Abstract
This article provides a broad overview of the interaction between neuropeptides and inflammatory mediators as it pertains to diabetic wound healing. Abnormal wound healing is a major complication of both type I and type II diabetes and is the most frequent cause of non-traumatic lower limb amputation. Wound healing requires the orchestrated integration of complex biological and molecular events. Inflammation, proliferation and migration of cells followed by angiogenesis and re-epithelization are essential phases of wound healing. The link between wound healing and the nervous system is clinically apparent as peripheral neuropathy is reported in 30–50% of diabetic patients and is the most common and sensitive predictor of foot ulceration. The bidirectional connection between the nervous and the immune systems and the role it plays in wound healing has emerged as one of the focal features of the wound healing dogma. The mediators of this connection include neuropeptides and the cytokines released from different cells including immune and cutaneous cells. Therefore, to develop successful wound healing therapies, it is vital to understand in depth the signaling pathways in the neuro-immune axis and their implication in diabetic wound healing.
Keywords: Diabetes, Inflammation, Neuropeptides, Wound-Healing, Foot Ulcers, Neuropathy, Cytokines
Introduction
According to the American Diabetes Association, 8% of the general population and 25% of people over the age of 65 are afflicted with diabetes in the United States (1). One of the most outward and debilitating complications of diabetes is the development of chronic non-healing foot ulcerations, occurring in 15% of diabetics. In its most unfavorable course, diabetic foot ulceration leads to 82,000 amputations per year and is the leading cause of non-traumatic lower extremity amputation in the U.S. (1, 2). The national economic burden of diabetic foot ulceration and amputation is correspondingly staggering, estimated near 11 billion dollars in the year 2001 (3).
The pathophysiologic trail from hyperglycemia to foot ulceration traverses a complex interplay between multiple dysfunctioning systems. Traditionally, ischemia, neuropathy, trauma, and infection were considered the culprits of the recurring chronic wound and treatment revolved largely around wound management and revascularization (4, 5). More recently, impairment of the cutaneous wound healing process itself has been recognized as a major contributor to the failure to heal, and wound healing has been appreciated as yet another biological system hindered by the metabolic, vascular, neurologic, and inflammatory alterations present in diabetes (6). In light of this recent understanding, numerous investigations are pursuing therapies for diabetic foot ulceration which act to restore the molecular and cellular processes required for successful wound healing.
Wound healing requires the well-orchestrated integration of the complex biological and molecular events of cell migration, cell proliferation, and extracellular matrix (ECM) deposition (6). Normal wound healing can be divided into four overlapping phases: hemostasis, inflammation, proliferation and remodeling. The cell types involved in each phase are responsible for mediating specific events that lead to wound closure. Hemostasis lasts for 2–3 hours, during which a fibrin plug is formed and aggregated platelets release pro-inflammatory mediators such as cytokines and growth factors. Cytokines recruit neutrophils and monocytes to the wound area triggering the inflammatory phase of wound healing, which lasts from hours to days. The proliferation phase involves several different cell types such as epithelial cells, endothelial cells (ECs) and fibroblasts. Migration of these cells to the wound site is again the result of cytokine stimulation. ECM deposition coupled with angiogenesis and re-epithelization lead to wound contraction and closure. Remodeling, the last step in wound healing, can last several weeks, and is marked by ECM remodeling and formation of scar tissue. Whereas acute wounds progress linearly through the phases of wound healing, chronic non-healing wounds become stalled in different phases at the same time and do not follow an orderly and reliable progression of healing (6).
The first observations regarding the impact of diabetes on wound healing focused on an impaired leukocyte function related to hyperglycemia (7, 8). However, additional factors common to other chronic wounds, such as decubitus ulcers and venous ulcers, were later found to participate in impaired diabetic wound healing (9). These abnormalities included the development of pericapillary fibrin cuffs (10), impaired expression of ECM (11), aberrant cellular infiltration (12), insufficient macrophage activation (13), impaired re-epithelialization and impaired angiogenesis (14, 15).
Traditionally, research in the field of diabetic wound healing focused on the role of growth factors and their therapeutic potential. However, to date only one growth factor, platelet derived growth factor (PDGF), has been approved by the FDA for treating diabetic wounds. Mediators of inflammation participate in all four phases of wound healing and represent an alternative therapeutic option for promoting proper healing. Numerous investigations have focused on the systemic implications of inflammation in diabetes yet few have examined the local role of inflammation in the wound microenvironment. The mechanism by which local inflammatory pathways are affected in diabetes is poorly understood, but an emerging hypothesis suggests the involvement of the neuroendocrine system in the inflammatory impairment present in diabetic wounds (16). Recently the role of nerves, their interaction with immune and cutaneous cells, and their contribution to wound healing has become evident, and neuropathy, an early and ubiquitous complication of diabetes, has been shown to impair wound healing by influencing inflammatory pathways. This review is aimed at summarizing the role of neuroinflammatory mediators, neuropeptides and cytokines, in diabetes and wound healing.
Inflammation and Wound Healing
Tissue injury triggers an acute inflammatory response, beginning with the arrival of neutrophils,monocytes/macrophages, and mast cells to the site of injury (17–19). These cells produce inflammatory cytokines and growth factors that coordinate wound repair (20, 21). Cytokines are a heterogeneous group of pleiotropic signaling proteins and glycoproteins that can generally be classified as pro-inflammatory or anti-inflammatory and allow an organism to respond rapidly to an immune challenge (22). Proper wound healing requires a sequential, self-limited, cytokine/immune cell interaction in order to achieve an adequate immune response necessary for bacterial clearance, organized tissue breakdown, and subsequent regeneration. An adult human study of wound healing showed that the cytokines interleukin (IL)-8, growth-related oncogene-α (GRO-α), macrophage chemoattractant protein (MCP)-1, interferon γ inducible protein (IP)-10, and mitogen inducible gene (MIG) are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets (17). Tumor necrosis factor (TNF)-α and IL-1β are early regulators of the immune response and both induce the release of secondary cytokines, such as IL-6 and IL-8 (23). Released cytokines, predominantly produced by monocytes and macrophages, activate and selectively guide various leukocyte subsets to specific microanatomical sites of skin wounds during the phases of tissue repair. Keratinocytes and dermal endothelial cells (ECs) are additional cell types that respond to and release molecular mediators that orchestrate wound healing. These cytokines mediate a variety of overlapping effects, and their actions can be additive. More recently, inflammatory mediators have been found to regulate angiogenesis, another critical process for wound repair (24).
Inflammation and Diabetes
The inflammatory alteration in diabetes is typically characterized by the chronic upregulation of pro-inflammatory cytokines. In type II diabetes, insulin resistance is associated with a state of chronic low-grade inflammation, and several mediators released from various cells, including immune cells and adipocytes, have been implicated in the development of insulin resistance. Among these are several pro-inflammatory cytokines such as IL-1, TNF-α, IL-6, RANTES and various others (25, 26). Similarly, another study found inflammatory markers correlate significantly with insulin resistance indices, HbA1c, lipid profile, hypertension, positive family history of type 2 diabetes, low physical fitness, and mixed high-fat and high-carbohydrate diet (27). This occurs not only in type II but also in type I diabetes. Cytokines such as IL-1α, IL-4, and IL-6 are acutely elevated during hyperglycemia in children with type 1 diabetes, and these elevations persist for hours after hyperglycemia has been corrected (28). In a similar study of monocyte function in type 1 diabetes increased levels of IL-6, IL-α and superoxide anion were identified, again suggesting that inflammatory complications are present in both type I and II diabetes (29). Finally, a study of lean young individuals found that even high-normal physiologic increases in blood glucose after meals can aggravate inflammatory processes by activation of the pro-inflammatory transcription factor nuclear factor kappa b (NF-κb) (30). This study reveals how inflammatory pathology is not simply a consequence of long-standing diabetes but can be triggered acutely by the hyperglycemic environment.
Inflammation and Diabetic Wound Healing
It has been questioned whether chronic systemic inflammation present in diabetes translates into chronic peripheral inflammation. In patients with metabolic syndrome, oral glucose tolerance testing was associated with increased levels of intercellular adhesion molecule 1 (ICAM-1), TNF-α, and IL-6 in peripheral blood leukocytes compared to patients without metabolic syndrome two hours after the test was performed. Similar results were obtained in vitro after seventy two hours of cultivation in high-glucose medium. Hence, it is thought metabolic syndrome may support peripheral inflammation by causing leukocytes to release pro-inflammatory mediators in response to glucose (31). In diabetes there is a pronounced imbalance of pro-/anti-inflammatory cytokines which are released in a non-sequential manner leading to impaired tissue repair and weakened cellular and humoral immune defense mechanisms (32, 33). These immune cells show abnormal activity and cytokine release profiles (34). Neutrophils that act as first-line-of-defense cells show reductions in functional activity contributing to the high susceptibility and severity of infections in diabetes (35). Clinical investigations in diabetic patients and experimental studies in diabetic rats and mice consistently demonstrate defects of neutrophil chemotactic, phagocytic, and microbicidal activities (36–38). Metabolic routes by which hyperglycemia is linked to neutrophil dysfunction include the advanced protein glycosylation reaction, the polyol pathway, oxygen-free radical formation, the nitric oxide-cyclic guanosine-3'-5'monophosphate pathway, and the glycolytic and glutaminolytic pathways. Lowering of blood glucose levels by insulin in diabetic patients or experimental animals has been reported to have significant correlation with improvement of neutrophil functional activity (39). A prospective clinical study that examined the phagocytic activity index and intracellular killing activity of neutrophils as well as IL-1β levels in a group of patients with healing and non-healing diabetic foot infections found a dysfunction of neutrophils and IL-1β regulation in patients with diabetic foot infections, possibly indicating ineffective immunological responses in diabetic patients (40). It has been shown in diabetes that immune cell infiltration is decreased in early stage wounds, followed by a persistence of neutrophils and macrophages in chronic, non-healing wounds. These changes in inflammatory cell recruitment are consistent with alterations in growth factor expression. Another consequence of diabetes is the formation of advanced glycation end (AGE) products which act via their receptor, RAGE (AGER), and are implicated in various diabetic complications including inflammation. It has been observed that S100b, an inflammatory protein as well as a specific RAGE ligand, significantly increased IP-10 mRNA and protein levels in THP-1 monocytes as well as peripheral blood monocytes (41). Similar to AGE but less characterized are advanced lipoxidation end (ALE) products which are also formed as a consequence of diabetes. A synthetic ALE has been shown to significantly increase the expression of IP-10, β1- and β2-integrins, cyclooxygenase-2 (COX-2), MCP-1, IL-6 and −8, and inducible nitric-oxide synthase (NOS), which are all associated with monocyte dysfunction (42).
Another feature of diabetes is that cytokine production is not limited to inflammatory cells but also comes from skin cells including keratinocytes and microvascular ECs (43). A study analyzing the expression of various chemotactic and growth factors in the margin of diabetic foot ulcers found elevations of TGF-β1 and its receptor, TGFβR1, in keratinocytes and significantly increased expression of MCP-1, CXCR1, and TGFβRI in ulcer dermal ECs. There was a decreased expression of IL-10, IL-15, and TGF-β1 in the ulcer dermal ECs and blunted up-regulation of IL-8, CCR2A, and IL-10 receptor in both keratinocytes and ECs. Finally, there was a lack of up-regulation of IL-10 and IL-15 in keratinocytes in the ulcer margins. The blunting of certain leukocyte chemotactic factors along with the reduced influx of immune cells led to poor formation of granulation tissue and chronicity of ulcer epithelialization (44). Similarly, it is now recognized that the cross-talk between adipocytes and adipose tissue stromal cells such as macrophages contributes to local and systemic inflammation. This interaction is mediated by adipocyte-derived free fatty acids (FFA) that enhance macrophage induced inflammation via IP-10, IL-8, MCP-1, COX-2, and MIG, and active NF-κB (45).
In addition to cytokines, nitrosative and oxidative stress contributed by monocytes and leukocytes also significantly contribute to wound healing problem. Nitric Oxide (NO), a known scavenger of superoxide (O2−), is the main component of oxidative stress and is also known to influence and enhance angiogenesis and proliferation. But under conditions of excessive and prolonged production of (O2−) in the wounds, NO infact leads to significant increase in the production of peroxynitrite (ONOO(-)) and peroxynitrous acid (ONOOH), both of which are highly reactive and damaging radicals significantly impairing wound healing (46). In a diabetic rat model of wound healing, lipoic acid, a known scavenger of several free oxygen radicals, accelerated wound healing and this effect of lipoic acid was attributed to its protection of endothelial cells from oxidant damage (47).
