Abstract
Background
Using 1998-2005 SEER-Medicare data, we examined the effect of diagnostic and treatment delays on all-cause and colorectal cancer (CRC)-specific death among U.S. adults aged ≥66 years with invasive colon or rectal cancer. We hypothesized that longer delays would be associated with a greater risk of death.
Methods
We defined diagnostic and treatment delays, respectively, as days between 1) initial medical consult for CRC symptoms and pathologically-confirmed diagnosis (maximum: 365 days) and 2) pathologically-confirmed diagnosis and treatment (maximum: 120 days). Cases (CRC deaths) and controls (deaths due to other causes or censored) were matched on survival time. Logistic regression analyses adjusted for sociodemographic, tumor, and treatment factors.
Results
Median diagnostic delays were 60 (colon) and 40 (rectal) days and treatment delays were 13 (colon) and 16 (rectal) days in 10,663 patients. Colon cancer patients with the longest diagnostic delays (8-12 months vs. 14-59 days) had higher odds of all-cause (aOR: 1.31 CI: 1.08-1.58) but not CRC-specific death. Colon cancer patients with the shortest treatment delays (<1 vs. 1-2 weeks) had higher odds of all-cause (aOR: 1.23 CI: 1.01-1.49) but not CRC-specific death. Among rectal cancer patients, delays were not associated with risk of all-cause or CRC-specific death.
Conclusions
Longer delays of up to 1 year after symptom onset and 120 days for treatment did not increase odds of CRC-specific death. There may be little clinical benefit in detecting and treating existing symptomatic disease earlier. Screening prior to symptom onset must remain the primary goal to reduce CRC incidence, morbidity, and mortality.
Keywords: colorectal cancer, delayed diagnosis, time factors, outcomes, survival, SEER-Medicare
INTRODUCTION
Colorectal cancer (CRC) accounted for an estimated 143,460 new cases of cancer in 2012 and is the second leading cause of cancer deaths in the U.S.[1] Early case finding of cancers prior to symptom onset using CRC screening results in dramatically improved survival.[2,3] However, while recommended for healthy asymptomatic adults aged 50 and over, only two-thirds (65.4%) of eligible adults met screening guidelines in 2010.[4] As a result, many CRC patients are not being tested or diagnosed until they experience symptoms.
Stage at diagnosis is the single most predictive factor for CRC survival.[1] By diagnosing and treating CRC cancers at earlier and less advanced stages, timely diagnosis and treatment of CRC may improve survival and other outcomes. However, the effect of diagnostic delays (time from medical consultation for CRC symptoms to diagnosis) and treatment delays (time from diagnosis to treatment initiation) on disease progression or mortality is uncertain.[5] In a systematic review, 20 of 26 studies on cancer delays showed no association between diagnostic or treatment delays and survival, while 4 showed that longer delay was associated with better prognosis and 2 showed an inverse association with worse prognosis.[6] In a companion meta-analysis, no statistically significant association was found between diagnostic and treatment delays and disease stage when considering colon and rectal cancers collectively. Analyzed separately, longer delays were associated with later stages for rectal cancer, but earlier stages for colon cancer.[7] More recently published studies demonstrated either no association of longer delays with stage or all-cause survival,[8,9] U-shaped relationships with higher all-cause mortality among patients with the shortest and the longest delays,[10,11] or differing results for colon and rectal cancer.[12,13] The mixed findings in this literature have been attributed in part to method limitations, including analyzing colon and rectal cancers together, having small, under-powered samples, assumptions of a monotonic association,[10] or failing to control for the confounding factor of tumor grade.[6,7,5,10]
The vast majority of existing research on this topic is based on European samples. [6,7,10] However, recent U.S. studies have examined some components of delay, such as the time between surgical consultation and surgery, or between referral for endoscopy and diagnosis, and found little evidence for an effect on outcomes.[14,15] To date, however, no population-based U.S. study has described the overall length of either diagnostic or treatment delays or explored the effect of these delays on risk of death.
Because the effect of delays on risk of death remains uncertain, and the wait times for cancer surgery have increased over 20% in the last decade in the U.S. and are projected to increase [16], the effect of timely cancer care deserves greater study. To address this critical knowledge gap, we examined, separately, the effect of the length of both diagnostic and treatment delays on the risk of death from colorectal cancer and from all causes in a population-based cohort of older U.S. adults with colon or rectal cancer using the linked Surveillance Epidemiology and End Results (SEER)-Medicare data. We hypothesized that longer delays would be associated with a greater risk of death.
METHODS
Data Sources
Data were obtained from an existing linkage of the 1998-2005 National Cancer Institute’s SEER program data with 1997-2006 Medicare claims files from the Centers for Medicare and Medicaid. As detailed elsewhere,[17] linked SEER-Medicare data provide a rich source of information on Medicare patients included in SEER, a nationally representative collection of population-based cancer registries. Ninety-four percent of cancer patients reported to SEER aged 65 years or older have been successfully linked with Medicare data.[17] Data for this study were available from 12 registries representing approximately 14% of the U.S. population,[18] including states (Connecticut, Hawaii, Iowa, New Mexico, and Utah), metropolitan areas (Atlanta, Detroit, Los Angeles, San Francisco-Oakland, San Jose-Monterey, and Seattle), and rural Georgia. This study was reviewed by the Institutional Review Board at Washington University and determined to be exempt.
Study Population
We selected all male and female patients aged ≥66 with a diagnosis of a first primary invasive colon or rectal cancer occurring from 1998 through 2005 who had full coverage by both Medicare Part A and Part B during this period. We excluded patients with in situ cancer because they may experience different symptoms and the urgency of their treatment differs from those with invasive disease. We excluded patients who had only autopsy or death certificate records, who were members of HMOs, or who had either un-staged or appendix cancer. We included only those aged ≥66 to allow for one-year of complete claims data prior to diagnosis to determine comorbidity.
We excluded patients with preexisting comorbid conditions (n= 2,540) of the gastrointestinal tract because they may experience shorter or longer delays in reporting or recognizing symptoms, obtaining appointments, or receiving endoscopy or a cancer diagnosis. Using International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes (see Supplementary Table 1), we excluded patients with ≥1 inpatient claims or ≥2 physician or outpatient claims occurring ≥30 days apart occurring any time in the year prior to diagnosis for any of the following: inflammatory bowel disease, ulcerative colitis, unspecified colitis, Crohn’s Disease, irritable bowel syndrome, diarrhea, diverticulitis, colon/anal/rectal polyps, personal history of malignant neoplasm of the lower intestinal tract, current benign neoplasm of lower intestinal tract, or family history of malignant neoplasm of gastrointestinal tract.
We excluded patients presenting for emergent procedures (n=5,123) in order to limit the potential for bias in which patients presenting with emergencies have the shortest delays and, given more advanced disease, might also exhibit higher mortality.[5] Patients with intestinal obstruction or perforation (ICD-9: 560.30, 560.89, 560.9, 569.83) or with an emergency room visit or admission, indicated using an algorithm described elsewhere, [19] within a week of either diagnosis or surgery were excluded.
Diagnostic and treatment delays were analyzed separately. Patients without identified CRC-related clinical manifestations or symptoms (n=994) in the year prior to diagnosis (presumably due to preventive screening) were excluded from the diagnostic delay analysis. Patients who did not receive any treatment in the 120 days after diagnosis (n=979) were excluded from the treatment delay analysis.
Study Design
In assessing the relationship between delays and a given outcome, biases and other errors may result when follow-up of groups does not begin at comparable time points in the natural history of a disease.[5,20-23] For example, patients’ delays in reporting symptoms, especially when disease is advanced or rapidly progressing, may prompt a provider to hasten diagnosis and treatment, but may nonetheless result in a worse outcome. Conversely, early detection through screening or early reporting of symptoms may result in longer provider delays but may nonetheless result in a more favorable outcome.
We conducted a matched case-control study rather than a survival analysis in order to avoid these potential errors, and because preliminary analyses indicated that our data did not meet the proportional hazard assumption. For assessing the CRC-specific risk of death, cases were CRC patients who died of either colorectal cancer during the study period and controls were CRC patients who died from other causes or who were alive at the end of follow-up (December 31, 2006). For the analysis of all-cause risk of death, cases included patients who died of any cause during the study period and controls were alive at the end of follow-up. Controls were matched to cases on survival time. Matching on survival time allowed cases and controls to have equal opportunity to experience clinical, treatment, and outcome events. Survival time was measured from the date of diagnosis to death/censoring date for diagnostic delay analyses and, to overcome immortal time bias, from the date of first treatment for treatment delay analyses. Because treatment delay patients who are alive are “immortal” between diagnosis and initial treatment, measuring survival from the time of diagnosis for these patients could result in an artificial survival advantage for those with longer treatment delays.[24-27]
Study Variables
We used SEER data to assess 2 outcomes: all-cause and CRC-specific (“colon excluding rectum” or “rectum and rectosigmoid junction”) risk of death. SEER cause of death data are highly valid and recent studies documented 85%-95% accuracy in studies of colorectal cancer.[28,29] Date of death was assessed using Medicare claims because only month and year of death are available in SEER data.
Delay
We examined diagnostic and treatment delays separately because they represent different factors that are more or less modifiable occurring across the cancer control continuum (detection of symptoms, presentation to provider, diagnostic and pathology work-up, diagnosis, treatment referral, treatment initiation, etc.) For example, long treatment delays may indicate limited surgical capacity whereas long diagnostic delays may indicate discontinuity of care during the transition between primary and specialty care.
