G1 phase. b Polyclonal HeLa cells stably transfected with pGL3-cyclin B-Luc (HeLa-cyclin B-Luc) were synchronized by growth in media containing 0.2 mM mimosine or 500 nM nocodazole for 18 h. Mimosine arrested 90 % of cells in the late G1 phase, while 500 nM nocodazole arrested 70 % of cells in G2/M phase.
To investigate whether cyclin B-Luc is regulated in a cell cycle-dependent manner, HeLa cells stably expressing cyclin B-Luc were blocked with the cell cycle-synchronizing reagents, mimosine (arresting cell cycle in the late G1 phase) or nocodazole (an M phase blocker). At 18 h after synchronization, 0.2 mM mimosine caused 90 % of HeLa-cyclin B-Luc cells in the late G1 phase, while 500 nM nocodazole blocked 70 % of cell cycle in the M phase (Fig. 1b). As expected, a luciferase assay demonstrated that mimosine downregulated the cyclin B-Luc reporter activity, while nocodazole upregulated its activity in a dose-dependent manner (Fig. 2a), consistent with cell cycle distribution. In addition to the reporter assay, Western blots were also performed to detect cyclin B-Luc protein levels (Fig. 2b). The results showed low cyclin B-Luc expression in mimosine-treated cells and high cyclin B-Luc expression with dose dependency in nocodazole-treated cells. That nocodazole-induced dose-dependent upregulation of cyclin B-Luc protein is similar to the induction of endogenous cyclin B1.
Fig. 2.
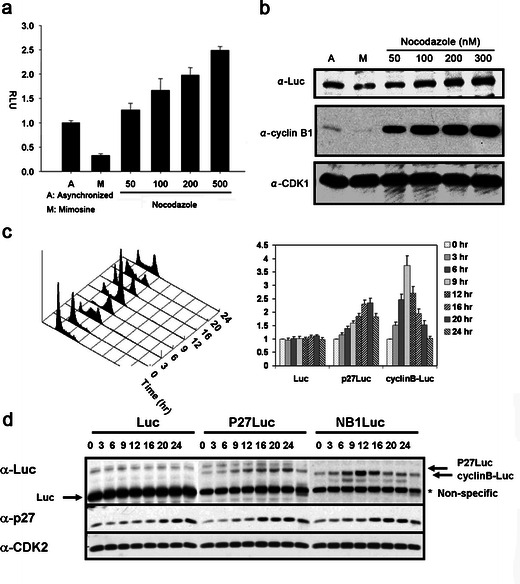
Induction of cyclin B-Luc by nocodazole, an M phase blocking reagent. a Luciferase assay was performed using HeLa-cyclin B-Luc cells treated with mimosine (0.2 mM) or nocodazole (at indicated concentrations). Normalized relative luciferase units (RLU) are shown. b Whole cell extracts from c were applied to Western blots for the assessment of luciferase, cyclin B1, and cdk1. c HeLa cells stably transfected to express luciferase or p27Luc or cyclin B-Luc were blocked in the late G1 phase by the treatment with mimosine. Cell cycle distribution was analyzed at various time points after release from mimosine treatment (left panel), and luciferase activity was assessed. d Luciferase or luciferase fusion protein expression was detected by Western blot after release from mimosine-synchronized cells.
To further investigate the cell cycle dependency of cyclin B-Luc protein turnover, mimosine was washed out to release the arrested HeLa-cyclin B-Luc from mimosine blocking. The cell lysates from various time points after mimosine removal were blotted with luciferase antibody. Cyclin B-Luc protein accumulated at 9 h after release from mimosine, which is in the M phase (Fig. 2c, d). In contrast, wild-type luciferase showed no change during the cell cycle shift, and a p27 luciferase fusion protein (p27-Luc), which we showed earlier accumulates in G1 [14], was increased at 20–24 h when the cells completed the cell cycle and reentered G1. Thus, the cyclin B-Luc reporter protein expression mimics the expression of endogenous cyclin B1, consistent with its transcriptional accumulation in the M phase and protein degradation in the G1 phase.