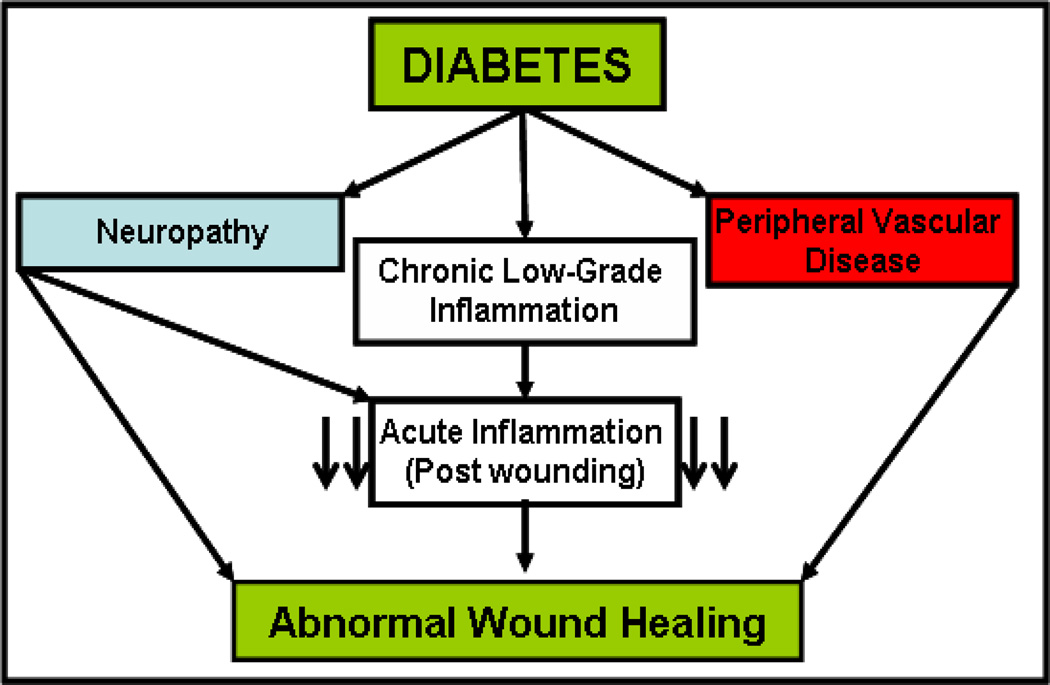
The aforementioned reports demonstrate how the chronic inflammatory environment of diabetes disturbs the interaction of pro- and anti-inflammatory cells and mediators at several levels to produce a persistent increase in pro-inflammatory cytokines by various immune and non-immune cells. It can therefore be hypothesized that the chronic inflammatory state of diabetes blunts the focused acute immune-cytokine response needed for the second phase of wound healing (Fig.1) (48−50). Rapid and effective leukocyte migration is pivotal during this early phase of wound healing. However, later observations show that leukocytes persist locally in poorly healing wounds of diabetic patients. These partly dysfunctional leukocytes (mainly neutrophils and macrophages) are believed to additionally promote the chronic inflammatory state. Further understanding of how to augment chronic low grade inflammation, the lack of acute-focused immune response, and the behavior of certain subpopulations of dysfunctional immune cells will aid the development of new approaches for treating non-healing wounds in diabetic patients.
Fig 1. Overview of diabetic wound healing.
Diabetes leads to neuropathy, peripheral vascular disease and chronic low grade inflammation, both systemically and peripherally. Neuropathy and chronic low grade inflammation together blunt the acute focused inflammatory response to injury and together with peripheral vascular disease lead to impaired wound healing.
Neuropathy and Diabetic Wound Healing
Diabetic neuropathy is an insidious, oftentimes unnoticed, long-term complication of diabetes characterized by the progressive loss of somatic and autonomic nerve fibers (51). Autonomic neuropathy results in anhidrosis, leading to altered neurogenic regulation of cutaneous blood flow and rendering the skin dry and vulnerable to cracks and fissures (51). Somatic diabetic neuropathy reported in about 30–50% of diabetic patients is the most common and sensitive predictor of foot ulceration (52–54). The consequent vulnerability to physical and thermal trauma increases the risk of foot ulceration by 7-fold, however, sensory loss must be profound before resulting in the loss of protective sensation (55, 56). It has been shown that loss of peripheral sensory and autonomic nerves along with diminished neuropeptide production precedes clinical symptoms of neuropathy (57). Peripheral sensory neuropathy is considered to be a major contributor of foot amputations and increases risk of foot amputations by 1.7-fold alone and by 36-fold if there is a prior history of ulceration (58). Skin biopsies from type II diabetic patients with and without peripheral sensory neuropathy were found to have severe denervation of the skin with active foot ulcers, irrespective of clinically identifiable sensory neuropathy (59).
Cutaneous Neuroimmunology
The contribution of peripheral nerves and cutaneous neuroimmunology to normal wound healing is of emerging importance. A bidirectional connection between the nervous and immune systems, together with signaling between the central and peripheral nervous systems with endocrine feedback, coalesce to produce complex immunomodulation (60). Both cutaneous nerve fibers and inflammatory cells are able to release neuromediators activating specific receptors on target cells in the skin or transient immunocompetent cells (61). Mediators of the neuro-immune axis include neuropeptides, neurotransmitters and cytokines. Neuropeptides commonly involved in immune regulation include substance P (SP), calcitonin gene related peptide (CRGP), neuropeptide Y (NPY), vasointestinal peptide (VIP) and proopiomelanocortin (POMC) derived peptides such as melanocyte stimulating hormone (MSH) which may be released from sensory or autonomic nerve fibers and several epidermal as well as dermal cells. Neurotransmitters include catecholamines and acetylcholine. Cytokines that are commonly activated by these neurohoromones include IL-1, IL-6, IL-8 and TNF-α, the same cytokines which are involved and dysregulated in diabetes. Sensory C nerve fibers are capable of inducing inflammation in response to a variety of irritants. This is achieved by the release of SP, CGRP, and other mediators from nerve terminals, which in turn induce mast cell discharge and increase the permeability of blood vessels (62). It is important to discuss the findings of the clinical study by Krishnan et. al showing that despite a significant reduction in the neurogenic flare response and dermal nerve fiber density the rate of wound closure was identical between control subjects and diabetic patients (63). In this study, the wounds are acute and surgically induced on the dorsum surface in well screened subjects that are not at risk of developing wound failure. The skin is different at the dorsum and plantar surface of the foot. In chronic diabetic ulcers, the wounds penetrate the underlying muscle and bone. Surgical wounds are known to heal even in patients with peripheral arterial disease following revascularization procedures (64). This further emphasizes the differences between acute surgical wounds and DFU that are chronic wounds accompanied by chronic inflammation, subclinical infection and considerable tissue damage during the period the ulcer develops.
Whereas neuropeptides have shown to modulate the cytokine response, there are studies implicating that inflammation in the form of nitrosative and oxidative stress can in turn directly impact nerve function. A recent study shows that peroxynitrite injures peripheral nerve and dorsal root ganglia leading to development of peripheral diabetic neuropathy (65). The same group has previously shown that activation of poly(ADP-ribose) polymerase (PARP) activation, a downstream effector of free radical and oxidant-induced DNA single-strand breakage is an obligatory step in functional and metabolic changes in the diabetic nerve leading to diabetic neuropathy (66) . In a mouse study of experimental diabetic peripheral neuropathy (DPN), deletion of the cyclooxygenase-2 gene, another inflammatory pathway enzyme, protected the mice from functional and biochemical deficits of experimental DPN and against nerve fiber loss (67). Not just hyperglycemia but high fat diet too has shown to induce neuropathy in pre-diabetic and obese mice by increased sorbitol pathway activity, oxidative-nitrosative stress, PARP activation, and 12/15-lipoxygenase activation (68).
Neuropeptides and Diabetic Wound Healing
It is argued that the occurrence of foot ulceration in those with neuropathy does not necessarily indicate a direct or mechanistic link between wound healing and neuropathy. One link that can directly connect neuropathy to wound healing is the neuropeptides. Neuropeptides exert their action by binding to specific receptors found on various cells in the skin, including immune cells, Langerhans cells, ECs, mast cells, fibroblasts and keratinocytes (69). They are known to activate these cutaneous cells through high affinity neuropeptide receptors or by direct activation of intracellular G-protein signaling cascades. The neuropeptides involved in wound healing are SP, NPY, CGRP, corticotropin releasing factor (CRF), α-melanocorticotropin releasing hormone (α-MSH) and neurotensin (NT). The roles for α-MSH and NT in diabetic wound healing await more thorough investigation (70–75).
Substance P
SP is a 10 amino acid peptide widely distributed in the central nervous system (CNS) and peripheral nervous system (PNS) (76, 77). In the PNS, SP is localized in primary sensory neurons and neurons intrinsic to the gastrointestinal, respiratory, and genitourinary tracts (78). There are three SP receptors, neurokinin (NK) −1, NK-2 and NK-3, of which the predominant SP receptor, NK-1, is a G-protein coupled receptor (79). NK-1 is present on variety of cell types including immune cells, ECs, neurons, epithelial cells and glial cells and couples to Gq/11, Gs and G0 proteins. The interaction of SP with NK-1 activates phospholipase C producing a net rise in intracellular [Ca2+] and cyclic adenosine monophosphate (cAMP) via adenylyl cyclase (80, 81).
Substance P and Diabetes
The first study investigating serum SP levels in type 1 diabetes showed a significant decrease in the diabetic group compared to control patients and even greater reductions in patients with diabetic neuropathy (82). Skin biopsies from both type I and type II diabetics showed reduced density of SP nerve fibers (57). Although corneal wound healing cannot be equated with skin wound healing because of the avascular nature of the cornea, in a study of diabetic rats, promotion of corneal epithelial wound healing was accelerated by the combination of an SP-derived peptide (FGLM-NH2) and insulin-like growth factor-1. This study suggests that these peptides may have effect on epithelial cells in addition to the vascular cell types (70). SP is metabolized by the enzyme enkephalinase, a zinc metalloprotease also known as neutral endopeptidase (NEP), present in the cell membrane of cells expressing NK-1R. NEP expression is found in keratinocytes, dermal appendages, the microvasculature of the skin (83), and cultured skin fibroblasts (84). NEP expression and activity has been shown to be increased in the skin of diabetic patients, leading to SP degradation (85). In the mouse diabetic model, use of an NEP inhibitor was shown to improve wound healing (86).
Substance P-Inflammatory Interaction and Wound Healing
SP is released from peripheral neurons upon noxious stimuli such as burn or pressure. Once released at the site of injury, SP binds to various cell types and initiates a cascade of events ensuring proper wound healing. In ECs, SP binds to NK-1 receptors and elicits vasodilation via nitric oxide, altering vascular permeability and enhancing the delivery and accumulation of leukocytes to tissues for the expression of the local immune response (87). SP is not only produced by immune cells such as T lymphocytes, macrophages, dendritic cells and eosinophils but is also a strong chemoattractant for lymphocytes, monocytes, neutrophils, and fibroblasts (88–95). SP can stimulate the production of TNF-α, IL-1β, IL-2, IL-8 and IL-6 from T-lymphocytes, macrophages and neutrophils (96). SP increases the expression of endothelial leukocyte adhesion molecule-1 on human microvascular endothelium and leukocyte function-associated antigen-1 (LFA-1) on murine ECs and lymphocytes (97, 98). SP has been shown to signal through and activate coincident NF-AT- and NF-kappa B pathways to induce ICAM and VCAM gene expression in microvascular ECs through intracellular [Ca2+] mobilization (99). By promoting vasodilatation, leukocyte chemotaxis, and leukocyte/endothelial cell adhesion, SP ensures the extravasation, migration, and subsequent accumulation of leukocytes at sites of injury. Induction of cell adhesion molecules on ECs is important for the recruitment and transmigration of immune cells through the endothelial monolayer. The accumulated leukocytes release cytokines creating a pro-inflammatory microenvironment leading to proliferation of ECs and angiogenesis. In intestinal wound healing, SP modulates epithelial cell restitution via the release of TGF-β from fibroblasts (100, 101). Endogenous SP release in rat knee synovium, a model of neurogenic inflammation, showed enhanced angiogenesis (102). In another study, exogenously administered SP stimulated fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing in rats (103). Similar results were found in the diabetic mouse model (db/db) of wound healing where less SP nerves were found compared to control mice, and exogenous treatment of wounds with SP shortened wound closure time (104). It is evident SP plays a major role in the inflammatory and angiogenesis phases of wound healing and dysregulation in the SP pathway in diabetes can significantly hinder wound repair.