In preliminary analyses, we confirmed previous reports [5,30,10] that suggested the association between delay and death is not linear and individuals with the shortest delays can have a higher risk of death. Therefore we categorized delay and selected a referent group that did not reflect the shortest length of delay. Because quartiles or quintiles would be model-and data-specific and less clinically meaningful, we categorized delays using more meaningful periods of time as measured in weeks or months.
We defined diagnostic delay as the period in days between initial consultation for a CRC-related clinical manifestation or symptom and pathologically-confirmed diagnosis (maximum of 365 days) as follows: <14 days, 14-59 days (referent), 2-4 months, 4-8 months, and ≥8 months. Treatment delay was defined as the period in days between pathologically-confirmed CRC diagnosis and date of first treatment (maximum of 120 days) as follows: <1 week, 1-2 weeks (referent), 2-4 weeks, and ≥4 weeks. While there are no common standards in the U.S. nor clinically recommended intervals for categorizing delay, these categorizations do reflect international standards that recommend treatment within either 2 weeks or one month after diagnosis.[31] In preliminary and sensitivity analyses we considered other categorizations. Figure 1 demonstrates the definitions of diagnostic and treatment delays.
Figure 1.
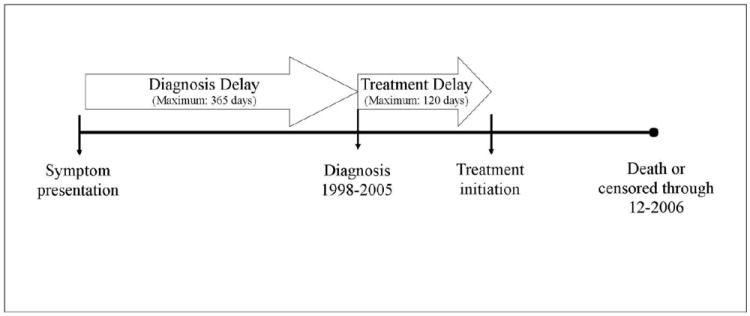
Diagnosis and Treatment Delays between Symptom Presentation and Treatment Initiation
The date of initial consultation was identified as the initial date on which a patient had a medical appointment for at least one clinical manifestation of CRC or CRC-related symptom within the year prior to the pathologically-confirmed CRC diagnosis. We searched outpatient, inpatient, and carrier claims for conditions relevant to the clinical signs of CRC. A comprehensive list of these conditions was initially developed using symptoms previously identified in systematic reviews of CRC symptoms[32,33] and in a validated algorithm designed to distinguish preventive screening from diagnostic colonoscopy,[34,35] and was subsequently adapted for our study with the consultation of two practicing gastroenterologists and two certified medical coders. The final list includes 41 ICD-9 codes grouped into 13 clinically distinct categories (see Supplementary Table 2).
We defined the date of diagnosis as the date of pathologically-confirmed cancer using the following procedure. We searched Medicare carrier claims for the first notation of a tissue exam by a pathologist of a colorectal biopsy (Healthcare Common Procedure Coding System [HCPCS][36] code 88305) with an associated diagnosis (line item diagnosis) assigned by the pathologist of invasive malignant CRC (ICD-9 codes 153.0-153.4; 153.6-154.8 or 209.12-209.17) occurring when either the claim first or last “line expense date” fell within a 60 day window (± 30 days) surrounding the SEER diagnosis date. These dates represent the first and last days on the billing statement covering services rendered to the beneficiary. Of patients otherwise eligible, we captured a CRC-positive pathology report for 61.8%. We used this method in lieu of the SEER diagnosis date in order to increase the precision of the diagnosis date; SEER diagnosis date is defined only as the month and year (no exact date is provided) in which the first diagnosis of cancer is made by a medical practitioner. We hypothesized that treatment may be delayed until a diagnosis is pathologically-confirmed.
We defined the treatment date as the date of the first of any type of CRC treatment (including definitive surgery [colectomy/proctectomy or pelvic exenteration], chemotherapy, or radiotherapy) by searching inpatient, outpatient, and carrier claims using previously identified HCPCS and/or ICD-9 codes.[37] To avoid capturing palliative procedures, which may be provided after longer delays, we included only those procedures occurring within the 4 months after diagnosis in the analysis. The length of treatment delay was calculated as the number of days between the date of pathologically-confirmed diagnosis and the first date of any treatment.
To calculate all symptom, diagnosis, and treatment dates, we used the line first expense date (the exact date on which a procedure was performed) that was associated with the relevant procedure when available. If not available, we used the claim “from” and “through” dates to define a single date. For the vast majority of claims for which the “from” date and “through” date matched (~94%), that date was used. To avoid excluding all inpatient or “bundled” claims, we included claims where the two dates represented spans of 1-6 days (~4%), and defined the midpoint of that span as the date of interest. However, to ensure adequate precision, we excluded patients (~2%) with relevant claims with spans ≥7 days.
Covariates
Multiple covariates, selected based on previous literature,[38,39] were examined. Covariates obtained from SEER data included: year of diagnosis, SEER registry, age (66-69, 70-74, 75-79, 80-84, ≥85), race/ethnicity (non-Hispanic white, non-Hispanic black, other, unknown), gender, marital status (married, unmarried, unknown), SEER historic stage (localized, regional, distant), tumor location (colon: proximal colon [cecum, ascending], transverse colon [hepatic flexure, transverse colon, splenic flexure], distal colon [descending and sigmoid colon]; rectum: rectosigmoid junction or rectum), histology (mucinous adenocarcinoma/signet ring cell, other adenocarcinoma, other, unknown), and tumor grade (low [well/moderately differentiated] or high [poorly differentiated/undifferentiated/anaplastic] or unknown).
Covariates obtained from Medicare claims included: treatment (surgery alone, surgery with neo/adjuvant chemotherapy or radiotherapy, adjuvant chemotherapy and/or radiotherapy only, and no treatment), number of endoscopies in the year prior to diagnosis, comorbidity, preventable hospitalizations, and eligibility for both Medicare and Medicaid (dual eligibility). Claims were searched for HCPCS codes indicating surgical resection, chemotherapy, or radiotherapy occurring in the 4-month period following diagnosis using codes reported elsewhere.[37] Following accepted practice,[40] we measured the total number of endoscopic procedures (both colonoscopies and sigmoidoscopies) in the year prior to diagnosis. To measure comorbidity, we searched inpatient or carrier claims for chronic conditions (e.g. myocardial infarction, diabetes, dementia) occurring between 1 and 12 months prior to diagnosis using the Klabunde adaptation of the Charlson comorbidity index.[41,42] We classified comorbidity as none, one, or two or more. Preventable hospitalizations identify poor ambulatory health care outcomes and can represent a breakdown in access to or the processes of primary care. Following methods described elsewhere,[43] we searched inpatient claims for the year prior to diagnosis for several potentially preventable hospitalizations, including asthma, hypertension, pneumonia, and compared those with one or more to those with none. Finally, dual-eligibility was defined as eligibility for Medicaid coverage for at least 1 month during the year before diagnosis.
Covariates at the census-tract level of the patient’s residence were obtained from the 2000 census and included: urban/rural status (metropolitan, micropolitan, or rural using Rural Urban Continuum Area codes), and the percent of population living in poverty (<9.9%, 10-19.9%, or ≥20%).
Statistical Analysis
We described patient characteristics by cancer type and case/control status using counts and proportions. Diagnostic and treatment delays were described using median and interquartile range along with counts and proportions.
Cases and controls were matched based on survival time and the association of delay with death was examined using logistic regression. We used Coarsened Exact Matching (CEM) to match cases and controls based on “coarsened” categories of survival time, which were strata of 1-month intervals. CEM performs exact matching by sorting observations into strata of survival time. Any strata that do not contain both ≥1 case and ≥1 control are discarded. For each observation, the proportion of cases to controls in the strata is used to create a CEM weight, which is used in all further analyses. Weights are based on the number of matched control observations per each case. The CEM method eliminates extreme values, restricting the matched data to areas of common empirical support and creating a counterfactual within the strata. The CEM approach offers advantages over other traditional 1:n matching methods, for example, that require a specific number of control subjects per each case.[44,45]
After matching, we calculated unadjusted (OR) and adjusted odds ratios (AOR) between categories of diagnostic and treatment delay and the two death outcomes (CRC-specific and all-other causes) using weighted logistic regression on the matched cases and controls. With the exception of age, gender, and race, which were retained in all models, covariates significant in bivariable analysis (p<.05) were entered into an initial multivariable logistic regression model and backward elimination based on the likelihood ratio test was used to trim the model. Given that stage may be a mediating factor between diagnostic delay and death and because treatment regimens are largely driven by stage, we stratified all models by stage, as suggested by others.[12]
To confirm the validity of our results, multiple sensitivity analyses were conducted. To check the effect of any misclassification due to our calculation of derived diagnosis and treatment dates, we re-analyzed all models using the midpoint of the month of the SEER diagnosis and SEER treatment dates. Next, we compared our results after matching cases and controls based on stage at diagnosis in addition to survival time as well as matching on survival time in 2-week intervals. Because there are no cutpoints that are universally accepted as clinically meaningful when measuring delays, we also re-analyzed the data using different cutpoints and categorizations (e.g. quintiles and using different referent categories). Notably, while multiple studies have examined the effect of treatment delays in excess of 3 months[6,7] very few (<1%) in our sample had such long delays and we were unable to examine such long treatment delay intervals. Next, we examined treatment delays separately by type of first treatment (surgery vs. neoadjuvant therapies) as well as separately for those who only had surgery. Finally, because symptoms may reflect the location or aggressiveness of a tumor and may influence the length of delay, we also stratified diagnostic delay models by the 4 most common presenting symptom types. All analyses were conducted using STATA 11.0 (College Station, TX).
