Abstract
The identification of a new class of potent and selective ROCK-II inhibitors is presented. Compound 5 (SR-3677) had an IC50 of ~3 nM in enzyme and cell based assays and had an off-target hit rate of 1.4% against 353 kinases, and inhibited only 3 out of 70 nonkinase enzymes and receptors. Pharmacology studies showed that 5 was efficacious in both, increasing ex vivo aqueous humor outflow in porcine eyes and inhibiting myosin light chain phosphorylation.
RhoA and its downstream kinase (ROCK) play an important role in the regulation of smooth muscle contraction1 and neurite growth retraction.2 The abnormal activation of the ROCK pathway has also been observed in many disorders of the central nervous system.3 Therefore, the inhibition of ROCK is a promising strategy for the treatment of various diseases such as hypertension,4 coronary and cerebral vasospasm,5 erectile dysfunction,6 glaucoma,7,8 asthma,9 multiple sclerosis (MS),3 atherosclerosis,10 stroke,11 and cancer.12
Most ROCK inhibitors reported in the literature contain isoquinoline (e.g., Fasudila and H-1152P),13,14 pyridine (such as Y-2763215 and Y-39983,8,16 which is in clinical trials for the treatment of glaucoma17), or indazole18–22 moieties. These compounds are either moderately potent in ROCK biochemical assays (IC50 > 100 nM) or have poor cell activities (IC50 values in cell-based assays normally in the range of 0.1 µM–10 µM). A promising new class of ROCK inhibitors (ROCK-I) based on aminofurazan-azabenzimidazoles was recently reported.23 Some compounds in this series were shown to have lower than 100 nM IC50 values in cell-based assays.
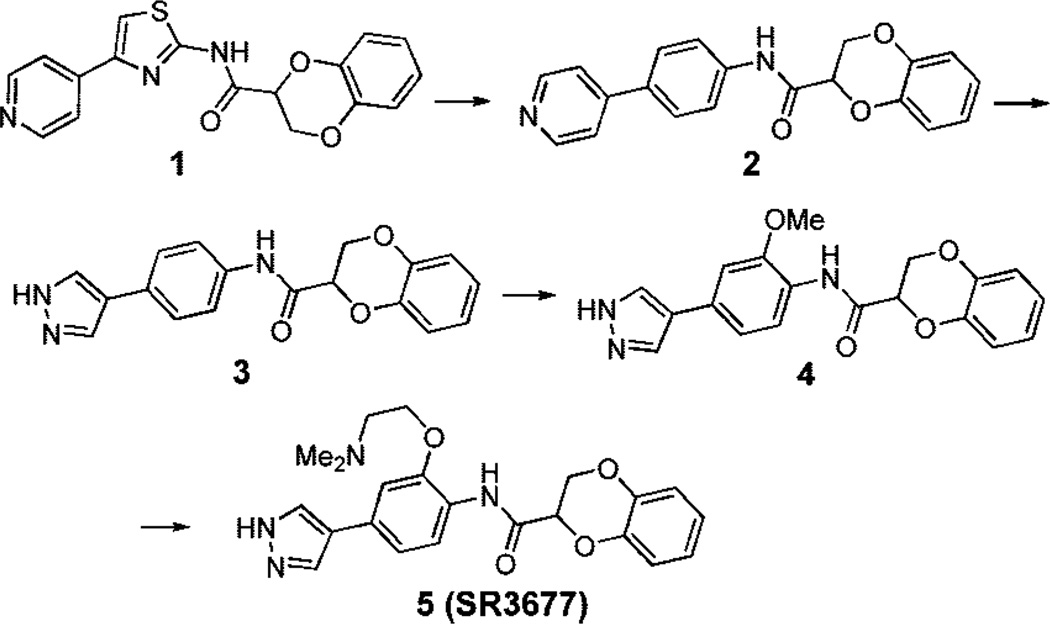
Our goal was to discover potent ROCK-II inhibitors with IC50 values less than 10 nM in biochemical assays and less than 100 nM in cell-based assays. In addition, these inhibitors should be highly selective against other kinases and nonkinase enzymes and receptors (ideally with IC50 values >1 µM). An in-house HTS campaign24 led to the discovery of compound 1 (Chart 1), a pyridine-thiazole based amide compound. Although 1 is a potent ROCK-II inhibitor (Table 1, IC50 = 7.2 nM) with good selectivity against a few other kinases, it has a relatively large shift in cell-based potency as assessed by myosin light chain bisphosphorylation (ppMLC) (IC50 = 137 nM).25 The optimization of 1 started by replacing the central thiazole group with a phenyl ring (Chart 1). As shown in Table 1, the potency of the resulting compound 2 was reduced in both enzyme and cell-based assays compared to that of 1. We reasoned that the pyridine-phenyl system in 2 might have perturbed the favorable geometry that was present in the pyridine-thiazole structure of 1. A molecule with a 5-membered nitrogen-containing hetero-cycle linked to the central phenyl ring might mimic the original pyridine-thiazole system. Thus, we prepared compound 3, which contained a pyrazole to replace the pyridine in 1 as the hinge binding element.