Neuropeptide Y (NPY)
NPY is a highly conserved 36 amino acid polypeptide, and is one of the most abundant neurotransmitters in the mammalian CNS and PNS. NPY has been shown to elicit diverse biological functions including hypothalamic control of food intake, anxiolysis, and sedation (105). Other non-neuronal cells have been shown to express NPY, including megakaryocytes, liver, heart, spleen, and ECs (106, 107). NPY activates members of the NPY receptor family of heptahelical G-protein coupled receptors. Four NPY Y receptor cDNAs have been cloned, Y1, Y2, Y4, and Y5. All of these receptors mediate their responses through pertussis toxin-sensitive G proteins, resulting in inhibition of adenylate cyclase activity and increase in intracellular [Ca2+] levels (108). Although NPY was isolated and characterized twenty years ago, little is known about the molecular mechanisms that regulate NPY receptor activity and the biological significance of NPY in the periphery. NPY is also co-stored and co-released with norepinephrine and ATP in the sympathetic nerve terminal and in the adrenal medulla with epinephrine. NPY regulates vascular tone by inducing contraction of blood vessels (109). It also stimulates growth of vascular smooth muscle cells and hypertrophy of ventricular cardiomyocytes (110, 111).
NPY and Diabetes
Most research related to NPY and diabetes is focused on the central effect of NPY on feeding and energy homeostasis where NPY is known to induce feeding and conservation of energy (112). Both insulin and the anti-obesity peptide leptin inhibit NPY expression in the hypothalamus. Although NPY levels in the hypothalamus have been shown to be increased in type I and type II diabetic and obese patients, NPY levels in the skin are reduced in patients with both type I and type II diabetics (113–115). Recently, NPY expression was also shown to be reduced in streptozotocin (STZ)-induced diabetic rats (116). The vascular smooth muscle contractile response to NPY was significantly reduced from rabbits with alloxan-induced diabetes mellitus in circular segments of isolated vessels in vitro compared to control rabbits (117).
NPY–Inflammatory Interaction and Wound Healing
NPY, acting through its Y1 receptor, has pleiotropic effects on both the innate and adaptive arms of the immune system, with effects ranging from the modulation of cell migration to macrophage and helper T cell cytokine release, antigen presentation, activation of natural killer cells and antibody production (118–120). In a study of rabbit ligament healing, NPY induced expression of IL-2 and TNF-α in specimens of injured ligament cultured two weeks after injury (121). Only recently, NPY has been reported to directly stimulate EC proliferation and migration through the Y2 and Y5 receptors, leading to angiogenesis (122–124). Deletion of Y2 in mice resulted in blockage of NPY-induced angiogenesis and delayed wound healing (72). NPY is also implicated in vascularization of neural crest-derived tumors (125). The enzyme that activates NPY, dipeptidyl peptidase IV (DPP IV), is expressed in ECs. It cleaves the 36 amino acid NPY to NPY3–36, the fragment which is agonistic to Y2 and Y5 angiogenic receptors (126). In aging mice it was shown that EC Y2 and DPP IV expression was significantly reduced (127).The molecular mechanisms mediating NPY-induced angiogenesis are not known. Similar to SP, NPY is also important for both the inflammatory and angiogenic phases of wound healing.
Calcitonin Gene Related Peptide (CGRP)
CGRP is a 37 amino acid peptide generated from the alternative splicing of the calcitonin gene, both in the CNS and the PNS (128). In the PNS, CGRP is co-stored and co-released with SP from capsaicin-sensitive peripheral afferent neurons (129, 130). It is also produced in non-neuronal tissues such as liver, lungs, kidneys, prostate and testis (131). CGRP receptors are found in the brain, including the cerebellum, as well as in various peripheral organs such as heart, vasculature, liver, spleen, skeletal muscle, lung and lymphocytes (128). The data regarding CGRP receptors and their structure is not very clear. It has been proposed that there are two CGRP receptors, CGRP R1 and CGRP R2. CGRP activates adenylate cyclase and/or increases in intracellular cAMP as seen in different tissues including liver, heart, skeletal muscle and astrocytes (128). It is one of the most potent vasodilators.
CGRP and Diabetes
Diabetes reduces CGRP in the hearts of mice and in the dorsal root ganglion and dura matter of rats (132–134). In diabetic rats, CGRP mediated vasodilation was significantly reduced (135–137). In a diabetic cardiomyopathy model, both CGRP and CGRP receptor expression were shown to be reduced (138, 139). It is clearly evident that diabetes decreases the expression, release, and action of CGRP.
CGRP-Inflammatory Interaction and Wound Healing
CGRP not only induces inflammatory pathways, but inflammation too can induce CGRP release (140, 141). Similar to SP, CGRP is known to induce neurogenic inflammation via release of histamine from mast cells leading to recruitment of neutrophils to the site of injury (142). CGRP also functions in autocrine fashion. It is known to be produced by macrophage-activated adipocytes and monocytes (143). CGRP has been shown to increase the release of IL-1α and IL-8 from keratinocytes (144). CGRP has been shown to be chemotactic towards T lymphocytes, modulate lymphocyte proliferation and inhibit IL-2 production (145, 146). In the airway epithelium, CGRP is known to increase IL-1, IL-8 and ICAM-1 expression leading to the accumulation of neutrophils (147). In humans CGRP induces IL-8 but not MCP-1 or RANTES in corneal epithelial cells (148). Both CGRP and SP increase the levels of IL-1β, IL-6, and TNF-α in dental pulp fibroblasts and IL-1β and TNF-α in macrophages (149, 150). Although the role of CGRP as a neuroinflammatory mediator is apparent this interaction is not yet directly linked to its effect on wound healing. More direct involvement of CGRP in wound healing is shown through its effect on angiogenesis via enhancement of VEGF release and the cAMP pathway (151, 152).
Hypothalamic-Pituitary-Adrenal Axis and Corticotropin Releasing Factor
The hypothalamic-pituitary-adrenal axis (HPA) comprised of the hypothalamus, the pituitary gland, and the adrenal glands, controls reactions to stress and regulates digestion, the immune system, mood and emotions, sexuality, and energy storage and expenditure. Corticotropin-releasing factor or hormone (CRF) is a 41-amino acid peptide derived from a 191-amino acid chain. CRF, along with the other members of the CRF family urocortin1, urocortin2 and urocortin3, is secreted by the paraventricular nucleus (PVN) of the hypothalamus in response to stress. In addition, CRF is synthesized and produced in multiple peripheral tissues and might be involved in many other biological functions, such as energy balance, metabolism, and regulation of the immune response (153, 154). CRF binds to and activates two known receptors, CRFR1 and CRFR2 (155). These receptors belong to the family of G-protein coupled receptors whose actions are mediated through activation of adenylate cyclase. The type-1 receptor is the predominant CRF receptor and is expressed in the brain, pituitary, gonads, and skin (156). CRF binding to the receptors in the anterior lobe of the pituitary gland causes release of adrenocorticotrophic hormone (ACTH). ACTH then activates the adrenal glands to release gluccocorticoids such as cortisol.
HPA Axis, CRH and Diabetes
Diabetes has been shown to modulate the HPA axis, and type II diabetic patients have been shown to have sub-clinical hypercortisolism (157–159). In a diabetic rat study, hyperactivation of the HPA axis was associated with an impaired stress response due to decreased pituitary and adrenal sensitivity; while the basal hyperactivation of the diabetic HPA axis in the morning was found to be due in part to decreased glucocorticoid negative feedback sensitivity (160, 161). The impairment in the HPA axis was corrected by insulin treatment (162). In an in vitro study, CRF-induced ACTH release from cultured anterior pituitary cells of short-term diabetic rats was increased in part by the cAMP system. In contrast, the decrease in the CRF-induced ACTH release from cultured anterior pituitary cells of long-term diabetic rats indicated a change in the properties of the L-type Ca2+ channel coupled with the CRF receptor, or the CRF receptor itself.
HPA Axis, CRF-Inflammatory Interaction and Wound Healing
HPA-axis dysfunction is generally caused by stress. Given that immune dysfunction is a known manifestation of chronic stress, it is not surprising that the HPA-axis leads to immune dysfunction. Also, cytokines have shown to directly lead to HPA-axis dysfunction, suggesting a bidirectional communication between inflammatory mediators and the HPA-axis (163). Glucocorticoids (GCs) such as cortisol are known to suppress inflammatory pathways and are known immunosupressants (164, 165). GCs secreted in response to acute, sub-acute and chronic stress suppress CD8+ cellular immunological responses but enhance CD4+ humoral-antibody immune responses (166, 167). GCs also affect the balance of the CD4+ T cell subsets of Th1 and Th2 (168). Th1 cells produce cytokines associated with cell-mediated immune responses against intracellular pathogens and induce organ-specific autoimmune diseases. Th2 cells produce cytokines that are associated with atopic and allergic conditions (169). Thus by affecting the TH1-TH2 balance, the hyperactivated HPA axis seen in diabetes can decrease resistance to viral and bacterial infections (170, 171). Immune cells including lymphocytes and monocytes express receptors for CRF, ACTH and cortisol (168, 172). CRF has been found to inhibit IFN-α release from Th1 cells and IFN-γ from monocytes (167, 173). Similar to SP and CGRP, CRF stimulates the activation and degranulation of mast cells via the CRFR1 leading to histamine release (174). GCs suppress the activity of activator protein 1 (AP-1) and the transcription factor NF-κB (164). GCs inhibit IL-1, IL-6 and TNF-α and upregulate IL-4 and IL-10 (164). In the CNS, CRF is known to induce IL-1 and in peripheral tissues stimulates the proliferation of B and T lymphocytes, induces IL-2 receptor expression, and releases IL-1 and IL-2 from mononuclear cells (164). Thus, it is predictable that CRF would be implicated in the pathology of inflammatory diseases such as rheumatoid arthritis, osteoarthritis, thyroiditis and ulcerative colitis (172, 175, 176). Chronic stress delays wound healing in humans and rodents which is mediated by the HPA axis and, in particular, increases in corticosteroids (177, 178). In a rhesus monkey model of wound healing, stress decreased the expression of IL-8 and MIP-1α (CCL-3) and impaired wound healing (179). In a restraint stress model of mouse wound healing, the expression of IL-β and PDGF was decreased accompanied by delayed wound healing. An antiglucocorticoid, androstenediol, inhibited this suppression of IL-β and PDGF and accelerated wound healing, suggesting glucocorticoids are responsible for stress-induced delayed wound healing (180). In a similar study, cutaneous expression of IL-1β and KGF-1 was shown to be reduced and was responsible for delayed wound healing (181). As mentioned earlier, activation of the HPA axis leads to immune suppression making wounds susceptible to opportunistic infections. Female mice subjected to restraint stress had delayed healing and a 2- to 5-log increase in opportunistic bacteria (e.g., Staphylococcus aureus) when compared to wounds from control animals. This effect was obliterated by the application of the glucocorticoid receptor antagonist RU486 (182).
In diabetes, neuropeptides SP, NPY, and CGRP are downregulated leading to immune dysfunction and impaired wound healing. But in the case of the HPA axis and CRF, there is hyperactivation which can have similar deleterious consequences on the immune system and wound healing. Therefore, whereas the sensory neuropeptides need to be substituted, the peptides of the HPA axis need to be suppressed to ensure successful wound healing.