RESULTS
Study Sample
Of all 10,663 eligible patients, 7,346 were diagnosed with colon and 3,317 with rectal cancer. Among colon cancer patients, 2,974 died; of these, 1,661 (55.9%) died of colorectal cancer. Among rectal cancer patients, 1,448 died; of these, 900 (62.2%) died of colorectal cancer. Because not all patients had a claim for a symptom of CRC in the year prior to their diagnosis, 6,702 (91.2%) of colon and 2,967 (89.4%) of rectal cancer patients were included in the diagnostic delay sample. Because not all patients had a cancer treatment claim, 6,698 (91.2%) and 2,986 (90.0%) of colon and rectal patients, respectively, were included in the treatment delay sample. Median follow-up after diagnosis was 29.9 months for both colon and rectal cancer patients. Characteristics of the study sample by cancer type and case/control status are provided in Tables 1 and 2.
Table 1.
Selected Characteristics of Colon Cancer Patients Included in Diagnosis Delay and Treatment Delay Samples by Case/Control Status (n=7346)
| Diagnostic Delay Sample n=6702 | Treatment Delay Sample n=6698 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Casesa n=2811 | Controls n=3891 | Casesa n=2634 | Controls n=4064 | |||||
|
| ||||||||
| n | % | n | % | n | % | N | % | |
| Colorectal cancer-specificdeath | 1576 | 56.1 | - | - | 1481 | 56.2 | - | - |
| Gender | ||||||||
| Male | 1229 | 43.7 | 1481 | 38.1 | 1168 | 44.3 | 1583 | 39.0 |
| Female | 1582 | 56.3 | 2410 | 61.9 | 1466 | 55.7 | 2481 | 61.1 |
| Age | ||||||||
| 66-69 | 287 | 10.2 | 665 | 17.1 | 287 | 10.9 | 743 | 18.3 |
| 70-74 | 513 | 18.3 | 913 | 23.5 | 508 | 19.3 | 963 | 23.7 |
| 75-79 | 641 | 22.8 | 1076 | 27.7 | 618 | 23.5 | 1142 | 28.1 |
| 80-84 | 689 | 24.5 | 795 | 20.4 | 631 | 24.0 | 807 | 19.9 |
| ≥85 | 681 | 24.2 | 442 | 11.4 | 590 | 22.4 | 409 | 10.1 |
| Race/ethnicity | ||||||||
| NH white | 2410 | 85.7 | 3352 | 86.2 | 2272 | 86.3 | 3521 | 86.6 |
| NH black | 199 | 7.1 | 212 | 5.5 | 176 | 6.7 | 218 | 5.4 |
| Other | 202 | 7.2 | 327 | 8.4 | 186 | 7.1 | 325 | 8.0 |
| Marital status | ||||||||
| Married | 1355 | 48.2 | 2172 | 55.8 | 1320 | 50.1 | 2327 | 57.3 |
| Unmarried | 1375 | 48.9 | 1604 | 41.2 | 1252 | 47.5 | 1631 | 40.1 |
| Unknown | 81 | 2.9 | 115 | 3.0 | 62 | 2.4 | 106 | 2.6 |
| Cancer stage | ||||||||
| Localized | 842 | 30.0 | 2052 | 52.7 | 731 | 27.8 | 2110 | 51.9 |
| Regional | 1091 | 38.8 | 1668 | 42.9 | 1106 | 42.0 | 1785 | 43.9 |
| Distant | 878 | 31.2 | 171 | 4.4 | 797 | 30.3 | 169 | 4.2 |
| Histology | ||||||||
| Mucinous adenocarcinoma/signet | 2350 | 83.6 | 3391 | 87.2 | 2184 | 82.9 | 3539 | 87.1 |
| Other adenocarcinoma | 421 | 15.0 | 471 | 12.1 | 418 | 15.9 | 495 | 12.2 |
| Other/unknown | 40 | 1.4 | 29 | 0.8 | 32 | 1.2 | 30 | 0.7 |
| Tumor grade | ||||||||
| Low | 1852 | 65.9 | 2931 | 75.3 | 1755 | 66.6 | 3101 | 76.3 |
| High | 805 | 28.6 | 766 | 19.7 | 781 | 29.7 | 801 | 19.7 |
| Unknown | 154 | 5.5 | 194 | 5.0 | 98 | 3.7 | 162 | 4.0 |
| Tumor location | ||||||||
| Proximal | 1368 | 48.7 | 1817 | 46.7 | 1291 | 49.0 | 1926 | 47.4 |
| Transverse | 518 | 18.4 | 675 | 17.4 | 507 | 19.3 | 747 | 18.4 |
| Distal | 925 | 32.9 | 1399 | 36.0 | 836 | 31.7 | 1391 | 34.2 |
| Treatment | ||||||||
| Surgery only | 1561 | 55.5 | 2500 | 64.3 | 1646 | 62.5 | 2836 | 69.8 |
| Surgery + adjuvant | 842 | 30.0 | 1088 | 28.0 | 882 | 33.5 | 1188 | 29.2 |
| Adjuvant only | 109 | 3.9 | 44 | 1.1 | 106 | 4.0 | 40 | 1.0 |
| No treatment | 299 | 10.6 | 259 | 6.7 | - | - | - | - |
| Comorbidity | ||||||||
| 0 | 1499 | 53.3 | 2525 | 64.9 | 1458 | 55.4 | 2704 | 66.5 |
| 1 | 701 | 24.9 | 902 | 23.2 | 636 | 24.2 | 892 | 22.0 |
| ≥2 | 539 | 19.2 | 394 | 10.1 | 472 | 17.9 | 390 | 9.6 |
| Unknown | 72 | 2.6 | 70 | 1.8 | 68 | 2.6 | 78 | 1.9 |
| Preventable hospitalizations | ||||||||
| ≥1 vs. none | 189 | 6.7 | 94 | 2.4 | 154 | 5.9 | 81 | 2.0 |
| Low income (Medicaid) | ||||||||
| Yes vs. no | 446 | 15.9 | 428 | 11.0 | 387 | 14.7 | 409 | 10.1 |
| Urban/rural status | ||||||||
| Metropolitan | 2306 | 82.0 | 3168 | 81.4 | 2166 | 82.2 | 3308 | 81.4 |
| Micropolitan | 178 | 6.3 | 252 | 6.5 | 163 | 6.2 | 259 | 6.4 |
| Rural | 327 | 11.6 | 471 | 12.1 | 305 | 11.6 | 497 | 12.2 |
| Poverty | ||||||||
| ≤9.9% | 1862 | 66.2 | 2710 | 69.7 | 1771 | 67.2 | 2852 | 70.2 |
| 10-19.9% | 653 | 23.2 | 872 | 22.4 | 599 | 22.7 | 892 | 22.0 |
| ≥20% | 296 | 10.5 | 308 | 7.9 | 264 | 10.0 | 319 | 7.9 |
All-cause death cases.
Table 2.