Cyclin B-Luc is Degraded Through Ubiquitination and Accumulates upon Mitotic Arrest
Upon mitotic exit, cyclin B1 degradation is mediated by the anaphase-promoting complex (APC), an E3 ligase complex. Cdh1-dependent APC activity targets mitotic cyclins from the end of mitosis to the G1 phase for ubiquitination-dependent degradation. We further addressed whether cyclin B-Luc is degraded via proteasome degradation. The protease inhibitor ALLN and proteasome inhibitor I, a universal proteasome inhibitor, caused a dose-dependent increase of cyclin B-Luc luciferase activity (Fig. 3a). To investigate whether cyclin B-Luc turnover is mediated by the APC complex, double-stranded siRNA specific to cdh1 was used to transiently transfect HCT116-cyclin B-Luc cells. Specific knock down of Cdh1 protein resulted in the accumulation of this reporter protein (Fig. 3b, c). In contrast, a control siRNA did not cause cyclin B-Luc accumulation. All together, these data demonstrate that cyclin B-Luc degradation is mediated by ubiquitination via the APC complex.
Fig. 3.
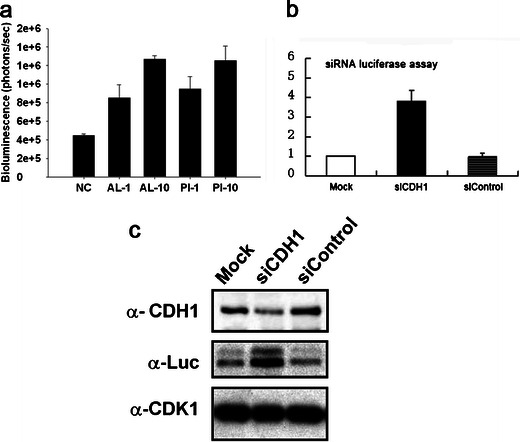
Stabilization of cyclin B-Luc by CDH1 siRNA. a HCT116 human colon carcinoma cells stably transfected with pGL3-cyclin B-Luc vector (HCT116-cyclin B-Luc) cells were treated with the proteasome inhibitors ALLN (AL) or proteasome inhibitor I (PI) at a concentration of 1 or 10 μM, as indicated for 6 h, and the luciferase activity was measured. b HCT116-cyclin B-Luc cells were transfected with either scrambled (siControl) or CDH1 siRNA. Luciferase assays were performed 48 h later. c Whole cell extracts from b were applied to Western blots for the assessment of CDH1, luciferase, and cdk1.
Bioluminescence Imaging of Mitotic Arrest In Vitro
To evaluate the effect of mitotic arrest compounds on this reporter, HeLa-cyclin B-Luc cells were treated with Taxotere or Taxol. Both Taxotere and Taxol interfere with microtubule breakdown, preventing depolymerization of tubulin, and compounds ultimately cause cell cycle arrest in the M phase. In vitro imaging showed that Taxotere and Taxol increased reporter activity in a dose-dependent manner (Fig. 4a, b). Next, hollow fibers were filled with HeLa-cyclin B-Luc and HCT116-cyclin B-Luc cells, and multilayer, rather than monolayer, cultivation was obtained. These hollow fibers containing reporter cells were then treated with Taxotere. At 48 h after treatment, 40 % of the population of Taxotere-treated cells was found to be in the M phase when retrieved and analyzed based on nuclear DNA content (Fig. 4c). Consistent with the cell cycle assay, in vitro imaging demonstrated luciferase induction in Taxotere-treated cells (Fig. 5a).
Fig. 4.