Melanocorticotropin Releasing Hormone (MSH)
Melanocortins (MCs), which are traditionally known to be responsible for the regulation of pigmentation and cortisol production, are structurally related peptides involved in different processes such as food intake, energy homeostasis, sexual behavior, exocrine gland function, inflammatory responses, and others (183). ACTH and α-MSH are MCs that are released from the anterior and pars intermedia region of the pituitary gland respectively. These two MCs are derived from pro-opiomelanocortin (POMC), which is processed by distinct members of the prohormone convertase family (184). Human skin is a potent source for α-MSH and contains all the necessary machinery for its production (185–187). CRF and inflammatory cytokines regulate expression of MCs including that of α-MSH. Different cell types including, melanocytes, keratinocytes, fibroblasts and endothelial cells not only synthesize α-MSH but also express MC receptors (MC-Rs), thus forming a complex endocrine, paracrine, and autocrine network (188). There are five known MC-Rs, MC-1R to MC-5R, which are seven transmembrane domain G-protein-coupled receptors. MC1-R is present on human monocytes, macrophages, neutrophils, mast cells, dendritic cells, microglia and astrocytes (189). On binding to the MC1-R, α-MSH activates adenylate cyclase which, in turn, causes an increase in intracellular cAMP. This is the classical pathway by which α-MSH is believed to mediate its melanogenic effects on melanocytes (188).
α- MSH and diabetes:
POMC levels have been shown to affect glucose metabolism. Homozygous POMC-null mice lack central as well as peripheral MSH signaling in addition to lacking adrenal glands. This mouse was unable to recover from insulin-induced hypoglycemia suggesting that the regulation of glucose homeostasis requires the integration of both central and peripheral melanocortin signaling systems (190). In mice, centrally-administered melanocortin agonists inhibited basal insulin release and altered glucose tolerance. Furthermore, increased plasma insulin levels occurred in the young lean MC4-R knockout (MC4-RKO) mouse (191). In diet induced obesity, long term activation of α-MSH reduced body weight, adiposity, and hepatic fat accumulation and improved glucose metabolism. These results suggest that long-term melanocortinergic activation could serve as a potential strategy for the treatment of obesity and its deleterious metabolic consequences (192). In a diabetic rat study, POMC mRNA in arcuate nucleus and pituitary was decreased and normalized after insulin treatment (193). In a similar study of diabetic rats, hypothalamic POMC mRNA expression was significantly reduced and was partially reversed by insulin treatment (194). These studies clearly demonstrate that MSH influences glucose metabolism and insulin sensitivity and similarly diabetes itself can affect the POMC-MSH pathway.
α-MSH-Inflammatory Interaction and Wound Healing:
α-MSH is known to be anti-inflammatory and has shown to attenuate inflammatory pathways that affect inflammatory bowel disease, heart transplantation, and brain inflammation (195–200). α-MSH modulates transcription factors AP-1 and NF- κB and regulates the expression of IL-8 in human dermal fibroblasts (201). α-MSH down-regulated CD86, a major T-cell costimulatory molecule, in LPS-stimulated monocytes (189). In human peripheral blood monocytes and cultured human monocytes,α-MSH increased the production and expression of the anti-inflammatory cytokine, IL-10. In the THP-1 monocytic cell line, α-MSH inhibited LPS-stimulated release of TNF-α. In septic patients, small concentrations of α-MSH added to LPS-stimulated whole blood samples inhibited TNF-α and IL-1β production (202–204). It inhibited nitric oxide production induced by LPS and IFN-γ in RAW264.7 mouse macrophages (205, 206). α-MSH also affects non-immune cells affecting production of inflammatory mediators in these cells. In ECs, α-MSH down-regulated LPS-induced expression of adhesion molecules VCAM-1 and E-Selectin, caused an increase in the expression and release of IL-8 in resting ECs, decreased IL-8 release in stimulated dermal fibroblasts and in human keratinocytes increased expression of IL-10 (201, 207–209). In corneal wound healing, topical administration of the COOH-terminal tripeptide sequence of α-MSH (α-MSH(11–13), KPV) with a mechanism that may have involved NO disposition in corneal tissue (210). Thus, similar to the other neuropeptides, MSH too affects inflammatory pathways and although MSH is abundantly found in cutaneous cells, the potential implication of this interaction needs a thorough investigation in cutaneous diabetic wound healing.
Neurotensin
Neurotensin (NT), a tridecapeptide, is localized predominantly in the CNS (predominantly hypothalamus and pituitary) and in endocrine cells (N cells) of the jejunum and ileum. In the CNS, NT functions by inhibiting dopaminergic pathways. In the periphery, NT stimulates growth of various GI tissues as well as adrenal gland, hepatocytes and fibroblasts (211). NT mediates its functions by binding to two specific G-protein coupled receptors, NT receptor 1 (NTR1), which is the predominant NT receptor, and NT receptor 2 (NTR2) (212) . There is a third receptor, NTR3 which is an intracellular receptor with a single transmembrane domain. All three receptors are found all throughout the CNS. NTR1 is functionally coupled to the phospholipase C and the inositol phosphate (IP) signaling pathway and it is also known to function through cyclic guanosine monophosphate (cGMP), cAMP, arachidonic acid production, mitogen-activated protein (MAP) kinase phosphorylation and inhibition of Akt activity (213). NTR2 receptors are known to produce ligand-induced internalization of receptor–ligand complexes which is associated with activation of extracellular signal–regulated kinases 1/2 (ERK1/2) but not Ca2+ mobilization (213). NTR3 may be involved in the NT-induced migration of human microglial cells via the stimulation of both MAP and phosphatidylinositol (PI)3-kinase-dependent pathways (213).
Neurotensin and Diabetes
Similar to α-MSH, neurotensin has been shown to play a role in the pathogenesis of diabetes. Raised concentrations and total contents of neurotensin were observed in the pancreas of obese (ob/ob) mice and the intestine of both ob/ob and diabetic (db/db) mice (214). In a similar study of ob/ob and db/db mice, pancreatic NT concentration was regulated by insulin, with elevated levels occurring in association with insulin deficiency and its metabolic consequences but not with insulin resistance (215). Daily administration of insulin to diabetic rats completely reversed this effect, and pancreatic neurotensin levels in these animals returned to control values. These findings suggest that elevated levels of pancreatic neurotensin may contribute to some of the metabolic and hormonal disturbances occurring in diabetes (216). However, these results were not consistent. In one study, NT levels were not different between lean and obese diabetic mice (217). Moreover, the results of these animal studies were not supported by human studies in which there was no difference in NT levels between non-diabetic subjects and lean and obese diabetic patients at pre- and post-prandial (218).
Neurotensin-Inflammatory Interaction and Wound Healing
NT has been reported to modulate cell functions of both innate and adaptive immunity (219–223). NT fibers and NT mRNA has been found in the skin where it is known to activate skin mast cells to release histamine (224, 225). Since NT is implicated in numerous GI tract diseases, most research focused on NT’s effect on inflammation is in the GI tract and investigations in the skin are limited. NT mediates acute intestinal inflammation in vivo and stimulates NF-κB-dependent IL-8 expression in non-transformed human colonocytes in vitro. It does so by stimulating IκBα phosphorylation and degradation, p65 phosphorylation and transcriptional activity, and Rho dependent pathways (226, 227). NT stimulates intestinal wound healing following chronic intestinal inflammation via inducing the COX-2 pathway (228). In a cerebral tissue wound-healing model and a chemotaxis assay, NT elicited the migration of the human microglial cell line C13NJ by a mechanism dependent on both phosphatidylinositol 3-kinase (PI 3-kinase) and mitogen-activated protein (MAP) kinase pathways via the receptor NT-R3 (229). From these studies once again it is apparent that NT is an important immunomodulator. At this time studies pertaining to the effect of diabetes on NT in the periphery are lacking along with the potential role it may play in wound healing. It could be expected that NT would enhance wound healing by increasing IL-8 expression and/or initiating mast cell degranulation.
Future Research into Neuroinflammatory Mediators
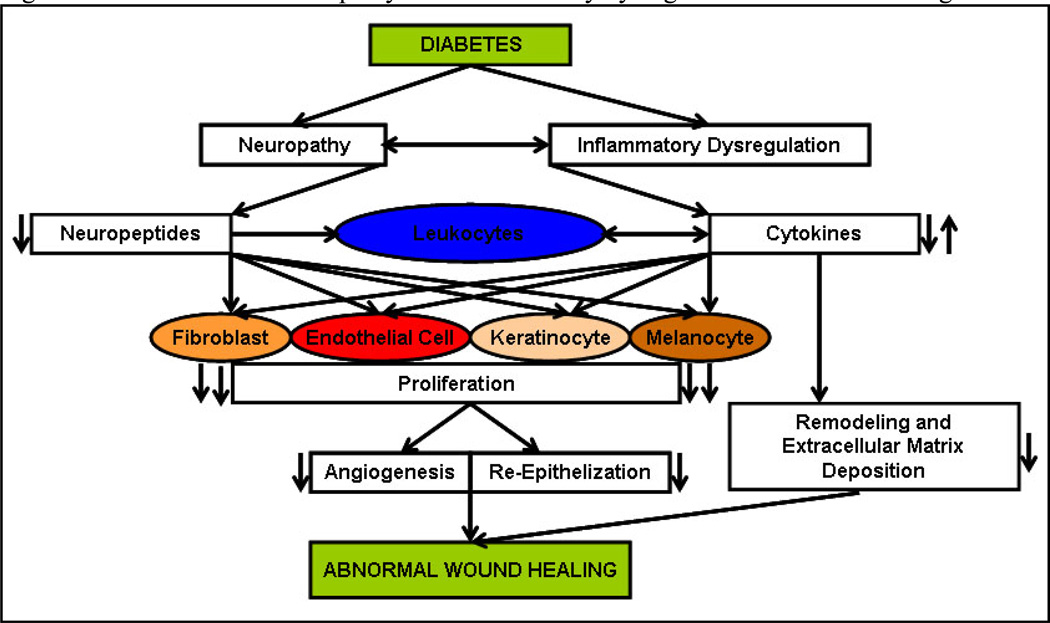
The studies presented here unmistakably point to the prominent role played by nerves and their mediators in inflammation. Nerves can no longer be viewed simply as transmitters of impulses but need to be recognized as integral components of the inflammatory cascade in various tissues including skin. Cutaneous nerves, both autonomic and sensory, participate in the immune modulation of the skin and can therefore influence wound healing (Fig.2.). They do so by affecting the function of immune and cutaneous cells such as keratinocytes, melanocytes, fibroblasts and dermal microvascular ECs. In the skin, neuropeptides and cytokines function in an endocrine, paracrine and in some cases autocrine fashion to ensure proper wound healing. Although some neuropeptides have received recognition as important players in cutaneous wound healing, few still need investigation (Table 1).
Fig. 2. Effect of diabetic neuropathy and inflammatory dysregulation on wound healing.
Diabetes leads to neuropathy and inflammatory dysregulation which manifests in decreased neuropeptide expression and imbalance in the inflammatory cytokine response. Neuropeptides directly affect leukocytes and monocytes thereby further contributing to the imbalance in cytokine expression. In addition, neuropeptides and cytokines also directly affect endothelial cells and keratinocytes thereby reducing their proliferation and leading to decreased angiogenesis, and re-epithelization. Along with decreased angiogenesis, re-epithelization and dysregulation in the remodeling and extracellular matrix deposition affected by disrupted cytokine expression; the final outcome is abnormal wound healing.
Table 1.
Phases of cutaneous wound healing affected by the neuropeptide-cytokine interaction
| Neuropeptide | Cytokines Affected | Phases of Cutaneous Wound Healing Affected |
|---|---|---|
| Substance P (SP) (96, 100, 101) | TNF-α, IL-1β, IL-2, IL-8, IL-6 and TGF-β | Early and Late Inflammation and Angiogenesis |
| Neuropeptide Y (NPY) (121) | IL-2 and TNF-α | Angiogenesis |
| Calcitonin Gene Related Peptide (CGRP) (144–150) | IL-1α, IL-1b, IL-8, IL-2, IL-6, and TNF-α | Needs Investigation |
| Corticotropin Releasing Factor (CRF) (164, 167, 173, 179, 181) | IL-1α, IL-1β, IL-2, IL-6, IL-4, IL-10, IL-8, IFN-α, IFN-γ, TNF-α, MIP-1α and KGF-1 | Early and Late Inflammation |
| α-Melanocyte Stimulating Hormone (α-MSH) (201 – 209) | IL-8, IL-10, TNF-α, IL- β, IFN-γ and IL-8 | Proliferation, Angiogenesis and Remodeling |
| Neurotensin (NT) (229) | IL-8 | Needs Investigation |
Diabetes, the endemic disease of the 21st century, and its complications are burdensome on the economy. Neuropathy and peripheral vascular disease result in impaired wound healing. The homeostasis maintained by the nerve-immune function in the skin is disrupted in diabetes. In diabetes some neuropeptides are downregulated (SP, NPY, CGRP) and others upregulated (CRF, α-MSH and NT) with the net effect being that downstream cytokines in the skin are dysregulated. Thus, it is not the gross upregulation or downregulation of cytokines that disrupts wound healing pathways but the disruption in the balance of cytokines that leads to impaired wound healing.