Selected Characteristics of Rectal Cancer Patients Included in Diagnostic Delay and Treatment Delay Samples by Case/Control Status (n=3317)
| Diagnostic Delay Sample n=2967 | Treatment Delay Sample n=2986 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Casesa n=1318 | Controls n=1649 | Casesa n=1277 | Controls n=1709 | |||||
|
| ||||||||
| n | % | n | % | n | % | n | % | |
| Colorectal cancer-specific death | 811 | 61.5 | - | - | 795 | 62.3 | - | - |
| Gender | ||||||||
| Male | 660 | 50.1 | 814 | 49.4 | 649 | 50.8 | 844 | 49.4 |
| Female | 658 | 49.9 | 835 | 50.6 | 628 | 49.2 | 865 | 50.6 |
| Age | ||||||||
| 66-69 | 148 | 11.2 | 380 | 23.0 | 162 | 12.7 | 405 | 23.7 |
| 70-74 | 313 | 23.8 | 512 | 31.1 | 316 | 24.8 | 528 | 30.9 |
| 75-79 | 295 | 22.4 | 388 | 23.5 | 286 | 22.4 | 406 | 23.8 |
| 80-84 | 297 | 22.5 | 250 | 15.2 | 291 | 22.8 | 253 | 14.8 |
| ≥85 | 265 | 20.1 | 119 | 7.2 | 222 | 17.4 | 117 | 6.9 |
| Race/ethnicity | ||||||||
| NH white | 1141 | 86.6 | 1414 | 85.8 | 1112 | 87.1 | 1481 | 86.7 |
| NH black | 63 | 4.8 | 59 | 3.6 | 58 | 4.5 | 52 | 3.0 |
| Other | 114 | 8.7 | 176 | 10.7 | 107 | 8.4 | 176 | 10.3 |
| Marital status | ||||||||
| Married | 645 | 48.9 | 1009 | 61.2 | 656 | 51.4 | 1060 | 62.0 |
| Unmarried | 636 | 48.3 | 616 | 37.4 | 591 | 46.3 | 625 | 36.6 |
| Unknown | 37 | 2.8 | 24 | 1.5 | 30 | 2.4 | 24 | 1.4 |
| Cancer stage | ||||||||
| Localized | 456 | 34.6 | 969 | 58.8 | 410 | 32.1 | 968 | 56.6 |
| Regional | 517 | 39.2 | 631 | 38.3 | 544 | 42.6 | 688 | 40.3 |
| Distant | 345 | 26.2 | 49 | 3.0 | 323 | 25.3 | 53 | 3.1 |
| Histology | ||||||||
| Mucinous adenocarcinoma/signet | 1181 | 89.6 | 1512 | 91.7 | 1146 | 89.7 | 1576 | 92.2 |
| Other adenocarcinoma | 101 | 7.7 | 92 | 5.6 | 102 | 8.0 | 100 | 5.9 |
| Other/unknown | 36 | 2.7 | 45 | 2.7 | 29 | 2.3 | 33 | 1.9 |
| Tumor grade | ||||||||
| Low | 933 | 70.8 | 1275 | 77.3 | 906 | 71.0 | 1340 | 78.4 |
| High | 262 | 19.9 | 209 | 12.7 | 266 | 20.8 | 230 | 13.5 |
| Unknown | 123 | 9.3 | 165 | 10.0 | 105 | 8.2 | 139 | 8.1 |
| Treatment | ||||||||
| Surgery only | 506 | 38.4 | 702 | 42.6 | 545 | 42.7 | 802 | 46.9 |
| Surgery + adjuvant | 404 | 30.7 | 626 | 38.0 | 440 | 34.5 | 692 | 40.5 |
| Adjuvant only | 278 | 21.1 | 203 | 12.3 | 292 | 22.9 | 215 | 12.6 |
| No treatment | 130 | 9.9 | 118 | 7.2 | - | - | - | - |
| Comorbidity | ||||||||
| 0 | 768 | 58.3 | 1125 | 68.2 | 749 | 58.7 | 1179 | 69.0 |
| 1 | 285 | 21.6 | 332 | 20.1 | 282 | 22.1 | 334 | 19.5 |
| ≥2 | 189 | 14.3 | 122 | 7.4 | 168 | 13.2 | 123 | 7.2 |
| Unknown | 76 | 5.8 | 70 | 4.2 | 78 | 6.1 | 73 | 4.3 |
| Preventable hospitalizations | ||||||||
| ≥1 vs. none | 60 | 4.6 | 37 | 2.2 | 33 | 1.9 | 50 | 3.9 |
| Low income (Medicaid) | ||||||||
| Yes vs. no | 203 | 15.4 | 191 | 11.6 | 184 | 10.8 | 181 | 14.2 |
| Urban/rural status | ||||||||
| Metropolitan | 1076 | 81.6 | 1302 | 79.0 | 1050 | 82.2 | 1337 | 78.2 |
| Micropolitan | 77 | 5.8 | 117 | 7.1 | 67 | 5.3 | 125 | 7.3 |
| Rural | 165 | 12.5 | 230 | 14.0 | 160 | 12.5 | 247 | 14.5 |
| Poverty | ||||||||
| 10-19.9% | 886 | 67.2 | 1128 | 68.4 | 858 | 67.2 | 1173 | 68.6 |
| ≥20% | 297 | 22.5 | 365 | 22.1 | 292 | 22.9 | 384 | 22.5 |
| ≤9.9% | 135 | 10.2 | 156 | 9.5 | 127 | 10.0 | 152 | 8.9 |
All-cause death cases.
Presenting Clinical Manifestations and Symptoms
Patients presented for medical care regarding multiple symptoms and clinical manifestations in the year prior to their CRC diagnosis. For both cancer types, the four most common included anemia, rectal bleeding or rectal/GI hemorrhage, abdominal pain, and fatigue (Table 3). Anemia was the most commonly diagnosed clinical manifestation among colon cancer patients at their first medical consultation for symptoms (33.7%) and at any time (52.0%). For rectal cancer patients, rectal bleeding or rectal/GI tract hemorrhage was the most commonly diagnosed symptom at the first medical consultation (41.6%) and at any time (66.1%). The length of delays varied by the first presenting symptom (Table 3).
Table 3.
Presenting Clinical Manifestations and Symptoms Suggestive of Colorectal Cancer in the Year Prior to CRC Diagnosis.
| Type of Symptom Diagnosed during First Medical Consultationa | Ever Visited Physician for Symptoma | Length of Diagnostic Delayb | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Colon n (%) | Rectum n (%) | Colon n (%) | Rectum n (%) | Colon Median (IQ) | Rectum Median (IQ) | |
|
| ||||||
| Abdominal pain | 1421 (19.3) | 432 (13.0) | 2470 (33.6) | 763 (23.0) | 40 (IQ: 14-158) | 36 (IQ: 13-143) |
| Abdominal pain, swelling, or mass | 125 (1.7) | 32 (1.0) | 525 (7.2) | 127 (3.8) | 13 (IQ: 6-30) | 15 (IQ: 6-31) |
| Abnormal stool | 343 (4.7) | 127 (3.8) | 1142 (15.6) | 351 (10.6) | 17 (IQ: 7-36) | 19 (IQ: 8-40) |
| Anal/rectal pain, anal spasm | 25 (0.3) | 31 (0.9) | 55 (0.8) | 57 (1.7) | 34 (IQ: 15-109) | 12 (IQ: 6-28) |
| Anemia | 2472 (33.7) | 602 (18.2) | 3817 (52.0) | 903 (27.2) | 49 (IQ: 15-200) | 63 (IQ: 15-230) |
| Anorexia or unexplained weight loss | 336 (4.6) | 168 (5.1) | 759 (10.3) | 271 (8.2) | 28 (IQ: 9-85) | 21 (IQ: 9-66) |
| Constipation | 339 (4.6) | 140 (4.2) | 720 (9.8) | 279 (8.4) | 40 (IQ: 15-142) | 29 (IQ: 10-76) |
| Fatigue | 1152 (15.7) | 387 (11.7) | 1737 (23.7) | 535 (16.1) | 94 (IQ: 28-235) | 119 (IQ: 34-233) |
| Flatulence, eructation, and gas pain | 42 (0.6) | 22 (0.7) | 139 (1.9) | 58 (1.8) | 27 (IQ: 10-87) | 27.5 (IQ: 12-132) |
| Observation for suspected neoplasm | 21 (0.3) | 18 (0.5) | 54 (0.7) | 28 (0.8) | 35.5 (IQ: 7-211) | 60.5 (IQ: 4.5-279) |
| Other GI symptoms, other bowel changes | 117 (1.6) | 193 (5.8) | 359 (4.9) | 495 (14.9) | 14 (IQ: 7-34) | 10 (IQ: 5-21) |
| Rectal bleeding or rectal/GI tract hemorrhage | 1520 (20.7) | 1380 (41.6) | 3392 (46.2) | 2193 (66.1) | 19 (IQ: 7-48) | 21 (IQ: 7-46) |
| Vomiting or nausea | 185 (2.5) | 50 (1.5) | 417 (5.7) | 99 (3.0) | 80 (IQ: 20-206) | 123 (IQ: 30-253) |
Percents will add to >100% because individuals may have presented with >1 symptom type during consultations for CRC symptoms.
Calculated from the date of the first symptom of that type until diagnosis. IQ: interquartile range.
Diagnostic Delays
Median diagnostic delays were 60 (colon) and 40 (rectal) days (Table 4). In all, 23.6% of colon cancer patients and 18.1% of rectal cancer patients had a diagnostic delay of ≥8 months. Median diagnostic delays differed by stage; among colon cancer patients, the longest delays occurred among patients with localized stage (median: 68 days) as compared to those with distant stage (median: 47 days). The same was true for rectal cancer patients where the longest delays occurred among patients with localized disease (median: 43 days) as compared to those with distant stage (median: 29 days).
Table 4.