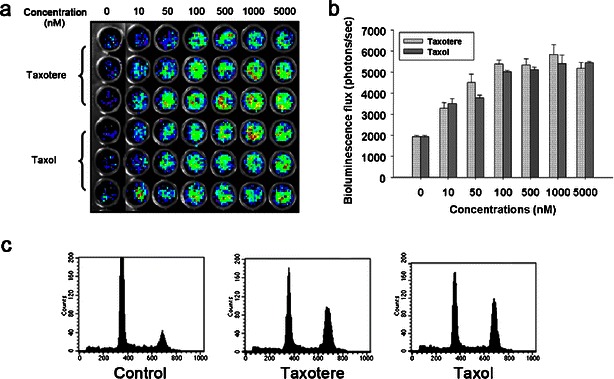
Cyclin B-Luc can be induced by antimitotic anticancer drugs in a dose-dependent manner. a HeLa-cyclin B-Luc cells were incubated with two antimitotic anticancer drugs, Taxotere and Taxol, in the indicated concentrations for 24 h and in vitro imaging was acquired. b Bioluminescence signals were quantified, showing that both Taxotere and Taxol induced the cyclin B-Luc reporter in a dose-dependent manner. c HCT116-cyclin B-Luc were filled into hollow fibers and treated with either Taxotere or Taxol at 100 nM for 24 h. Uncontaminated pure HCT116-cyclin B-Luc cells were retrieved from these hollow fibers after either vehicle or taxane treatment, and cell cycle distribution was determined by flow cytometry.
Fig. 5.
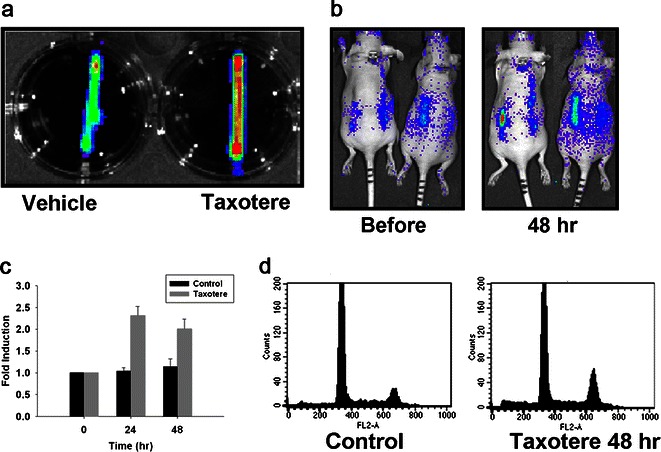
Induction of cyclin B-Luc by Taxotere in hollow fibers. a Bioluminescence images of hollow fibers containing HCT116-cyclin B-Luc cells after vehicle or Taxotere treatment. b–d Nude mice bearing hollow fibers filled with HCT116-cyclin B-Luc cells were either vehicle-treated or treated with Taxotere (i.p., 20 mg/kg). Bioluminescence images (b) were acquired before and at 48 h after treatment, and fold induction is expressed as a ratio of posttreatment versus pretreatment bioluminescence (c) data from five mice with ten fibers; error bars standard error. Cell cycle distribution was determined by flow cytometry with cells retrieved from hollow fibers at 48 h after Taxotere treatment (d).
Bioluminescence Imaging of Mitotic Arrest In Vivo
Next, we attempted to determine if this reporter can monitor mitotic arrest in vivo. First, as a rapid and accurate in vivo approach, the hollow fiber model was used to evaluate the reporter. Mice implanted with hollow fibers filled with HCT116-cyclin B-Luc reporter cells were treated with Taxotere by i.p. injection. As shown in Fig. 5b, c, at 24–48 h after Taxotere administration, bioluminescence emitted from hollow fibers increased more than twofold. To confirm that the reporter induction is indeed caused by cell cycle change, cells were retrieved from these hollow fibers and were assessed with the cell cycle (FACS) assay. At 48 h after Taxotere treatment, 30 % of cells were arrested in the M phase (Fig. 5d), suggesting that the reporter induction is caused by mitotic arrest.