Current principles of treatment for diabetic wounds include debridement, pressure off-loading, infection control, judicious wound care, tight glycemic control, and optimization of cholesterol, nutrition, and other lifestyle factors (230–232). Despite this current multifaceted approach, treatment success is limited. Therefore, future therapeutics need to rely on the successful integration of mediators of different pathways involved in wound healing. A combination of deficient neuropeptides and cytokines and inhibitors of upregulated neuropeptides and cytokines could be one such approach. It is extremely important to underscore the complex molecular events that underlie successful wound healing and thus monotherapy is doomed to fail. In the past, growth factors were considered as high potential targets for therapy but to this day the only growth factor approved for treatment of diabetic wounds is PDGF. Combining different growth factors or using growth factors in concert with neuropeptides may be a key strategy in achieving high rates of wound healing in the clinic. Another limiting factor in treating diabetic wounds is the delivery of wound healing agents. Efflux of these agents and degradation by proteases of protein-based agents in the wound microenvironment is problematic. Delivery of protein based agents by gene therapy using viral vectors, although promising, poses a serious risk of local and systemic inflammation. Non-viral plasmids could prove to be better vectors. However, in vivo plasmid delivery using chemical transfection reagents is difficult to achieve. Alternatively, physical methods such as electroporation are easy, feasible, safe and efficacious (233–236). Transdermal delivery of keratinocyte growth factor using these methods in a rat model successfully healed wounds (236). Thus, identification of therapeutic mediators and therapeutic feasibility are equally important. We have already recognized the importance of the neuropeptide-immune axis mediators in diabetic wound healing and now the future lies in the successful translation of these mediators into treatments for this insidious complication.
ACKNOWLEDGEMENTS
This work was partly supported by National Institutes of Health Grants R01-HL075678, R01-NS046710 and R01 DK076937 to AV.
Abbreviations
- TNF-α
Tumor Necrosis Factor – alpha
- IL-1β
Interleukin-1 beta
- IL-2
Interleukin-2
- IL-8
Interleukin-8
- IL-6
Interleukin-6
- TGF-β
Transforming Growth Factor – b
- IL-1a
Interleukin-1 alpha
- IL-4
Interleukin-4
- IL-10
Interleukin-10
- IFN-β
Interferon – beta
- IFN-γ
Interferon – gamma
- MIP-1α
Macrophage Inflammatory Protein – 1alpha
- KGF
Keratinocyte Growth Factor
Footnotes
FURTHER READING:
Publications:
Blakytny, R., Jude, E (2006) The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med: 23(6), 594–608.
This review article examines the work done at the molecular level on chronic diabetic ulcers, as well as considering changes seen in diabetes in general, both in humans and animal models, that may in turn contribute to ulcer formation.
Vinik, A., Ullal, J., Parson, H..K. and Casellini, C.M. (2006) Diabetic neuropathies: clinical manifestations and current treatment options. Nat Clin Pract Endocrinol Metab: 2(5), 269–281.
This review article describes the different conditions that embrace the diagnosis of diabetic neuropathies
Veves, A., Giurini, J. M. and LoGerfo, F. W. (2002) The diabetic foot : medical and surgical management. Humana Press, Totowa, N.J.
This book provides detailed information on the medical and surgical management of the diabetic foot
Website:
Following is the link to the National Institutes of Digestive Diabetes Kidney (NIDDK) Diabetes in America, 2nd Edition which is a 733-page compilation and assessment of epidemiologic, public health, and clinical data on diabetes and its complications in the United States, http://diabetes.niddk.nih.gov/dm/pubs/america/contents.htm
REFERENCES
- 1.Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.Consensus Development Conference on Diabetic Foot Wound Care: 7–8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care. 22(8):1354–1360. doi: 10.2337/diacare.22.8.1354. [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJ, et al. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 4.Edmonds ME, et al. Improved survival of the diabetic foot: the role of a specialized foot clinic. Q J Med. 1986;60(232):763–771. [PubMed] [Google Scholar]
- 5.Thomson F, et al. A team approach to diabetic foot care--the Manchester experience. The Foot. 1991;1(2):75–82. [Google Scholar]
- 6.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 7.Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23(1):9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Nolan CM, Beaty HN, Bagdade JD. Further characterization of the impaired bactericidal function of granulocytes in patients with poorly controlled diabetes. Diabetes. 1978;27(9):889–894. doi: 10.2337/diab.27.9.889. [DOI] [PubMed] [Google Scholar]
- 9.Falanga V. Chronic wounds: pathophysiologic and experimental considerations. J Invest Dermatol. 1993;100(5):721–725. doi: 10.1111/1523-1747.ep12472373. [DOI] [PubMed] [Google Scholar]
- 10.Claudy AL, et al. Detection of undegraded fibrin and tumor necrosis factor-alpha in venous leg ulcers. J Am Acad Dermatol. 1991;25(4):623–627. doi: 10.1016/0190-9622(91)70242-t. [DOI] [PubMed] [Google Scholar]
- 11.Loots MA, et al. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111(5):850–857. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosner K, et al. Immunohistochemical characterization of the cutaneous cellular infiltrate in different areas of chronic leg ulcers. Apmis. 1995;103(4):293–299. doi: 10.1111/j.1699-0463.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 13.Moore K, Ruge F, Harding KG. T lymphocytes and the lack of activated macrophages in wound margin biopsies from chronic leg ulcers. Br J Dermatol. 1997;137(2):188–194. doi: 10.1046/j.1365-2133.1997.18041895.x. [DOI] [PubMed] [Google Scholar]
- 14.Herrick SE, et al. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol. 1992;141(5):1085–1095. [PMC free article] [PubMed] [Google Scholar]
- 15.Duraisamy Y, et al. Effect of glycation on basic fibroblast growth factor induced angiogenesis and activation of associated signal transduction pathways in vascular endothelial cells: possible relevance to wound healing in diabetes. Angiogenesis. 2001;4(4):277–288. doi: 10.1023/a:1016068917266. [DOI] [PubMed] [Google Scholar]
- 16.Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52(1–2):40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Engelhardt E, et al. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153(6):1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebda PA, Collins MA, Tharp MD. Mast cell and myofibroblast in wound healing. Dermatol Clin. 1993;11(4):685–696. [PubMed] [Google Scholar]
- 19.Trautmann A, et al. Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol. 2000;190(1):100–106. doi: 10.1002/(SICI)1096-9896(200001)190:1<100::AID-PATH496>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Riches DWH. Macrophage involvement in wound repair, remodeling and fibrosis. 2nd edition. London: Plenum New York; 1996. [Google Scholar]
- 21.Sunderkotter C, et al. Macrophages and angiogenesis. J Leukoc Biol. 1994;55(3):410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 22.Guest CB, et al. The implication of proinflammatory cytokines in type 2 diabetes. Front Biosci. 2008;13:5187–5194. doi: 10.2741/3074. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrand F, Pape HC, Krettek C. [The importance of cytokines in the posttraumatic inflammatory reaction] Unfallchirurg. 2005;108(10):793–794. 796–803. doi: 10.1007/s00113-005-1005-1. [DOI] [PubMed] [Google Scholar]
- 24.Belperio JA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68(1):1–8. [PubMed] [Google Scholar]
- 25.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3–4):222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogdanski P, et al. Influence of insulin therapy on expression of chemokine receptor CCR5 and selected inflammatory markers in patients with type 2 diabetes mellitus. Int J Clin Pharmacol Ther. 2007;45(10):563–567. doi: 10.5414/cpp45563. [DOI] [PubMed] [Google Scholar]
- 27.Syrenicz A, et al. Low-grade systemic inflammation and the risk of type 2 diabetes in obese children and adolescents. Neuro Endocrinol Lett. 2006;27(4):453–458. [PubMed] [Google Scholar]
- 28.Rosa JS, et al. Sustained IL-1alpha, IL-4, and IL-6 elevations following correction of hyperglycemia in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9(1):9–16. doi: 10.1111/j.1399-5448.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 29.Devaraj S, et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55(3):774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson S, et al. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. Am J Clin Nutr. 2008;87(5):1188–1193. doi: 10.1093/ajcn/87.5.1188. [DOI] [PubMed] [Google Scholar]
- 31.Kempf K, et al. The metabolic syndrome sensitizes leukocytes for glucose-induced immune gene expression. J Mol Med. 2007;85(4):389–396. doi: 10.1007/s00109-006-0132-7. [DOI] [PubMed] [Google Scholar]
- 32.Fisman EZ, Adler Y, Tenenbaum A. Biomarkers in cardiovascular diabetology: interleukins and matrixins. Adv Cardiol. 2008;45:44–64. doi: 10.1159/000115187. [DOI] [PubMed] [Google Scholar]
- 33.Hatanaka E, et al. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol. 2006;146(3):443–447. doi: 10.1111/j.1365-2249.2006.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract. 2007;76(1):44–50. doi: 10.1016/j.diabres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Stegenga ME, et al. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med. 2008;25(2):157–164. doi: 10.1111/j.1464-5491.2007.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alba-Loureiro TC, et al. Diabetes causes marked changes in function and metabolism of rat neutrophils. J Endocrinol. 2006;188(2):295–303. doi: 10.1677/joe.1.06438. [DOI] [PubMed] [Google Scholar]
- 37.Mastej K, Adamiec R. Neutrophil surface expression of CD11b and CD62L in diabetic microangiopathy. Acta Diabetol. 2008;45(3):183–190. doi: 10.1007/s00592-008-0040-0. [DOI] [PubMed] [Google Scholar]
- 38.Marhoffer W, et al. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res Clin Pract. 1993;19(3):183–188. doi: 10.1016/0168-8227(93)90112-i. [DOI] [PubMed] [Google Scholar]
- 39.Alba-Loureiro TC, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40(8):1037–1044. doi: 10.1590/s0100-879x2006005000143. [DOI] [PubMed] [Google Scholar]
- 40.Oncul O, et al. Effect of the function of polymorphonuclear leukocytes and interleukin-1 beta on wound healing in patients with diabetic foot infections. J Infect. 2007;54(3):250–256. doi: 10.1016/j.jinf.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Shanmugam N, Ransohoff RM, Natarajan R. Interferon-gamma-inducible protein (IP)-10 mRNA stabilized by RNA-binding proteins in monocytes treated with S100b. J Biol Chem. 2006;281(42):31212–31221. doi: 10.1074/jbc.M602445200. [DOI] [PubMed] [Google Scholar]
- 42.Shanmugam N, et al. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes. 2008;57(4):879–888. doi: 10.2337/db07-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular. 2007;15(6):350–355. doi: 10.2310/6670.2007.00056. [DOI] [PubMed] [Google Scholar]
- 44.Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14(5):558–565. doi: 10.1111/j.1743-6109.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 45.Laine PS, et al. Palmitic acid induces IP-10 expression in human macrophages via NF-kappaB activation. Biochem Biophys Res Commun. 2007;358(1):150–155. doi: 10.1016/j.bbrc.2007.04.092. [DOI] [PubMed] [Google Scholar]
- 46.Soneja A, Drews M, Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep. 2005;(57 Suppl):108–119. [PubMed] [Google Scholar]
- 47.Lateef H, et al. Pretreatment of diabetic rats with lipoic acid improves healing of subsequently-induced abrasion wounds. Arch Dermatol Res. 2005;297(2):75–83. doi: 10.1007/s00403-005-0576-6. [DOI] [PubMed] [Google Scholar]