Length of Diagnostic and Treatment Delays in Days by Cancer Type and Stage
| Localized n (%) | Regional n (%) | Distant n (%) | Total n (%) | |
|---|---|---|---|---|
| Colon Cancer (n=7346) | ||||
|
| ||||
| Diagnostic Delaya | ||||
| <2 weeks | 504 (17.4) | 506 (18.3) | 229 (21.8) | 1239 (18.5) |
| 14-59 days | 877 (30.3) | 877 (31.8) | 343 (32.7) | 2097 (31.3) |
| 2-4 months | 364 (12.6) | 302 (11.0) | 129 (12.3) | 795 (11.9) |
| 4-8 months | 441 (15.2) | 415 (15.0) | 135 (12.9) | 991 (14.8) |
| ≥8 months | 708 (24.5) | 659 (23.9) | 213 (20.3) | 1580 (23.6) |
| Median: 68 (IQ: 21-237) | Median: 59 (IQ: 19-231) | Median: 47 (IQ: 15-193) | Median: 60 (IQ: 19-230) | |
| Treatment Delayb | ||||
| <1 week | 776 (27.3) | 1043 (36.1) | 420 (43.5) | 2239 (33.4) |
| 1-2 weeks | 512 (18.0) | 543 (18.8) | 199 (20.6) | 1254 (18.7) |
| 2-4 weeks | 1023 (36.0) | 918 (31.8) | 247 (25.6) | 2188 (32.7) |
| ≥4 weeks | 530 (18.7) | 387 (13.4) | 100 (10.4) | 1017 (15.2) |
| Median: 15 (IQ: 6-26) | Median: 12 (IQ: 2-21) | Median: 8 (IQ: 2-19) | Median: 13 (IQ: 3-23) | |
|
| ||||
| Rectal Cancer (n=3317) | ||||
|
| ||||
| Diagnostic Delay | ||||
| <2 weeks | 304 (21.3) | 304 (26.5) | 123 (31.2) | 731 (24.6) |
| 14-59 days | 499 (35.0) | 351 (30.6) | 138 (35.0) | 988 (33.3) |
| 2-4 months | 167 (11.7) | 129 (11.2) | 27 (6.9) | 323 (10.9) |
| 4-8 months | 182 (12.8) | 160 (13.9) | 45 (11.4) | 387 (13.0) |
| ≥8 months | 273 (19.2) | 204 (17.8) | 61 (15.5) | 538 (18.1) |
| Median: 43 (IQ: 15-189) | Median: 40.5 (IQ: 13-173.5) | Median: 29 (IQ: 9-128) | Median: 40 (IQ: 14-174) | |
| Treatment Delay | ||||
| <1 week | 319 (23.2) | 305 (24.8) | 103 (27.4) | 727 (24.4) |
| 1-2 weeks | 208 (15.1) | 234 (19.0) | 86 (22.9) | 528 (17.7) |
| 2-4 weeks | 492 (35.7) | 426 (34.6) | 119 (31.7) | 1037 (34.7) |
| ≥4 weeks | 359 (26.1) | 267 (21.7) | 68 (18.1) | 694 (23.2) |
| Median: 18 (IQ: 7-30) | Median: 15 (IQ: 7-28) | Median: 13 (IQ: 6-26) | Median: 16 (IQ: 7-29) | |
Maximum diagnostic delay: 365 days;
Maximum treatment delay: 120 days;
IQ: interquartile range
Treatment Delays
Median treatment delays were shorter than diagnostic delays. Median treatment delays were 13 and 16 days for colon and rectal cancer patients, respectively (Table 4). In all, 33.4% of colon cancer patients and 24.4% of rectal cancer patients had a treatment delay of less than a week. However, 15.2% of colon cancer patients and 23.3% of rectal cancer patients had a treatment delay of longer than one month. As with diagnostic delays, treatment delays were longer among those with localized stage compared with distant stage for both colon (median: 15 vs. 8 days) and rectal (median: 18 vs. 13 days) cancer.
Matching
Using the CEM routine, we matched controls to cases in one-month intervals of survival time separately by cancer type and outcome. On average, 2.8 (range: 1.3-5.5) controls were matched to each case. On average, 1.1 (range: 0-5) cases and 280 controls (range: 29-643) controls were unmatched in each model.
Colon Cancer Delays and Mortality
Results from colon cancer matched logistic regression analyses by stage and for the whole sample are presented in Table 5. In adjusted analyses, compared to those with diagnostic delays of 14-59 days, colon cancer patients with the longest diagnostic delays (8-12 months) had higher odds of all-cause (AOR: 1.31; CI: 1.08-1.58) but not CRC-specific death.
Table 5.
The Unadjusted and Adjusted Association of Diagnostic and Treatment Delays of Colon Cancer on CRC-Specific and All-Cause Risk of Death by Stage
| All-Cause Death | CRC-Specific Death | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | Unadjusted OR (95% CI) | Adjustedb OR (95% CI) | |
| Diagnostic Delay | ||||
| Total | (n=6666)c | (n=6365)c | ||
| <2 weeks | 1.23 (1.06-1.42) | 1.07 (0.88-1.31) | 1.27 (1.08-1.49) | 1.13 (0.92-1.39) |
| 14-59 days | 1 | 1 | 1 | 1 |
| 2-4 months | 1.11 (0.94-1.32) | 1.11 (0.88-1.41) | 0.98 (0.81-1.20) | 1.11 (0.87-1.42) |
| 4-8 months | 1.05 (0.90-1.23) | 0.94 (0.75-1.16) | 0.76 (0.63-0.92) | 0.88 (0.70-1.11) |
| ≥8 months | 1.33 (1.17-1.52) | 1.31 (1.08-1.58) | 0.82 (0.71-0.96) | 1.04 (0.85-1.26) |
| Local Stage | (n=2841) | (n=2114) | ||
| <2 weeks | 1.21 (0.94-1.56) | 0.96 (0.71-1.31) | 1.33 (0.86-2.04) | 1.05 (0.66-1.67) |
| 14-59 days | 1 | 1 | 1 | 1 |
| 2-4 months | 1.15 (0.87-1.53) | 1.09 (0.77-1.55) | 0.99 (0.59-1.65) | 0.94 (0.54-1.62) |
| 4-8 months | 1.30 (1.01-1.69) | 1.08 (0.78-1.49) | 1.03 (0.65-1.64) | 0.93 (0.57-1.53) |
| ≥8 months | 1.65 (1.33-2.06) | 1.23 (0.93-1.63) | 1.11 (0.75-1.66) | 0.91 (0.59-1.42) |
| Regional Stage | (n=2667) | (2523) | ||
| <2 weeks | 1.18 (0.94-1.48) | 1.13 (0.84-1.51) | 1.34 (1.03-1.75) | 1.19 (0.88-1.59) |
| 14-59 days | 1 | 1 | 1 | 1 |
| 2-4 months | 1.11 (0.84-1.46) | 1.01 (0.71-1.43) | 0.92 (0.65-1.29) | 0.89 (0.61-1.28) |
| 4-8 months | 1.22 (0.96-1.55) | 0.97 (0.71-1.32) | 0.97 (0.72-1.31) | 0.93 (0.67-1.29) |
| ≥8 months | 1.47 (1.19-1.81) | 1.31 (1.00-1.72) | 0.99 (0.77-1.27) | 1.00 (0.76-1.32) |
| Distant Stage | (n=964) | (n=1011) | ||
| <2 weeks | 1.19 (0.75-1.88) | 1.17 (0.59-2.32) | 1.06 (0.70-1.60) | 0.97 (0.63-1.51) |
| 14-59 days | 1 | 1 | 1 | 1 |
| 2-4 months | 1.60 (0.87-2.94) | 2.23 (0.89-5.58) | 1.77 (0.99-3.17) | 1.99 (1.07-3.67) |
| 4-8 months | 0.76 (0.46-1.25) | 0.52 (0.24-1.16) | 0.53 (0.34-0.83) | 0.69 (0.42-1.13) |
| ≥8 months | 2.78 (1.52-5.11) | 2.37 (1.01-5.56) | 1.00 (0.66-1.52) | 1.31 (0.82-2.11) |
| Treatment Delay | ||||
| Total | (n=6669)c | (n=6238)c | ||
| <1 week | 1.55 (1.35-1.79) | 1.23 (1.01-1.49) | 1.30 (1.10-1.53) | 1.07 (0.87-1.31) |
| 1-2 weeks | 1 | 1 | 1 | 1 |
| 2-4 weeks | 0.75 (0.65-0.87) | 1.03 (0.85-1.26) | 0.66 (0.55-0.78) | 0.92 (0.74-1.14) |
| ≥4 weeks | 0.84 (0.71-1.00) | 1.00 (0.79-1.27) | 0.57 (0.46-0.70) | 0.80 (0.62-1.05) |
| Local Stage | (n=2802) | (n=2035) | ||
| <1 week | 1.74 (1.34-2.26) | 1.43 (1.04-1.96) | 1.32 (0.82-2.13) | 1.14 (0.69-1.89) |
| 1-2 weeks | 1 | 1 | 1 | 1 |
| 2-4 weeks | 0.96 (0.74-1.24) | 1.18 (0.86-1.62) | 0.78 (0.48-1.25) | 0.84 (0.51-1.38) |
| ≥4 weeks | 1.13 (0.85-1.50) | 1.15 (0.80-1.64) | 0.77 (0.45-1.32) | 0.71 (0.40-1.25) |
| Regional Stage | (n=2791) | (n=2593) | ||
| <1 week | 1.45 (1.16-1.80) | 1.24 (0.94-1.63) | 1.43 (1.10-1.86) | 1.26 (0.95-1.67) |
| 1-2 weeks | 1 | 1 | 1 | 1 |
| 2-4 weeks | 0.91 (0.72-1.13) | 1.13 (0.85-1.49) | 0.90 (0.69-1.18) | 1.04 (0.78-1.40) |
| ≥4 weeks | 1.11 (0.85-1.45) | 1.06 (0.75-1.50) | 0.76 (0.54-1.07) | 0.78 (0.54-1.14) |
| Distant Stage | (n=855) | (n=921) | ||
| <1 week | 0.72 (0.43-1.21) | 0.54 (0.26-1.13) | 0.67 (0.44-1.04) | 0.71 (0.45-1.14) |
| 1-2 weeks | 1 | 1 | 1 | 1 |
| 2-4 weeks | 0.57 (0.33-0.99) | 0.61 (0.27-1.37) | 0.78 (0.48-1.27) | 0.87 (0.52-1.47) |
| ≥4 weeks | 0.88 (0.41-1.86) | 0.73 (0.26-2.07) | 0.97 (0.51-1.83) | 1.10 (0.54-2.24) |
All-cause models adjusted for: stage, comorbidity, treatment type, preventable hospitalizations, year of diagnosis, low income, race, gender, age, marital status, tumor grade.
CRC-specific models adjusted for: stage, comorbidity, treatment type, year of diagnosis, race, gender, age, tumor grade, tumor location (in diagnostic delay model only), histology (in treatment delay model only).
Stage-specific numbers may not add up to equal the total sample, because matching was conducted separately for each model, and unmatched cases and controls were dropped.
OR=Odds ratio; CI=Confidence interval. Bold font indicates p<.05.