Next, xenograft tumor models were established by subcutaneous injection of HCT116-cyclin B-Luc cells. As shown in Fig. 6a, more than twofold induction in bioluminescence was detected at 24 h after Taxotere administration with in vivo imaging, with maximum induction (about threefold) at 48 h (Fig. 6b). After in vivo bioluminescence imaging, the tumors were removed for immunohistochemical staining to detect endogenous cyclin B1. The rabbit MoAb used in this study reacts with an epitope in the C terminus of cyclin B1 and, thus, is able to specifically detect endogenous cyclin B1. Tumor samples from Taxotere-treated mice confirmed robust induction of cyclin B1 compared to vehicle-treated mice (Fig. 6c, d).
Fig. 6.
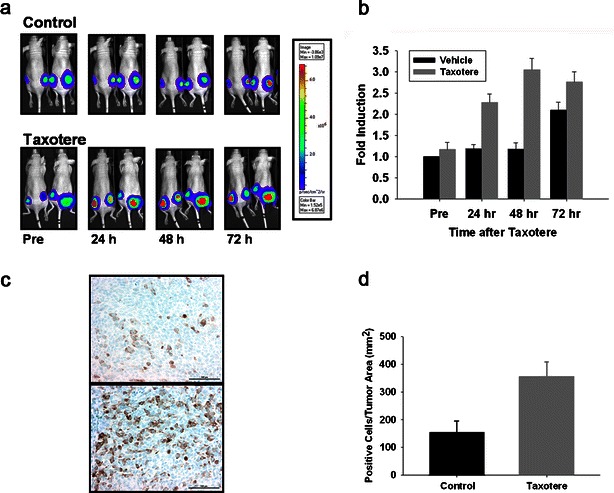
Induction of cyclin B-Luc by Taxotere in subcutaneous xenografts in vivo. a, b Nude mice bearing HCT116-cyclin B-Luc subcutaneous tumors were either vehicle-treated or treated with Taxotere (i.p., 20 mg/kg). Bioluminescence images were acquired at the time points indicated (a), and fold induction is expressed as a ratio of posttreatment versus pretreatment bioluminescence (b) data from five mice with ten tumors; error bars standard error. c, d Tumor samples at 48 h after vehicle treatment (upper panel of c) or Taxotere treatment (lower panel of c) were immunohistochemically stained with anti-cyclin B1 antibody. Cyclin B1-positive cells per tumor area were counted with the Ariol SL-50 software (d) (error bars standard error; n = 4).
Induction of Cyclin B-Luc Reporter by KSP Inhibitor
KSP is a kinesin motor protein required to establish mitotic spindle bipolarity, and KSPi have been reported to cause mitotic arrest with a monopolar spindle. A KSPi, identified with a high-throughput screen, has been shown to activate spindle assembly checkpoint and mitotic slippage and, ultimately, to induce apoptosis [15]. To determine whether this reporter can predict mitotic arrest caused by a KSPi, the HCT116-cyclin B-Luc tumor xenograft model was used to evaluate this KSPi. At 18 h, mice treated with KSPi showed approximately fourfold brighter bioluminescence in comparison to vehicle-treated mice (Fig. 7a, b). After the removal of HCT116-cyclin B-Luc tumors, immunohistochemical staining was performed to detect phosphorylation of histone H3 specific to mitosis. As shown in Fig. 7c, d, significant levels of phospho-histone H3 was detected in tumors treated with KSPi. In contrast, in mice treated with 5-FU, an antitumor drug to block DNA replication in the S phase, no bioluminescence induction was observed. All together, these data demonstrate that this cyclin B-Luc reporter can specifically indicate the ability of a compound to arrest the cell cycle in the M phase.
Fig. 7.