- 48.Straino S, et al. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol. 2008;128(6):1545–1553. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 49.Corrales JJ, et al. Decreased production of inflammatory cytokines by circulating monocytes and dendritic cells in type 2 diabetic men with atherosclerotic complications. J Diabetes Complications. 2007;21(1):41–49. doi: 10.1016/j.jdiacomp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Maruyama K, et al. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urbancic-Rovan V. Causes of diabetic foot lesions. Lancet. 2005;366(9498):1675–1676. doi: 10.1016/S0140-6736(05)67673-8. [DOI] [PubMed] [Google Scholar]
- 52.Sheehan P, et al. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26(6):1879–1882. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- 53.Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17(6):557–560. doi: 10.2337/diacare.17.6.557. [DOI] [PubMed] [Google Scholar]
- 54.Adler AI, et al. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20(7):1162–1167. doi: 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- 55.Reiber GE, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22(1):157–162. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- 56.Yao JS, et al. Interleukin-6 triggers human cerebral endothelial cells proliferation and migration: the role for KDR and MMP-9. Biochem Biophys Res Commun. 2006;342(4):1396–1404. doi: 10.1016/j.bbrc.2006.02.100. [DOI] [PubMed] [Google Scholar]
- 57.Lindberger M, et al. Nerve fibre studies in skin biopsies in peripheral neuropathies. I. Immunohistochemical analysis of neuropeptides in diabetes mellitus. J Neurol Sci. 1989;93(2–3):289–296. doi: 10.1016/0022-510x(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 58.Vinik AI, et al. Diabetic neuropathies. Diabetologia. 2000;43(8):957–973. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- 59.Galkowska H, et al. Neurogenic factors in the impaired healing of diabetic foot ulcers. J Surg Res. 2006;134(2):252–258. doi: 10.1016/j.jss.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Quattrini C, Jeziorska M, Malik RA. Small fiber neuropathy in diabetes: clinical consequence and assessment. Int J Low Extrem Wounds. 2004;3(1):16–21. doi: 10.1177/1534734603262483. [DOI] [PubMed] [Google Scholar]
- 61.Luger TA. Neuromediators--a crucial component of the skin immune system. J Dermatol Sci. 2002;30(2):87–93. doi: 10.1016/s0923-1811(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 62.Berczi I, et al. The immune effects of neuropeptides. Baillieres Clin Rheumatol. 1996;10(2):227–257. doi: 10.1016/s0950-3579(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan ST, et al. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care. 2007;30(12):3058–3062. doi: 10.2337/dc07-1421. [DOI] [PubMed] [Google Scholar]
- 64.Pomposelli FB, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37(2):307–315. doi: 10.1067/mva.2003.125. [DOI] [PubMed] [Google Scholar]
- 65.Vareniuk I, Pavlov IA, Obrosova IG. Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia. 2008 doi: 10.1007/s00125-008-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Obrosova IG, et al. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54(12):3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kellogg AP, et al. Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes. 2007;56(12):2997–3005. doi: 10.2337/db07-0740. [DOI] [PubMed] [Google Scholar]
- 68.Obrosova IG, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of "healthy" diet and aldose reductase inhibition. Diabetes. 2007;56(10):2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 69.Roosterman D, et al. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86(4):1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura M, et al. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia. 2003;46(6):839–842. doi: 10.1007/s00125-003-1105-9. [DOI] [PubMed] [Google Scholar]
- 71.Movafagh S, et al. Neuropeptide Y induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. Faseb J. 2006;20(11):1924–1926. doi: 10.1096/fj.05-4770fje. [DOI] [PubMed] [Google Scholar]
- 72.Ekstrand AJ, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003;100(10):6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuo LE, Abe K, Zukowska Z. Stress, NPY and vascular remodeling: Implications for stress-related diseases. Peptides. 2007;28(2):435–440. doi: 10.1016/j.peptides.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delgado AV, McManus AT, Chambers JP. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp Biol Med (Maywood) 2005;230(4):271–280. doi: 10.1177/153537020523000407. [DOI] [PubMed] [Google Scholar]
- 75.Zukowska Z, Grant DS, Lee EW. Neuropeptide Y: a novel mechanism for ischemic angiogenesis. Trends Cardiovasc Med. 2003;13(2):86–92. doi: 10.1016/s1050-1738(02)00232-3. [DOI] [PubMed] [Google Scholar]
- 76.Hokfelt T, et al. Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res. 1975;100(2):235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- 77.Hokfelt T, et al. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975;190(4217):889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- 78.Maggi CA. The troubled story of tachykinins and neurokinins. Trends Pharmacol Sci. 2000;21(5):173–175. doi: 10.1016/s0165-6147(00)01463-2. [DOI] [PubMed] [Google Scholar]
- 79.Maggi CA. The mammalian tachykinin receptors. Gen Pharmacol. 1995;26(5):911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- 80.Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol. 1996;28(7):721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- 81.Harrison S, Geppetti P. Substance p. Int J Biochem Cell Biol. 2001;33(6):555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 82.Kunt T, et al. Serum levels of substance P are decreased in patients with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2000;108(3):164–167. doi: 10.1055/s-2000-7738. [DOI] [PubMed] [Google Scholar]
- 83.Olerud JE, et al. Neutral endopeptidase expression and distribution in human skin and wounds. J Invest Dermatol. 1999;112(6):873–881. doi: 10.1046/j.1523-1747.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- 84.Bou-Gharios G, et al. Expression of ectopeptidases in scleroderma. Ann Rheum Dis. 1995;54(2):111–116. doi: 10.1136/ard.54.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Antezana M, et al. Neutral endopeptidase activity is increased in the skin of subjects with diabetic ulcers. J Invest Dermatol. 2002;119(6):1400–1404. doi: 10.1046/j.1523-1747.2002.19618.x. [DOI] [PubMed] [Google Scholar]
- 86.Spenny ML, et al. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen. 2002;10(5):295–301. doi: 10.1046/j.1524-475x.2002.10504.x. [DOI] [PubMed] [Google Scholar]
- 87.Pernow B. Substance P. Pharmacol Rev. 1983;35(2):85–141. [PubMed] [Google Scholar]
- 88.Ho WZ, et al. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159(11):5654–5660. [PubMed] [Google Scholar]
- 89.Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86(1):80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 90.Lai JP, et al. Quantification of substance p mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin Diagn Lab Immunol. 2002;9(1):138–143. doi: 10.1128/CDLI.9.1.138-143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lambrecht BN. Immunologists getting nervous: neuropeptides, dendritic cells and T cell activation. Respir Res. 2001;2(3):133–138. doi: 10.1186/rr49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lambrecht BN, et al. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol. 1999;29(12):3815–3825. doi: 10.1002/(SICI)1521-4141(199912)29:12<3815::AID-IMMU3815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 93.Weinstock JV, et al. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J Immunol. 1988;141(3):961–966. [PubMed] [Google Scholar]
- 94.O'Connor TM, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201(2):167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 95.Schratzberger P, et al. Differential chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. J Immunol. 1997;158(8):3895–3901. [PubMed] [Google Scholar]
- 96.Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides. 2003;37(6):355–361. doi: 10.1016/j.npep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Matis WL, Lavker RM, Murphy GF. Substance P induces the expression of an endothelial-leukocyte adhesion molecule by microvascular endothelium. J Invest Dermatol. 1990;94(4):492–495. doi: 10.1111/1523-1747.ep12874665. [DOI] [PubMed] [Google Scholar]
- 98.Vishwanath R, Mukherjee R. Substance P promotes lymphocyte endothelial cell adhesion preferentially via LFA-1/ICAM-1 interactions. J Neuroimmunol. 1996;71(1–2):163–171. doi: 10.1016/s0165-5728(96)00143-9. [DOI] [PubMed] [Google Scholar]
- 99.Quinlan KL, et al. Substance P activates coincident NF-AT- and NF-kappa B-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J Immunol. 1999;163(10):5656–5665. [PubMed] [Google Scholar]
- 100.Bulut K, et al. Sensory neuropeptides and epithelial cell restitution: the relevance of SP- and CGRP-stimulated mast cells. Int J Colorectal Dis. 2008;23(5):535–541. doi: 10.1007/s00384-008-0447-7. [DOI] [PubMed] [Google Scholar]
- 101.Felderbauer P, et al. Substance P induces intestinal wound healing via fibroblasts--evidence for a TGF-beta-dependent effect. Int J Colorectal Dis. 2007;22(12):1475–1480. doi: 10.1007/s00384-007-0321-z. [DOI] [PubMed] [Google Scholar]
- 102.Seegers HC, et al. Enhancement of angiogenesis by endogenous substance P release and neurokinin-1 receptors during neurogenic inflammation. J Pharmacol Exp Ther. 2003;306(1):8–12. doi: 10.1124/jpet.103.050013. [DOI] [PubMed] [Google Scholar]
- 103.Burssens P, et al. Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing. Foot Ankle Int. 2005;26(10):832–839. doi: 10.1177/107110070502601008. [DOI] [PubMed] [Google Scholar]
- 104.Gibran NS, et al. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108(1):122–128. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 105.Blomqvist AG, Herzog H. Y-receptor subtypes--how many more? Trends Neurosci. 1997;20(7):294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- 106.Ericsson A, et al. Detection of neuropeptide Y and its mRNA in megakaryocytes: enhanced levels in certain autoimmune mice. Proc Natl Acad Sci U S A. 1987;84(16):5585–5589. doi: 10.1073/pnas.84.16.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Strand FL. Neuropeptides : regulators of physiological processes. MIT Press: Cambridge, Mass; 1999. [Google Scholar]
- 108.Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996;65(3):165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- 109.Franco-Cereceda A, Lundberg JM, Dahlof C. Neuropeptide Y and sympathetic control of heart contractility and coronary vascular tone. Acta Physiol Scand. 1985;124(3):361–369. doi: 10.1111/j.1748-1716.1985.tb07671.x. [DOI] [PubMed] [Google Scholar]
- 110.Erlinge D, Brunkwall J, Edvinsson L. Neuropeptide Y stimulates proliferation of human vascular smooth muscle cells: cooperation with noradrenaline and ATP. Regul Pept. 1994;50(3):259–265. doi: 10.1016/0167-0115(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 111.Zukowska-Grojec Z, et al. Mitogenic effect of neuropeptide Y in rat vascular smooth muscle cells. Peptides. 1993;14(2):263–268. doi: 10.1016/0196-9781(93)90040-n. [DOI] [PubMed] [Google Scholar]
- 112.Frankish HM, et al. Neuropeptide Y, the hypothalamus, and diabetes: insights into the central control of metabolism. Peptides. 1995;16(4):757–771. doi: 10.1016/0196-9781(94)00200-p. [DOI] [PubMed] [Google Scholar]
- 113.Ahlborg G, Lundberg JM. Exercise-induced changes in neuropeptide Y, noradrenaline and endothelin-1 levels in young people with type I diabetes. Clin Physiol. 1996;16(6):645–655. doi: 10.1111/j.1475-097x.1996.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 114.Wallengren J, et al. Innervation of the skin of the forearm in diabetic patients: relation to nerve function. Acta Derm Venereol. 1995;75(1):37–42. doi: 10.2340/00015555753742. [DOI] [PubMed] [Google Scholar]
- 115.Levy DM, et al. Depletion of cutaneous nerves and neuropeptides in diabetes mellitus: an immunocytochemical study. Diabetologia. 1989;32(7):427–433. doi: 10.1007/BF00271262. [DOI] [PubMed] [Google Scholar]