Longer treatment delays among colon cancer patients did not increase the risk of all-cause or CRC-specific death. In adjusted analyses, compared to those with delays of 1-2 weeks, colon cancer patients with the shortest treatment delays (<1 week) were more likely to die of all-causes (AOR: 1.23; CI: 1.01-1.49) but not CRC-specific death. Analyses conducted by stage do not demonstrate substantively different findings.
Rectal Cancer Delays and Mortality
Results from rectal cancer matched logistic regression analyses by stage and for the whole sample are presented in Table 6. For rectal cancer patients, neither diagnostic nor treatment delays were associated with risk of all-cause or CRC-specific death in adjusted models. Analyses conducted by stage do not demonstrate substantively different findings.
Table 6.
The Unadjusted and Adjusted Association of Diagnostic and Treatment Delays of Rectal Cancer on CRC-Specific and All-Cause Risk of Death by Stage
| All-Cause Death | CRC-Specific Death | |||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | Unadjusted OR (95% CI) | Adjustedb OR (95% CI) | |||
| Diagnostic Delay | (n=2933)c | (n=2632)c | ||||
| <2 weeks | 1.30 (1.07-1.58) | 1.16 (0.88-1.53) | 1.15 (0.92-1.43) | 0.94 (0.72-1.23) | ||
| 14-59 days | 1 | 1 | 1 | 1 | ||
| 2-4 months | 0.93 (0.72-1.21) | 1.05 (0.74-1.50) | 0.77 (0.57-1.04) | 0.94 (0.66-1.35) | ||
| 4-8 months | 1.14 (0.90-1.44) | 0.96 (0.68-1.35) | 1.05 (0.80-1.37) | 1.05 (0.76-1.45) | ||
| ≥8 months | 1.12 (0.91-1.39) | 1.09 (0.81-1.47) | 0.87 (0.68-1.11) | 1.01 (0.76-1.36) | ||
| Local Stage | (n=1343) | (n=1059) | ||||
| <2 weeks | 1.56 (1.14-2.13) | 1.55 (1.04-2.29) | 1.02 (0.66-1.59) | 0.82 (0.50-1.34) | ||
| 14-59 days | 1 | 1 | 1 | 1 | ||
| 2-4 months | 1.48 (1.01-2.16) | 1.71 (1.06-2.76) | 0.94 (0.55-1.63) | 0.89 (0.49-1.60) | ||
| 4-8 months | 1.27 (0.88-1.86) | 0.96 (0.60-1.55) | 0.72 (0.41-1.30) | 0.51 (0.27-0.96) | ||
| ≥8 months | 1.57 (1.1-2.16) | 1.46 (0.97-2.20) | 1.07 (0.68-1.67) | 1.00 (0.62-1.62) | ||
| Regional Stage | (n=1102) | (n=996) | ||||
| <2 weeks | 1.21 (0.88-1.66) | 1.05 (0.69-1.60) | 1.13 (0.79-1.62) | 0.93 (0.62-1.41) | ||
| 14-59 days | 1 | 1 | 1 | 1 | ||
| 2-4 months | 1.00 (0.66-1.52) | 0.87 (0.50-1.52) | 0.97 (0.61-1.54) | 1.09 (0.64-1.85) | ||
| 4-8 months | 1.25 (0.86-1.81) | 1.08 (0.65-1.80) | 1.54 (1.02-2.32) | 1.58 (0.99-2.52) | ||
| ≥8 months | 1.04 (0.73-1.47) | 0.87 (0.55-1.38) | 0.91 (0.61-1.35) | 1.02 (0.66-1.59) | ||
| Distant Stage | (n=201) | (n=335) | ||||
| <2 weeks | 0.84 (0.31-2.30) | 1.26 (0.21-7.42) | 1.26 (0.64-2.48) | 1.10 (0.53-2.30) | ||
| 14-59 days | 1 | 1 | 1 | 1 | ||
| 2-4 months | 0.12 (0.03-0.36) | 0.09 (0.01-0.88) | 0.41 (0.16-1.04) | 0.35 (0.12-0.99) | ||
| 4-8 months | 0.66 (0.17-2.53) | 0.51 (0.04-6.05) | 0.60 (0.28-1.29) | 0.70 (0.31-1.60) | ||
| ≥8 months | 1.26 (0.33-4.74) | 1.52 (0.21-10.96) | 0.95 (0.44-2.05) | 0.83 (0.36-1.91) | ||
| Treatment Delay | ||||||
| Total | (n=2942)c | (n=2670)c | ||||
| <1 week | 1.01 (0.80-1.27) | 1.20 (0.86-1.66) | 0.94 (0.73-1.21) | 1.09 (0.80-1.49) | ||
| 1-2 weeks | 1 | 1 | 1 | 1 | ||
| 2-4 weeks | 0.75 (0.60-0.93) | 0.94 (0.69-1.27) | 0.72 (0.56-0.91) | 0.93 (0.69-1.25) | ||
| ≥4 weeks | 0.69 (0.55-0.87) | 0.94 (0.68-1.31) | 0.57 (0.44-0.74) | 0.83 (0.60-1.15) | ||
| Local Stage | (n=1295) | (n=1025) | ||||
| <1 week | 1.20 (0.80-1.80) | 1.50 (0.90-2.51) | 1.32 (0.70-2.49) | 1.55 (0.77-3.10) | ||
| 1-2 weeks | 1 | 1 | 1 | 1 | ||
| 2-4 weeks | 1.09 (0.74-1.60) | 1.49 (0.93-2.40) | 1.33 (0.73-2.41) | 1.52 (0.80-2.92) | ||
| ≥4 weeks | 1.06 (0.71-1.58) | 1.45 (0.88-2.40) | 1.40 (0.76-2.57) | 1.63 (0.83-3.18) | ||
| Regional Stage | (n=1189) | (n=1066) | ||||
| <1 week | 1.25 (0.88-1.77) | 1.11 (0.70-1.76) | 1.08 (0.74-1.58) | 1.02 (0.65-1.58) | ||
| 1-2 weeks | 1 | 1 | 1 | 1 | ||
| 2-4 weeks | 0.82 (0.59-1.13) | 0.79 (0.51-1.22) | 0.80 (0.56-1.16) | 0.83 (0.54-1.26) | ||
| ≥4 weeks | 1.03 (0.72-1.48) | 1.05 (0.65-1.70) | 0.73 (0.49-1.10) | 0.74 (0.46-1.19) | ||
| Distant Stage | (n=199) | (n=346) | ||||
| <1 week | 0.59 (0.19-1.84) | 1.04 (0.18-6.02) | 0.58 (0.25-1.35) | 0.73 (0.29-1.81) | ||
| 1-2 weeks | 1 | 1 | 1 | 1 | ||
| 2-4 weeks | 0.55 (0.19-1.60) | 0.88 (0.17-4.40) | 0.52 (0.23-1.18) | 0.54 (0.23-1.30) | ||
| ≥4 weeks | 0.34 (0.11-1.05) | 0.88 (0.13-5.90) | 0.27 (0.12-0.62) | 0.32 (0.12-0.83) | ||
All-cause models adjusted for: stage, comorbidity, treatment type, year of diagnosis, low income, race, gender, age, marital status, tumor grade.
CRC-specific models adjusted for: stage, treatment type, year of diagnosis, race, gender, age, tumor grade, histology (in treatment delay model only).
Stage-specific numbers may not add up to equal the total sample, because matching was conducted separately for each model, and unmatched cases and controls were dropped.
OR=Odds ratio; CI=Confidence interval. Bold font indicates p<.05.
Sensitivity Analyses
We found good concordance of our calculated Medicare exact diagnosis date with the SEER dates (defined as midpoint of the month and year). For date of diagnosis, the mean difference was 1.3 days (SD=9.7); for date of first treatment, the mean difference was 2.1 days (SD=18.6). Using SEER diagnosis dates, we re-ran all analyses and the results did not substantively change.
We next tested the effect of using different matching methods: first matching on survival time in more precise, 2-week intervals, and second, matching on stage in addition to survival time. These strategies resulted in fewer matched strata and a larger number of unmatched patients. Next, to test for any bias resulting from our selected cutpoints and reference categories, we re-analyzed all models using different categorizations of delay, (including model-specific quartiles and quintiles) and different reference categories. Given that the vast majority of our sample had surgery as a first-line treatment, stratifying by first treatment type made no difference to the results. Analyzing treatment delays for those who only received surgical treatment also did not change the findings. Finally, we stratified diagnostic delay models by type of presenting symptom. Results of these analyses by symptom type did not substantively change the findings.
DISCUSSION
Summary of Findings
We examined diagnostic and treatment delays spanning the time between first presentation to a physician for clinical manifestations or symptoms of colorectal cancer until first treatment and assessed associations with all-cause and CRC-specific risk of death. Median diagnostic delays in our study were 60 days and 40 days for colon and rectal cancer, respectively. Median treatment delays were 13 days for colon and 16 days for rectal cancer patients. Contrary to our hypothesis, we found little evidence to suggest that longer diagnostic (up to 12 months) or treatment (up to 4 months) delays were associated with greater odds of death.
Our results showed that diagnostic delays of 8-12 months were associated with higher odds of all-cause death among colon cancer patients. However, this association was not found for CRC-specific deaths among these same patients, suggesting that an unmeasured confounding factor contributed to the association between longer delays and other causes of death. It is possible that the long (8-12 months) diagnostic delays in this situation do not directly affect the higher odds of all-cause death but are simply a consequence of prognostic factors such as performance status or a result of patient preferences that are not captured in the SEER-Medicare data.