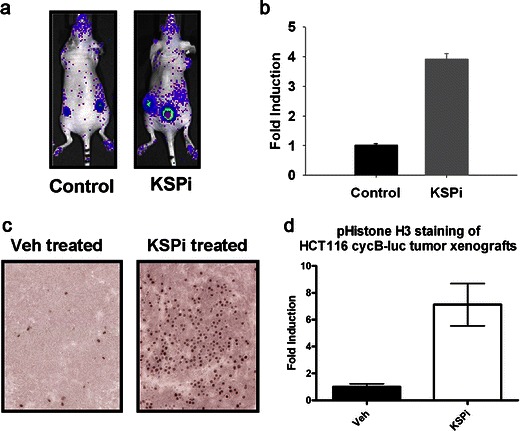
Induction of cyclin B-Luc in subcutaneous tumors treated with KSPi correlates with phospho-histone H3. Nude mice implanted with subcutaneous HCT116-cyclin B-Luc tumor xenografts were either vehicle-treated or treated with KSPi. a Bioluminescence images were acquired at 18 h after KSPi treatment. b Relative fold induction was calculated using normalized bioluminescence in the control group (data represent five mice implanted with ten tumors; error bars standard error). c At 18 h after KSPi treatment, subcutaneous tumors were removed and paraffin-embedded sections were stained with anti-phospho-histone H3 antibody. d Quantitative analysis for phospho-histone H3. Fold induction is expressed by normalization of KSPi-treated tumors versus control tumors.
Discussion
Cell cycle distribution is typically detected by flow cytometry to determine nuclear DNA contents or is visualized under microscope to look at mitotic characteristics, such as chromatin condensation and spindle separation. Visualization of the cell cycle in living subjects has long been a big challenge. Green fluorescent protein (GFP) fusion protein has been used to generate a reporter to monitor cell cycle change previously. For example, Hadjantonakis et al. fused histone 2B into GFP to localize chromatin and track embryonic cells in transgenic mice [16]. Although this approach has been proved to be useful in embryos and isolated organs with fluorescence imaging, it is hard to conduct whole-body imaging, in particular, for quantitative purpose.
The bioengineered luciferase reporter has been widely used for in vivo imaging of various proteins, such as p27-luciferase, p53-luciferase, and ubiquitin–luciferase [14, 17, 18]. In our attempt to generate a mitotic reporter, the N-terminal region of cyclin B1 was used to make a reporter construct fused to luciferase. During the cell cycle progression, a large protein complex called the “anaphase-promoting complex (APC)” initiates chromosome segregation and exit from mitosis by degrading anaphase inhibitors and mitotic cyclin B [19]. The N terminus of cyclin B1 contains a conserved nine-amino-acid motif (RTALGDIGN) called the destruction box (D-box) that is necessary for cyclin B1 ubiquitination and subsequent degradation [20]. Thus, we hypothesized that if the cyclin B D-box is grafted onto otherwise stable proteins, those proteins become unstable in mitosis (i.e., the D-box is portable).
During the S and G2 phases, cyclin B1 shuttles between the nucleus and the cytoplasm because constitutive nuclear import is counteracted by rapid nuclear export during these phases. At the M phase, cyclin B1 is phosphorylated in the cytoplasmic retention signal (CRS) sequence [21]. The nuclear export sequence (a region of the CRS) is then inactivated and the protein moves rapidly to the nucleus. Phosphorylation of the CRS is required for nuclear trafficking of cyclin B1 [21]. Lukas et al. has reported a fusion reporter of N terminus 119 amino acids of cyclin B1 linked to luciferase to measure Cdh1 ligase activity [22]. In the present study, the N terminus of cyclin B1 includes the CRS, different from that reported by Lukas et al. [22], so the fusion protein is able to be transported into the cytoplasm for ubiquitination-dependent degradation. Furthermore, the cyclin B-Luc protein is controlled by the cyclin B promoter which assures that this protein is transcriptionally regulated similar to endogenous cyclin B1. In addition to the previously mentioned features, the cdk1 binding site located at the C terminus of cyclin B1 was excluded from our fusion protein so that the reporter molecule would not bind or activate cdk1 and, therefore, would not affect the cell cycle (data not shown).