- 116.Kuncova J, et al. Heterogenous changes in neuropeptide Y, norepinephrine and epinephrine concentrations in the hearts of diabetic rats. Auton Neurosci. 2005;121(1–2):7–15. doi: 10.1016/j.autneu.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 117.Andersson D, et al. Diminished contractile responses to neuropeptide Y of arteries from diabetic rabbits. J Auton Nerv Syst. 1992;37(3):215–222. doi: 10.1016/0165-1838(92)90043-g. [DOI] [PubMed] [Google Scholar]
- 118.Wheway J, Herzog H, Mackay F. NPY and receptors in immune and inflammatory diseases. Curr Top Med Chem. 2007;7(17):1743–1752. doi: 10.2174/156802607782341046. [DOI] [PubMed] [Google Scholar]
- 119.Groneberg DA, et al. Neuropeptide Y (NPY) Pulm Pharmacol Ther. 2004;17(4):173–180. doi: 10.1016/j.pupt.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Bedoui S, et al. Relevance of neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol. 2003;134(1–2):1–11. doi: 10.1016/s0165-5728(02)00424-1. [DOI] [PubMed] [Google Scholar]
- 121.Salo P, et al. Neuropeptides regulate expression of matrix molecule, growth factor and inflammatory mediator mRNA in explants of normal and healing medial collateral ligament. Regul Pept. 2007;142(1–2):1–6. doi: 10.1016/j.regpep.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 122.Ghersi G, et al. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides. 2001;22(3):453–458. doi: 10.1016/s0196-9781(01)00340-0. [DOI] [PubMed] [Google Scholar]
- 123.Marion-Audibert AM, et al. [Effects of endocrine peptides on proliferation, migration and differentiation of human endothelial cells] Gastroenterol Clin Biol. 2000;24(6–7):644–648. [PubMed] [Google Scholar]
- 124.Zukowska-Grojec Z, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83(2):187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 125.Kitlinska J, et al. Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Res. 2005;65(5):1719–1728. doi: 10.1158/0008-5472.CAN-04-2192. [DOI] [PubMed] [Google Scholar]
- 126.Zukowska Z, et al. Neuropeptide Y: a new mediator linking sympathetic nerves, blood vessels and immune system? Can J Physiol Pharmacol. 2003;81(2):89–94. doi: 10.1139/y03-006. [DOI] [PubMed] [Google Scholar]
- 127.Kitlinska J, et al. Neuropeptide Y-induced angiogenesis in aging. Peptides. 2002;23(1):71–77. doi: 10.1016/s0196-9781(01)00581-2. [DOI] [PubMed] [Google Scholar]
- 128.van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21(5):649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 129.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 130.Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45(1):1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- 131.Russwurm S, et al. Procalcitonin and CGRP-1 mrna expression in various human tissues. Shock. 2001;16(2):109–112. doi: 10.1097/00024382-200116020-00004. [DOI] [PubMed] [Google Scholar]
- 132.Song JX, et al. Impaired transient receptor potential vanilloid 1 in streptozotocin-induced diabetic hearts. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2007.12.081. [DOI] [PubMed] [Google Scholar]
- 133.Dux M, et al. Loss of capsaicin-induced meningeal neurogenic sensory vasodilatation in diabetic rats. Neuroscience. 2007;150(1):194–201. doi: 10.1016/j.neuroscience.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 134.Adeghate E, et al. Pattern of distribution of calcitonin gene-related Peptide in the dorsal root ganglion of animal models of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:296–303. doi: 10.1196/annals.1372.030. [DOI] [PubMed] [Google Scholar]
- 135.Oltman CL, et al. Vascular and neural dysfunction in Zucker diabetic fatty rats: a difficult condition to reverse. Diabetes Obes Metab. 2008;10(1):64–74. doi: 10.1111/j.1463-1326.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 136.Oltman CL, et al. Treatment of Zucker diabetic fatty rats with AVE7688 improves vascular and neural dysfunction. Diabetes Obes Metab. 2008 doi: 10.1111/j.1463-1326.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sheykhzade M, et al. The effect of long-term streptozotocin-induced diabetes on contractile and relaxation responses of coronary arteries: selective attenuation of CGRP-induced relaxations. Br J Pharmacol. 2000;129(6):1212–1218. doi: 10.1038/sj.bjp.0703159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chottova Dvorakova M, et al. Cardiomyopathy in streptozotocin-induced diabetes involves intra-axonal accumulation of calcitonin gene-related peptide and altered expression of its receptor in rats. Neuroscience. 2005;134(1):51–58. doi: 10.1016/j.neuroscience.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 139.Yorek MA, et al. Sensory nerve innervation of epineurial arterioles of the sciatic nerve containing calcitonin gene-related peptide: effect of streptozotocin-induced diabetes. Exp Diabesity Res. 2004;5(3):187–193. doi: 10.1080/15438600490486732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ambalavanar R, et al. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience. 2006;143(3):875–884. doi: 10.1016/j.neuroscience.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 141.Ambalavanar R, et al. Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain. 2006;120(1–2):53–68. doi: 10.1016/j.pain.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 142.Hu CL, Xiang JZ, Hu FF. Vanilloid receptor TRPV1, sensory C-fibers, and activation of adventitial mast cells A novel mechanism involved in adventitial inflammation. Med Hypotheses. 2008;71(1):102–103. doi: 10.1016/j.mehy.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 143.Linscheid P, et al. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32(8):1715–1721. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- 144.Dallos A, et al. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006;40(4):251–263. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 145.Wang F, et al. Calcitonin gene-related peptide inhibits interleukin 2 production by murine T lymphocytes. J Biol Chem. 1992;267(29):21052–21057. [PubMed] [Google Scholar]
- 146.Foster CA, et al. Calcitonin gene-related peptide is chemotactic for human T lymphocytes. Ann N Y Acad Sci. 1992;657:397–404. doi: 10.1111/j.1749-6632.1992.tb22785.x. [DOI] [PubMed] [Google Scholar]
- 147.Zhang JS, et al. Regulatory peptides modulate adhesion of polymorphonuclear leukocytes to bronchial epithelial cells through regulation of interleukins, ICAM-1 and NF-kappaB/IkappaB. Acta Biochim Biophys Sin (Shanghai) 2006;38(2):119–128. doi: 10.1111/j.1745-7270.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 148.Tran MT, et al. Calcitonin gene-related peptide induces IL-8 synthesis in human corneal epithelial cells. J Immunol. 2000;164(8):4307–4312. doi: 10.4049/jimmunol.164.8.4307. [DOI] [PubMed] [Google Scholar]
- 149.Yamaguchi M, et al. Neuropeptides stimulate production of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in human dental pulp cells. Inflamm Res. 2004;53(5):199–204. doi: 10.1007/s00011-003-1243-z. [DOI] [PubMed] [Google Scholar]
- 150.Yaraee R, et al. Neuropeptides (SP and CGRP) augment pro-inflammatory cytokine production in HSV-infected macrophages. Int Immunopharmacol. 2003;3(13–14):1883–1887. doi: 10.1016/S1567-5769(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 151.Toda M, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008 doi: 10.1016/j.biopha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 152.Haegerstrand A, et al. Calcitonin gene-related peptide stimulates proliferation of human endothelial cells. Proc Natl Acad Sci U S A. 1990;87(9):3299–3303. doi: 10.1073/pnas.87.9.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Richard D, Huang Q, Timofeeva E. The corticotropin-releasing hormone system in the regulation of energy balance in obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S36–S39. doi: 10.1038/sj.ijo.0801275. [DOI] [PubMed] [Google Scholar]
- 154.Baigent SM. Peripheral corticotropin-releasing hormone and urocortin in the control of the immune response. Peptides. 2001;22(5):809–820. doi: 10.1016/s0196-9781(01)00395-3. [DOI] [PubMed] [Google Scholar]
- 155.Carlin KM, Vale WW, Bale TL. Vital functions of corticotropin-releasing factor (CRF) pathways in maintenance and regulation of energy homeostasis. Proc Natl Acad Sci U S A. 2006;103(9):3462–3467. doi: 10.1073/pnas.0511320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann N Y Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 157.Chiodini I, et al. Association of subclinical hypercortisolism with type 2 diabetes mellitus: a case-control study in hospitalized patients. Eur J Endocrinol. 2005;153(6):837–844. doi: 10.1530/eje.1.02045. [DOI] [PubMed] [Google Scholar]
- 158.Ghizzoni L, et al. Adrenal steroid and adrenocorticotropin responses to human corticotropin-releasing hormone stimulation test in adolescents with type I diabetes mellitus. Metabolism. 1993;42(9):1141–1145. doi: 10.1016/0026-0495(93)90271-o. [DOI] [PubMed] [Google Scholar]
- 159.Roy MS, et al. The ovine corticotropin-releasing hormone-stimulation test in type I diabetic patients and controls: suggestion of mild chronic hypercortisolism. Metabolism. 1993;42(6):696–700. doi: 10.1016/0026-0495(93)90235-g. [DOI] [PubMed] [Google Scholar]
- 160.Chan O, et al. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002;143(5):1761–1768. doi: 10.1210/endo.143.5.8809. [DOI] [PubMed] [Google Scholar]
- 161.Chan O, et al. Hyperglycemia does not increase basal hypothalamo-pituitary-adrenal activity in diabetes but it does impair the HPA response to insulin-induced hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R235–R246. doi: 10.1152/ajpregu.00674.2004. [DOI] [PubMed] [Google Scholar]
- 162.Chan O, et al. Diabetes impairs hypothalamo-pituitary-adrenal (HPA) responses to hypoglycemia, and insulin treatment normalizes HPA but not epinephrine responses. Diabetes. 2002;51(6):1681–1689. doi: 10.2337/diabetes.51.6.1681. [DOI] [PubMed] [Google Scholar]
- 163.Turnbull AV, Rivier CL. Sprague-Dawley rats obtained from different vendors exhibit distinct adrenocorticotropin responses to inflammatory stimuli. Neuroendocrinology. 1999;70(3):186–195. doi: 10.1159/000054475. [DOI] [PubMed] [Google Scholar]
- 164.Lozovaya N, Miller AD. Chemical neuroimmunology: health in a nutshell bidirectional communication between immune and stress (limbic-hypothalamic-pituitary-adrenal) systems. Chembiochem. 2003;4(6):466–484. doi: 10.1002/cbic.200200492. [DOI] [PubMed] [Google Scholar]
- 165.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 166.Elenkov IJ, et al. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann N Y Acad Sci. 1999;876:1–11. doi: 10.1111/j.1749-6632.1999.tb07618.x. discussion 11–13. [DOI] [PubMed] [Google Scholar]
- 167.Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999;10(9):359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 168.Webster EL, Elenkov IJ, Chrousos GP. Corticotropin-releasing hormone acts on immune cells to elicit pro-inflammatory responses. Mol Psychiatry. 1997;2(5):345–346. doi: 10.1038/sj.mp.4000314. [DOI] [PubMed] [Google Scholar]
- 169.Monney L, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 170.Brown DH, Zwilling BS. Activation of the hypothalamic-pituitary-adrenal axis differentially affects the anti-mycobacterial activity of macrophages from BCG-resistant and susceptible mice. J Neuroimmunol. 1994;53(2):181–187. doi: 10.1016/0165-5728(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 171.Sheridan JF, et al. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann N Y Acad Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Webster EL, et al. Corticotropin-releasing hormone and inflammation. Ann N Y Acad Sci. 1998;840:21–32. doi: 10.1111/j.1749-6632.1998.tb09545.x. [DOI] [PubMed] [Google Scholar]
- 173.Karalis K, et al. CRH and the immune system. J Neuroimmunol. 1997;72(2):131–136. doi: 10.1016/s0165-5728(96)00178-6. [DOI] [PubMed] [Google Scholar]
- 174.Theoharides TC, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139(1):403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 175.Karalis K, et al. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254(5030):421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 176.Crofford LJ, et al. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol. 1993;151(3):1587–1596. [PubMed] [Google Scholar]