Colon cancer patients with the shortest (≤1 week) treatment delays had higher odds of all-cause death. Previous literature has also demonstrated that CRC patients with shorter delays have worse prognosis.[5,30,10] Although we attempted to limit our sample to nonemergent cases only, it is likely that this finding indicates higher odds of death among patients with emergent or urgent situations that were not excluded using our algorithm.
Policy and Research Context of Findings
The Institute of Medicine’s Crossing the Quality Chasm report identified timeliness of care as one of six aims of quality improvement in healthcare.[46] To our knowledge, no studies have been conducted in the U.S. to test specific benchmarks regarding the timeliness of CRC care. In a comprehensive review of quality CRC care measures, two measures of timeliness were identified: 1) Time from patient presentation with symptoms to physician diagnosis and 2) Proportion of (diagnostic) colonoscopies that were completed in a timely fashion.[47] The review group concluded that neither of these benchmarks improved survival or other outcomes of interest, were appropriately validated, or based on evidence-based guidelines. Nevertheless, the U.S. Veterans Affairs and other health systems abroad have adopted timeliness guidelines. For example, UK guidelines state that patients with suspected cancer should see a specialist within 2 weeks and treatment should begin within a month of diagnosis.[48,49] Notably, a systematic review determined that all identified international benchmarks for cancer care were established solely on the basis of expert opinion only.[31]
Comparing estimates of delay across studies is difficult due to differing definitions of delay.[5] Nevertheless, other U.S. studies also demonstrate longer diagnostic[16,15] and shorter treatment[16] delays for colon (vs. rectal) cancer patients. Longer diagnostic delays in colon cancer could result from missed diagnoses or longer diagnostic workups or due to the more insidious nature of colon cancer symptoms.[50] Neoadjuvant treatments, which are more common in rectal cancer, require medical oncology referrals, which may delay initial rectal cancer treatment. Notably, we found a significant trend in increasing delays over time in our study (data not shown), which is consistent with other studies.[16,51] Delays are expected to continue to increase given the continued growth of the elderly population, regionalization of surgical care, impending surgeon shortage, and increasing use of complex, multimodal treatments.[16] The effect of these predicted increases of delay over time on patient outcomes is uncertain.
The lack of an association between longer delays and increased risk of CRC-specific death in our study supports many, but not all, previously published studies.[52,9,12,5,6,8,10,11] While somewhat counterintuitive, our finding is consistent with biological models of colorectal carcinogenesis wherein the majority of cancers arise in a temporally predictable sequence in which an average of 10-15 years elapse between the development of an adenomatous polyp and invasive cancer.[53,54] Most symptoms are unlikely to present before the development of invasive cancer, such that the vast majority of the natural history of CRC is asymptomatic. We also found, for both cancers, that patients with longer diagnostic delays actually had earlier stage disease, refuting the common assumption that stage is an intermediate factor in the causal chain between diagnostic delay and survival.[55] Previous research has produced mixed results in regard to the effect of diagnostic delays on stage.[13,7,8] However, our matched case-control results may not be comparable to previous studies utilizing survival analysis.
Opportunities to decrease delays are available. In the Veterans Affairs system, for example, one-third of CRC patients had one or more missed opportunities to initiate an earlier diagnostic or endoscopic test.[56] Although intuitively appealing, policies and interventions designed to reduce delays in CRC could potentially cause harm in certain circumstances. For example, evidence suggests that timeliness and quality of CRC care are not synonymous. A Canadian study found that achieving the recommended 4-week benchmark of time from diagnosis to surgery was more likely when patients did not receive recommended procedures such as preoperative staging imaging or neoadjuvant radiotherapy.[57] In the UK, the maximum 2-week wait policy, designed to fast-track patients with suspected CRC for endoscopic evaluation, failed to positively impact patient outcomes. The policy also adversely lengthened waiting times for CRC patients diagnosed outside of the urgent referral system.[58]
Strengths and Limitations
Our results should be interpreted in light of several limitations. First, our sample included only patients aged ≥66 years insured with Medicare. Therefore we cannot generalize to younger patients or those uninsured patients who likely experience longer delays as a result of reduced access to care. However the effect of delays on risk of death is likely the same in other populations regardless of insurance status. Second, although we searched claims for diagnoses that were thought to represent CRC symptoms, we cannot confirm that these represented CRC symptoms exclusively, rather than other conditions. Additionally, we may have missed some clinically-relevant CRC symptoms that were not captured by billing data. Third, by limiting delays to a maximum of 365 days (diagnostic) and 120 days (treatment), we may be artificially capping longer delays therefore biasing our results toward the null. However, based on previous research,[16,59,51,9,60] we suspect that the vast majority of patients, particularly a Medicare-insured population, have diagnostic delays within this range. Fourth, in our attempt to be as precise as possible when identifying the diagnosis date, we applied a strict algorithm, which limited the size of our sample and potentially the generalizability of our findings. Fifth, when studying diagnostic delay, survival time should ideally be measured from the date of first symptom, [20,6,22] rather than the date of diagnosis, but this was not possible in retrospective analyses of administrative data. Finally, although not associated with death, extended delays may contribute to other patient outcomes. Future research should explore the effect of delays on postoperative complications, hospital stay, hospital readmission, cost, quality of life, and psychosocial outcomes such as anxiety.
Despite these limitations, our study has several advantages over previous studies. For example, we examined delays using a large population-based sample and estimated delay periods using administrative data, without relying on potentially unreliable patient estimates. In addition, we controlled for the confounding effect of tumor aggressiveness by including tumor grade in our models, and we further analyzed colon and rectal cancers separately. Notably, our sample size (n=10,663) represents more than a 3-fold increase over the number of patients (n=3,187) from all six previously published U.S. studies combined on this topic.[9,6] As the second leading cause of U.S. cancer death, even a small reduction in CRC mortality could have a large societal impact. Because delays are potentially preventable and because they are projected to continue to increase in the future, [16] delays in cancer care should continue to be monitored.
Conclusions
In this, the first U.S. population-based study to explore both diagnostic and treatment delays among CRC patients, we found that long delays of up to 1 year for diagnosis after the onset of symptoms and up to 120 days for treatment did not appear to increase risk of death. However, because our population was symptomatic or already diagnosed with cancer, our results should not be interpreted as casting doubts on the value of timely early detection via screening of pre-symptomatic individuals. Rather, evidence suggests that preventive screening prior to symptom onset for adults aged ≥50 is highly effective and must remain the public health priority in efforts to reduce CRC incidence, morbidity, and mortality.
Supplementary Material
Acknowledgments
We gratefully acknowledge James Struthers for his data management and programming services. We thank Margie Olsen, PhD and Jean Wang, MD for their assistance and helpful advice and Leah Akers and Chris Davenport for their help with medical coding. We also thank the Center for Administrative Data Research of the Institute of Clinical and Translational Sciences and the Alvin J. Siteman Cancer’s Health Behavior, Communications, and Outreach Core at the Washington University School of Medicine. This work was supported by grants from the National Cancer Institute (CA112159); the National Center for Research Resources Washington University-ICTS (KL2 RR024994); and the Health Behavior, Communication and Outreach Core; the Core is supported in part by the National Cancer Institute Cancer Center Support Grant (P30 CA91842) to the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri. NOD was supported by grants DK-56260, HL-38180, and DDRCC DK-52574. This study used the linked SEER-Medicare database. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. Contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
References
- 1.American Cancer Society Cancer Facts and Figures. [7-27-12];2012 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf.