The reporter, as expected, is tightly regulated in a cell cycle-dependent manner, evidenced by downregulation in the late G1 phase, and upregulation in the G2/M phase after synchronization of tumor cells expressing cyclin B-Luc. The trend of the protein change is extremely similar to that of endogenous cyclin B1 [20]. Furthermore, cyclin B-Luc accumulates after the blockade of the proteasomal degradation pathway by proteasome inhibitors, suggesting that the turnover of this reporter is ubiquitination-dependent. Moreover, siRNA targeting the APC complex significantly upregulated cyclin B-Luc, while control siRNA did not, suggesting that cyclin B-Luc is degraded via APC E3 ligase. By testing a few inhibitors of mitosis, cyclin B-Luc was shown to report the cell cycle arrest in the M phase in vitro. All together, these results indicate that the cyclin B-Luc reporter mimics the protein change of endogenous cyclin B1, showing accumulation upon mitotic arrest.
Lack of a noninvasive mitotic reporter has necessitated the use of large numbers of small animals when determining the fraction of tumor cells in the M phase after treatment with anticancer agents [23]. The purpose of this study was to develop an optical reporter able to detect the in vivo pharmacodynamics of novel anticancer drugs that are able to block mitosis. To take advantage of bioluminescence imaging with its low background and high signal-to-noise ratio, cell lines stably expressing cyclin B-Luc were used to generate tumor xenograft or hollow fiber models in vivo. Administration of antimitotic anticancer drugs increased the bioluminescence emitted from the treated tumors, consistent with the M phase arrest caused by the same compounds. Thus, the development of the mitotic reporter enables noninvasive monitoring of mitotic arrest in the whole body. Sakaue-Sawano et al. used Cdt1-RFP (G1 phase) or Gemenin-GFP (S/G2/M phase) fusion protein to determine the spatiotemporal dynamics of the cell cycle change and showed the cell cycle distribution during the development of neural tissue in transgenic mice [24]. However, the fusion reporter applied in their study was not affected by transcriptional regulation and exhibited a limitation in showing quantitative analysis. Especially, Gemenin-GFP can be accumulated in either S, G2, or M phase, showing degradation only in the G1 phase. In contrast, cyclin B-Luc is specifically accumulated to mitotic arrest and upregulated by blocking mitosis.
One use of a stable cell line expressing the cyclin B-Luc construct is to screen for antimitotic or antiproliferative compounds that would be expected to arrest a proportion of cells in a certain phase of the cell cycle. A KSPi, known to arrest the cell cycle in the M phase, was used to validate this reporter [12, 15]. KSPi caused arrest in the M phase, evidenced by the increase in phospho-histone H3, a biomarker of mitotic arrest [15], and correlated with the induction of bioluminescence. In further support of new mitotic targets, it will be helpful to visualize the therapeutic window, in order to verify target inhibition in the tumor and optimize dose and schedule and to determine the potential role for these agents in the treatment for cancers. Thus, this imaging reporter would be useful in target validation, dose optimization, and clinical development. However, the cyclin B-Luc reporter has its limitation of translation to clinical studies and may be mainly used in animal experiments.
In conclusion, this reporter is an indicator of cyclin B turnover. It can be used to monitor the mitotic arrest with noninvasive bioluminescence imaging and, therefore, has a number of potential applications for drug discovery and research in cell signaling. For instance, this cyclin B-Luc reporter can be used to screen for antiproliferative compounds that may arrest cell cycle in the M phase and then trigger apoptosis, as well as in secondary screening and lead optimization studies to detect undesirable toxic side effects of lead compounds. Finally, this novel and unique cyclin B-Luc reporter system stably expressed in tumor cells could be used in in vitro and in vivo assays to investigate basic processes of cell cycle and their dependence and modulation by intracellular pathways and receptor signaling.