- 177.Glasper ER, Devries AC. Social structure influences effects of pair-housing on wound healing. Brain Behav Immun. 2005;19(1):61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 178.Detillion CE, et al. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29(8):1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 179.Kalin NH, et al. Stress decreases, while central nucleus amygdala lesions increase, IL-8 and MIP-1alpha gene expression during tissue healing in non-human primates. Brain Behav Immun. 2006;20(6):564–568. doi: 10.1016/j.bbi.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 180.Head CC, et al. Androstenediol reduces the anti-inflammatory effects of restraint stress during wound healing. Brain Behav Immun. 2006;20(6):590–596. doi: 10.1016/j.bbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 181.Mercado AM, et al. Altered kinetics of IL-1 alpha, IL-1 beta, and KGF-1 gene expression in early wounds of restrained mice. Brain Behav Immun. 2002;16(2):150–162. doi: 10.1006/brbi.2001.0623. [DOI] [PubMed] [Google Scholar]
- 182.Rojas IG, et al. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun. 2002;16(1):74–84. doi: 10.1006/brbi.2000.0619. [DOI] [PubMed] [Google Scholar]
- 183.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284(3):E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 184.Seidah NG, et al. The subtilisin/kexin family of precursor convertases. Emphasis on PC1, PC2/7B2, POMC and the novel enzyme SKI-1. Ann N Y Acad Sci. 1999;885:57–74. doi: 10.1111/j.1749-6632.1999.tb08665.x. [DOI] [PubMed] [Google Scholar]
- 185.Thody AJ, et al. MSH peptides are present in mammalian skin. Peptides. 1983;4(6):813–816. doi: 10.1016/0196-9781(83)90072-4. [DOI] [PubMed] [Google Scholar]
- 186.Slominski A, et al. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med. 1993;122(6):658–666. [PubMed] [Google Scholar]
- 187.Mazurkiewicz JE, Corliss D, Slominski A. Spatiotemporal expression, distribution, and processing of POMC and POMC-derived peptides in murine skin. J Histochem Cytochem. 2000;48(7):905–914. doi: 10.1177/002215540004800703. [DOI] [PubMed] [Google Scholar]
- 188.Bohm M, et al. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126(9):1966–1975. doi: 10.1038/sj.jid.5700421. [DOI] [PubMed] [Google Scholar]
- 189.Bhardwaj R, et al. Evidence for the differential expression of the functional alpha-melanocyte-stimulating hormone receptor MC-1 on human monocytes. J Immunol. 1997;158(7):3378–3384. [PubMed] [Google Scholar]
- 190.Hochgeschwender U, et al. Altered glucose homeostasis in proopiomelanocortin-null mouse mutants lacking central and peripheral melanocortin. Endocrinology. 2003;144(12):5194–5202. doi: 10.1210/en.2003-1008. [DOI] [PubMed] [Google Scholar]
- 191.Fan W, et al. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141(9):3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- 192.Lee M, et al. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab. 2007;293(1):E121–E131. doi: 10.1152/ajpendo.00555.2006. [DOI] [PubMed] [Google Scholar]
- 193.Kim EM, et al. STZ-induced diabetes decreases and insulin normalizes POMC mRNA in arcuate nucleus and pituitary in rats. Am J Physiol. 1999;276(5 Pt 2):R1320–R1326. doi: 10.1152/ajpregu.1999.276.5.R1320. [DOI] [PubMed] [Google Scholar]
- 194.Havel PJ, et al. Effects of streptozotocin-induced diabetes and insulin treatment on the hypothalamic melanocortin system and muscle uncoupling protein 3 expression in rats. Diabetes. 2000;49(2):244–252. doi: 10.2337/diabetes.49.2.244. [DOI] [PubMed] [Google Scholar]
- 195.Abou-Mohamed G, et al. HP-228, a novel synthetic peptide, inhibits the induction of nitric oxide synthase in vivo but not in vitro. J Pharmacol Exp Ther. 1995;275(2):584–591. [PubMed] [Google Scholar]
- 196.Rajora N, et al. alpha-MSH modulates local and circulating tumor necrosis factor-alpha in experimental brain inflammation. J Neurosci. 1997;17(6):2181–2186. doi: 10.1523/JNEUROSCI.17-06-02181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rajora N, et al. alpha-MSH modulates experimental inflammatory bowel disease. Peptides. 1997;18(3):381–385. doi: 10.1016/s0196-9781(96)00345-2. [DOI] [PubMed] [Google Scholar]
- 198.Catania A, et al. alpha-MSH in systemic inflammation. Central and peripheral actions. Ann N Y Acad Sci. 1999;885:183–187. doi: 10.1111/j.1749-6632.1999.tb08675.x. [DOI] [PubMed] [Google Scholar]
- 199.Gatti S, et al. alpha-Melanocyte-stimulating hormone protects the allograft in experimental heart transplantation. Transplantation. 2002;74(12):1678–1684. doi: 10.1097/00007890-200212270-00005. [DOI] [PubMed] [Google Scholar]
- 200.Catania A, et al. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56(1):1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 201.Bohm M, et al. Alpha-melanocyte-stimulating hormone modulates activation of NF-kappa B and AP-1 and secretion of interleukin-8 in human dermal fibroblasts. Ann N Y Acad Sci. 1999;885:277–286. doi: 10.1111/j.1749-6632.1999.tb08685.x. [DOI] [PubMed] [Google Scholar]
- 202.Bhardwaj RS, et al. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. J Immunol. 1996;156(7):2517–2521. [PubMed] [Google Scholar]
- 203.Taherzadeh S, et al. alpha-MSH and its receptors in regulation of tumor necrosis factor-alpha production by human monocyte/macrophages. Am J Physiol. 1999;276(5 Pt 2):R1289–R1294. doi: 10.1152/ajpregu.1999.276.5.R1289. [DOI] [PubMed] [Google Scholar]
- 204.Catania A, et al. Plasma concentrations and anti-L-cytokine effects of alpha-melanocyte stimulating hormone in septic patients. Crit Care Med. 2000;28(5):1403–1407. doi: 10.1097/00003246-200005000-00024. [DOI] [PubMed] [Google Scholar]
- 205.Star RA, et al. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1995;92(17):8016–8020. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mandrika I, Muceniece R, Wikberg JE. Effects of melanocortin peptides on lipopolysaccharide/interferon-gamma-induced NF-kappaB DNA binding and nitric oxide production in macrophage-like RAW 264.7 cells: evidence for dual mechanisms of action. Biochem Pharmacol. 2001;61(5):613–621. doi: 10.1016/s0006-2952(00)00583-9. [DOI] [PubMed] [Google Scholar]
- 207.Kalden DH, et al. Mechanisms of the antiinflammatory effects of alpha-MSH. Role of transcription factor NF-kappa B and adhesion molecule expression. Ann N Y Acad Sci. 1999;885:254–261. doi: 10.1111/j.1749-6632.1999.tb08682.x. [DOI] [PubMed] [Google Scholar]
- 208.Hartmeyer M, et al. Human dermal microvascular endothelial cells express the melanocortin receptor type 1 and produce increased levels of IL-8 upon stimulation with alpha-melanocyte-stimulating hormone. J Immunol. 1997;159(4):1930–1937. [PubMed] [Google Scholar]
- 209.Redondo P, et al. Alpha-MSH regulates interleukin-10 expression by human keratinocytes. Arch Dermatol Res. 1998;290(8):425–428. doi: 10.1007/s004030050330. [DOI] [PubMed] [Google Scholar]
- 210.Bonfiglio V, et al. Effects of the COOH-terminal tripeptide alpha-MSH(11–13) on corneal epithelial wound healing: role of nitric oxide. Exp Eye Res. 2006;83(6):1366–1372. doi: 10.1016/j.exer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 211.Evers BM. Neurotensin and growth of normal and neoplastic tissues. Peptides. 2006;27(10):2424–2433. doi: 10.1016/j.peptides.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 212.Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(7):918–932. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 213.St-Gelais F, Jomphe C, Trudeau LE. The role of neurotensin in central nervous system pathophysiology: what is the evidence? J Psychiatry Neurosci. 2006;31(4):229–245. [PMC free article] [PubMed] [Google Scholar]
- 214.Sheppard MC, et al. Immunoreactive neurotensin in spontaneous syndromes of obesity and diabetes in mice. Acta Endocrinol (Copenh) 1985;108(4):532–536. doi: 10.1530/acta.0.1080532. [DOI] [PubMed] [Google Scholar]
- 215.Berelowitz M, Frohman LA. The role of neurotensin in the regulation of carbohydrate metabolism and in diabetes. Ann N Y Acad Sci. 1982;400:150–159. doi: 10.1111/j.1749-6632.1982.tb31566.x. [DOI] [PubMed] [Google Scholar]
- 216.Fernstrom MH, et al. Immunoreactive neurotensin levels in pancreas: elevation in diabetic rats and mice. Metabolism. 1981;30(9):853–855. doi: 10.1016/0026-0495(81)90063-9. [DOI] [PubMed] [Google Scholar]
- 217.El-Salhy M. Neuroendocrine peptides of the gastrointestinal tract of an animal model of human type 2 diabetes mellitus. Acta Diabetol. 1998;35(4):194–198. doi: 10.1007/s005920050130. [DOI] [PubMed] [Google Scholar]
- 218.Service FJ, et al. Neurotensin in diabetes and obesity. Regul Pept. 1986;14(1):85–92. doi: 10.1016/0167-0115(86)90207-7. [DOI] [PubMed] [Google Scholar]
- 219.Goldman R, et al. Enhancement of phagocytosis by neurotensin, a newly found biological activity of the neuropeptide. Adv Exp Med Biol. 1982;155:133–141. doi: 10.1007/978-1-4684-4394-3_11. [DOI] [PubMed] [Google Scholar]
- 220.Koff WC, Dunegan MA. Modulation of macrophage-mediated tumoricidal activity by neuropeptides and neurohormones. J Immunol. 1985;135(1):350–354. [PubMed] [Google Scholar]
- 221.Lemaire I. Neurotensin enhances IL-1 production by activated alveolar macrophages. J Immunol. 1988;140(9):2983–2988. [PubMed] [Google Scholar]
- 222.Garrido JJ, et al. Modulation by neurotensin and neuromedin N of adherence and chemotaxis capacity of murine lymphocytes. Regul Pept. 1992;41(1):27–37. doi: 10.1016/0167-0115(92)90511-r. [DOI] [PubMed] [Google Scholar]
- 223.Evers BM, et al. Characterization of functional neurotensin receptors on human lymphocytes. Surgery. 1994;116(2):134–139. discussion 139–140. [PubMed] [Google Scholar]
- 224.Hartschuh W, Weihe E, Reinecke M. Peptidergic (neurotensin, VIP, substance P) nerve fibres in the skin. Immunohistochemical evidence of an involvement of neuropeptides in nociception, pruritus and inflammation. Br J Dermatol. 1983;109(Suppl 25):14–17. doi: 10.1111/j.1365-2133.1983.tb06811.x. [DOI] [PubMed] [Google Scholar]
- 225.Donelan J, et al. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci U S A. 2006;103(20):7759–7764. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhao D, et al. Neurotensin stimulates interleukin-8 expression through modulation of I kappa B alpha phosphorylation and p65 transcriptional activity: involvement of protein kinase C alpha. Mol Pharmacol. 2005;67(6):2025–2031. doi: 10.1124/mol.104.010801. [DOI] [PubMed] [Google Scholar]
- 227.Zhao D, et al. Neurotensin stimulates IL-8 expression in human colonic epithelial cells through Rho GTPase-mediated NF-kappa B pathways. Am J Physiol Cell Physiol. 2003;284(6):C1397–C1404. doi: 10.1152/ajpcell.00328.2002. [DOI] [PubMed] [Google Scholar]
- 228.Brun P, et al. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288(4):G621–G629. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 229.Martin S, Vincent JP, Mazella J. Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia. J Neurosci. 2003;23(4):1198–1205. doi: 10.1523/JNEUROSCI.23-04-01198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dinh TL, Veves A. Treatment of diabetic ulcers. Dermatol Ther. 2006;19(6):348–355. doi: 10.1111/j.1529-8019.2006.00093.x. [DOI] [PubMed] [Google Scholar]
- 231.Steed DL, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14(6):680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 232.Leena Pradhan NDA, Christoph Nabzdyk, Frank W LoGerfo, Aristidis Veves. Wound Healing Abnormalities in Diabetes and New Therapeutic Interventions. U. S. Endocrine Disease. 2007;2:68–72. [Google Scholar]
- 233.Denet AR, Vanbever R, Preat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56(5):659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 234.Vanbever R, Preat VV. In vivo efficacy and safety of skin electroporation. Adv Drug Deliv Rev. 1999;35(1):77–88. doi: 10.1016/s0169-409x(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 235.Lee PY, Chesnoy S, Huang L. Electroporatic delivery of TGF-beta1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Invest Dermatol. 2004;123(4):791–798. doi: 10.1111/j.0022-202X.2004.23309.x. [DOI] [PubMed] [Google Scholar]
- 236.Lin MP, et al. Delivery of plasmid DNA expression vector for keratinocyte growth factor-1 using electroporation to improve cutaneous wound healing in a septic rat model. Wound Repair Regen. 2006;14(5):618–624. doi: 10.1111/j.1743-6109.2006.00169.x. [DOI] [PubMed] [Google Scholar]