- 2.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326(10):653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 4.Vital signs: colorectal cancer screening, incidence, and mortality --- United States, 2002--2010. MMWR Morb Mortal Wkly Rep. 2011;60(26):884–889. [PubMed] [Google Scholar]
- 5.Neal RD. Do diagnostic delays in cancer matter? Br J Cancer. 2009;101(Suppl 2):S9–S12. doi: 10.1038/sj.bjc.6605384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos M, Esteva M, Cabeza E, Campillo C, Llobera J, Aguilo A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: a review. Eur J Cancer. 2007;43(17):2467–2478. doi: 10.1016/j.ejca.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Ramos M, Esteva M, Cabeza E, Llobera J, Ruiz A. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur J Cancer. 2008;44(4):510–521. doi: 10.1016/j.ejca.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Terhaar sive Droste JS, Oort FA, van der Hulst RW, Coupe VM, Craanen ME, Meijer GA, Morsink LM, Visser O, van Wanrooij RL, Mulder CJ. Does delay in diagnosing colorectal cancer in symptomatic patients affect tumor stage and survival? A population-based observational study. BMC Cancer. 10:332. doi: 10.1186/1471-2407-10-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher DA, Zullig LL, Grambow SC, Abbott DH, Sandler RS, Fletcher RH, El-Serag HB, Provenzale D. Determinants of medical system delay in the diagnosis of colorectal cancer within the Veteran Affairs Health System. Dig Dis Sci. 2010;55(5):1434–1441. doi: 10.1007/s10620-010-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P. Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol. 2012;65(6):669–678. doi: 10.1016/j.jclinepi.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Torring ML, Frydenberg M, Hansen RP, Olesen F, Hamilton W, Vedsted P. Time to diagnosis and mortality in colorectal cancer: a cohort study in primary care. Br J Cancer. 2011;104(6):934–940. doi: 10.1038/bjc.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iversen LH, Antonsen S, Laurberg S, Lautrup MD. Therapeutic delay reduces survival of rectal cancer but not of colonic cancer. Br J Surg. 2009;96(10):1183–1189. doi: 10.1002/bjs.6700. [DOI] [PubMed] [Google Scholar]
- 13.Jullumstro E, Lydersen S, Moller B, Dahl O, Edna TH. Duration of symptoms, stage at diagnosis and relative survival in colon and rectal cancer. Eur J Cancer. 2009;45(13):2383–2390. doi: 10.1016/j.ejca.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Simunovic M, Rempel E, Theriault ME, Baxter NN, Virnig BA, Meropol NJ, Levine MN. Influence of delays to nonemergent colon cancer surgery on operative mortality, disease-specific survival and overall survival. Can J Surg. 2009;52(4):E79–E86. [PMC free article] [PubMed] [Google Scholar]
- 15.Wattacheril J, Kramer JR, Richardson P, Havemann BD, Green LK, Le A, El-Serag HB. Lagtimes in diagnosis and treatment of colorectal cancer: determinants and association with cancer stage and survival. Aliment Pharmacol Ther. 2008;28(9):1166–1174. doi: 10.1111/j.1365-2036.2008.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Ko CY, Tomlinson JS, Stewart AK, Talamonti MS, Hynes DL, Winchester DP, Bentrem DJ. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779–785. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 18.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BKe, editors. National Cancer Institute; Bethesda, MD: 2010. SEER Cancer Statistics Review, 1975-2007. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 19.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coates AS. Breast cancer: delays, dilemmas, and delusions. Lancet. 1999;353(9159):1112–1113. doi: 10.1016/S0140-6736(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 21.Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79(5-6):858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 23.Porter MP. Examining the association between delay in diagnosis and decreased survival in bladder cancer. Cancer. 2010;116(22):5122–5125. doi: 10.1002/cncr.25290. [DOI] [PubMed] [Google Scholar]
- 24.Ray WA. Observational studies of drugs and mortality. N Engl J Med. 2005;353(22):2319–2321. doi: 10.1056/NEJMp058267. [DOI] [PubMed] [Google Scholar]
- 25.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16(3):241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 26.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 27.Jung SY, Sereika SM, Linkov F, Brufsky A, Weissfeld JL, Rosenzweig M. The effect of delays in treatment for breast cancer metastasis on survival. Breast Cancer Res Treat. 2011;130(3):953–964. doi: 10.1007/s10549-011-1662-4. [DOI] [PubMed] [Google Scholar]
- 28.Hu C, Xing Y, Cormier JN, Chang GJ. The validity of cause of death coding within the Surveillance, Epidemiology, and End Results (SEER) Registry. Journal of Clinical Oncology. 2009;27(15s):6544. [Google Scholar]
- 29.Lund JL, Harlan LC, Yabroff KR, Warren JL. Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease. Cancer investigation. 2010;28(7):758–764. doi: 10.3109/07357901003630959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguire A, Porta M, Malats N, Gallen M, Pinol JL, Fernandez E. Cancer survival and the duration of symptoms. An analysis of possible forms of the risk function. ISDS II Project Investigators. Eur J Cancer. 1994;30A(6):785–792. doi: 10.1016/0959-8049(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 31.Taylor M, Turner D, Latosinsky S, Noseworthy T. Determining acceptable waiting times for the surgical treatment of solid organ malignancies – A systematic review. [11-17-2011];2005 http://www.cancercare.mb.ca/cancercare_resources/EPI/pdfs/CIHR_WaitTime_Report2_111005.pdf Toward Canadian benchmarks for health services wait times? Evidence, application and research priorities (Report 2)
- 32.Ford AC, Veldhuyzen van Zanten SJ, Rodgers CC, Talley NJ, Vakil NB, Moayyedi P. Diagnostic utility of alarm features for colorectal cancer: systematic review and meta-analysis. Gut. 2008;57(11):1545–1553. doi: 10.1136/gut.2008.159723. [DOI] [PubMed] [Google Scholar]
- 33.Jellema P, van der Windt DA, Bruinvels DJ, Mallen CD, van Weyenberg SJ, Mulder CJ, de Vet HC. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ. 340:c1269. doi: 10.1136/bmj.c1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Serag HB, Petersen L, Hampel H, Richardson P, Cooper G. The use of screening colonoscopy for patients cared for by the Department of Veterans Affairs. Arch Intern Med. 2006;166(20):2202–2208. doi: 10.1001/archinte.166.20.2202. [DOI] [PubMed] [Google Scholar]
- 35.Fisher DA, Grubber JM, Castor JM, Coffman CJ. Ascertainment of colonoscopy indication using administrative data. Dig Dis Sci. 55(6):1721–1725. doi: 10.1007/s10620-010-1200-y. [DOI] [PubMed] [Google Scholar]
- 36.Physicians’ Current Procedural Terminology CPT 2002. American Medical Association; Chicago: 2001. [Google Scholar]
- 37.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100(12):888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001;93(7):501–515. doi: 10.1093/jnci/93.7.501. [DOI] [PubMed] [Google Scholar]
- 39.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006;24(14):2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 40.Schenck AP, Klabunde CN, Warren JL, Peacock S, Davis WW, Hawley ST, Pignone M, Ransohoff DF. Data sources for measuring colorectal endoscopy use among Medicare enrollees. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2118–2127. doi: 10.1158/1055-9965.EPI-07-0123. [DOI] [PubMed] [Google Scholar]
- 41.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 42.National Cancer Institute SEER-Medicare: Calculation of comorbidity weights. [7-18-2011]; http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 43.Parchman ML, Culler SD. Preventable hospitalizations in primary care shortage areas. An analysis of vulnerable Medicare beneficiaries. Arch Fam Med. 1999;8(6):487–491. doi: 10.1001/archfami.8.6.487. [DOI] [PubMed] [Google Scholar]
- 44.Iacus SM, King G, Porro G. Multivariate matching methods that are monotonic imbalance bounding. Journal of the American Statistical Association. 2011;106:345–361. Avail at: http://gking.harvard.edu/files/abs/cem-math-abs.shtml. [Google Scholar]
- 45.Iacus SM, King G, Porro G. Causal inference without balance checking: Coarsened exact matching. 2011 Avail at: http://gking.harvard.edu/files/abs/cem-plus-abs.shtml.
- 46.National Academy Press; Washington, D.C.: Institute of Medicine Crossing the quality chasm: A new health system for the 21st century. Available at: http://books.nap.edu/catalog.php?record_id=10027. [PubMed] [Google Scholar]
- 47.Patwardhan MB, S GP, McCrory DC, Fisher DA, Mantyh CR, Morse MA, Prosnitz RG, Cline KE, Gray RN. Evidence Report/Technology Assessment No 138 (Prepared by the Duke Evidence based Practice Center under Contract No 290-02-0025) AHRQ Publication No 06-E002. Agency for Healthcare Research and Quality; Rockville, MD: 2006. Cancer Care Quality Measures: Diagnosis and Treatment of Colorectal Cancer. [PMC free article] [PubMed] [Google Scholar]
- 48.Department of Health (2000) Referral guidelines for suspected cancer. [11-30-11]; http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4014421.pdf.
- 49.Department of Health The NHS cancer plan. [11-29-11]; http://www.thh.nhs.uk/documents/_Departments/Cancer/NHSCancerPlan.pdf.
- 50.Korsgaard M, Pedersen L, Sorensen HT, Laurberg S. Reported symptoms, diagnostic delay and stage of colorectal cancer: a population-based study in Denmark. Colorectal Dis. 2006;8(8):688–695. doi: 10.1111/j.1463-1318.2006.01014.x. [DOI] [PubMed] [Google Scholar]
- 51.Singh H, De Coster C, Shu E, Fradette K, Latosinsky S, Pitz M, Cheang M, Turner D. Wait times from presentation to treatment for colorectal cancer: a population-based study. Can J Gastroenterol. 24(1):33–39. doi: 10.1155/2010/692151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer. 2005;92(11):1959–1970. doi: 10.1038/sj.bjc.6602587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH, Rosen L, Zapka JG, Olsen SJ, Giardiello FM, Sisk JE, Van Antwerp R, Brown-Davis C, Marciniak DA, Mayer RJ. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112(2):594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 54.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361(25):2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porta M, Fernandez E, Alguacil J. Semiology, proteomics, and the early detection of symptomatic cancer. J Clin Epidemiol. 2003;56(9):815–819. doi: 10.1016/s0895-4356(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 56.Singh H, Daci K, Petersen LA, Collins C, Petersen NJ, Shethia A, El-Serag HB. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol. 2009;104(10):2543–2554. doi: 10.1038/ajg.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McConnell YJ, Inglis K, Porter GA. Timely access and quality of care in colorectal cancer: are they related? Int J Qual Health Care. 2010;22(3):219–228. doi: 10.1093/intqhc/mzq010. [DOI] [PubMed] [Google Scholar]
- 58.Rai S, Kelly MJ. Prioritization of colorectal referrals: a review of the 2-week wait referral system. Colorectal Dis. 2007;9(3):195–202. doi: 10.1111/j.1463-1318.2006.01107.x. [DOI] [PubMed] [Google Scholar]
- 59.Siminoff LA, Rogers HL, Thomson MD, Dumenci L, Harris-Haywood S. Doctor, what’s wrong with me? Factors that delay the diagnosis of colorectal cancer. Patient Educ Couns. 2011;84(3):352–358. doi: 10.1016/j.pec.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu CY, Delclos GL, Chan W, Du XL. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2010 doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