Acknowledgments
This research was partly supported by grants from the Major State Basic Research Development Program of China (no. 2011CB707705), the National Natural Science Foundation of China (no. 30973377), and Special Fund for Recruited Talents of Guangdong Province. We would thank Dr. Stanley Lin for his critical and careful reading of the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Loog M, Morgan DO. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434:104–108. doi: 10.1038/nature03329. [DOI] [PubMed] [Google Scholar]
- 2.Lukas J, Bartek J. Cell division: the heart of the cycle. Nature. 2004;432:564–567. doi: 10.1038/432564a. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt M, Bastians H. Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug Resist Updat. 2007;10:162–181. doi: 10.1016/j.drup.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? J Cell Sci. 2009;122:2579–2585. doi: 10.1242/jcs.039719. [DOI] [PubMed] [Google Scholar]
- 6.Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov. 2003;2:123–131. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- 7.Monga M, Sausville EA. Developmental therapeutics program at the NCI: molecular target and drug discovery process. Leukemia. 2002;16:520–526. doi: 10.1038/sj.leu.2402464. [DOI] [PubMed] [Google Scholar]
- 8.Bednar B, Zhang GJ, Williams DL, Hargreaves R, Sur C. Optical molecular imaging in drug discovery and clinical development. Expert Opin Drug Discov. 2007;2:65–85. doi: 10.1517/17460441.2.1.65. [DOI] [PubMed] [Google Scholar]
- 9.Glotzer M. Cell cycle. The only way out of mitosis. Curr Biol. 1995;5:970–972. doi: 10.1016/S0960-9822(95)00190-4. [DOI] [PubMed] [Google Scholar]
- 10.Murray A. Cyclin ubiquitination: the destructive end of mitosis. Cell. 1995;81:149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- 11.Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 12.Garbaccio RM, Tasber ES, Neilson LA, Coleman PJ, Fraley ME, et al. Kinesin spindle protein (KSP) inhibitors. Part 7: design and synthesis of 3,3-disubstituted dihydropyrazolobenzoxazines as potent inhibitors of the mitotic kinesin KSP. Bioorg Med Chem Lett. 2007;17:5671–5676. doi: 10.1016/j.bmcl.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 13.Zhang GJ, Chen TB, Bednar B, Connolly BM, Hargreaves R, et al. Optical imaging of tumor cells in hollow fibers: evaluation of the antitumor activities of anticancer drugs and target validation. Neoplasia. 2007;9:652–661. doi: 10.1593/neo.07421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang GJ, Safran M, Wei W, Sorensen E, Lassota P, et al. Bioluminescent imaging of Cdk2 inhibition in vivo. Nat Med. 2004;10:643–648. doi: 10.1038/nm1047. [DOI] [PubMed] [Google Scholar]
- 15.Tao W, South VJ, Zhang Y, Davide JP, Farrell L, et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49–59. doi: 10.1016/j.ccr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Hadjantonakis AK, Papaioannou VE. Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol. 2004;4:33. doi: 10.1186/1472-6750-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, El-Deiry WS. Bioluminescent molecular imaging of endogenous and exogenous p53-mediated transcription in vitro and in vivo using an HCT116 human colon carcinoma xenograft model. Cancer Biol Ther. 2003;2:196–202. doi: 10.4161/cbt.2.2.347. [DOI] [PubMed] [Google Scholar]
- 18.Luker GD, Pica CM, Song J, Luker KE, Piwnica-Worms D. Imaging 26S proteasome activity and inhibition in living mice. Nat Med. 2003;9:969–973. doi: 10.1038/nm894. [DOI] [PubMed] [Google Scholar]
- 19.King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, et al. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 20.Yamano H, Tsurumi C, Gannon J, Hunt T. The role of the destruction box and its neighbouring lysine residues in cyclin B for anaphase ubiquitin-dependent proteolysis in fission yeast: defining the D-box receptor. EMBO J. 1998;17:5670–5678. doi: 10.1093/emboj/17.19.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagting A, Jackman M, Simpson K, Pines J. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr Biol. 1999;9:680–689. doi: 10.1016/S0960-9822(99)80308-X. [DOI] [PubMed] [Google Scholar]
- 22.Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, et al. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- 23.Agnese V, Bazan V, Fiorentino FP, Fanale D, Badalamenti G, et al. The role of Aurora-A inhibitors in cancer therapy. Ann Oncol. 2007;18(Suppl 6):vi47–vi52. doi: 10.1093/annonc/mdm224. [DOI] [PubMed] [Google Scholar]
- 24.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]