Chart 1.
SAR Evolution
Table 1.
Data for Biochemical and Cell-Based Assays
| kinase IC50 (µM) | |||||
|---|---|---|---|---|---|
| cmpd | ROCK-II IC50 (µM) |
ppMLC IC50 (µM) |
PKA | MRCK | Akt1 |
| 1 | 0.0072 ± 0.0023 | 0.137 | 0.637 ± 0.119 | 4.850 ± 0.212 | >20 |
| (n = 2) | (n = 1)a | (n = 2) | (n = 2) | (n = 2) | |
| 2 | 0.046 ± 0.032 | 0.657 ± 0.119 | 0.692 ± 0.021 | 7.050 ± 2.900 | 6.436 ± 2.834 |
| (n = 2) | (n = 2) | (n = 2) | (n = 2) | (n = 4) | |
| 3 | 0.0015 ± 0.0007 | 0.072 ± 0.057 | 0.186 ± 0.016 | 1.190 ± 0.057 | 1.410 ± 0.646 |
| (n = 2) | (n = 4) | (n = 2) | (n = 2) | (n = 4) | |
| 4 | 0.0033 ± 0.002 | 0.030 | 0.940 ± 0.019 | 0.367 ± 0.188 | 8.81 ± 2.000 |
| (n = 2) | (n = 1)a | (n = 2) | (n = 2) | (n = 2) | |
| 5 | 0.0032 ± 0.0023 | 0.0035 | 3.968 ± 1.680 | 1.190 ± 0.509 | 7.491 ± 1.402 |
| (n = 9) | (n = 1)a | (n = 5) | (n = 2) | (n = 2) | |
The compound was tested as replicates on the plate, and the IC50 data was calculated by drawing a curve from both data sets.
The new pyrazole-phenyl scaffold of 3 was even more potent (Table 1) than compound 1. Introduction of additional functionality on the central aryl ring was used to improve the inhibitor’s overall pharmaceutical properties and/or to enhance the selectivity. We thus prepared compound 4. This methoxy substituted ROCK inhibitor showed good selectivity against PKA (~300-fold) and Akt1 (~2700-fold), and its cell potency was also improved (IC50 = 30 nM vs IC50 = 72 nM in 3). However, the selectivity against MRCK (IC50 = 367 nM), the most closely related kinase to ROCK that we tested, decreased (110-fold vs 790-fold in 3). Finally, a dimethylaminoethoxy moiety was introduced to replace the methoxy group to give compound 5. This novel ROCK-II inhibitor showed high potency in biochemical and cell-based assays as well as high selectivity against all kinases tested (Table 1 and Supporting Information). The IC50 value of 5 for ROCK-I was 56 ± 12 nM (n = 4). The equal potency between biochemical and cell-based assays for 5 could be due to cell accumulation, differences in cell permeability, or enzyme kinetics. It should be pointed out that compounds 1–5 are all racemates.
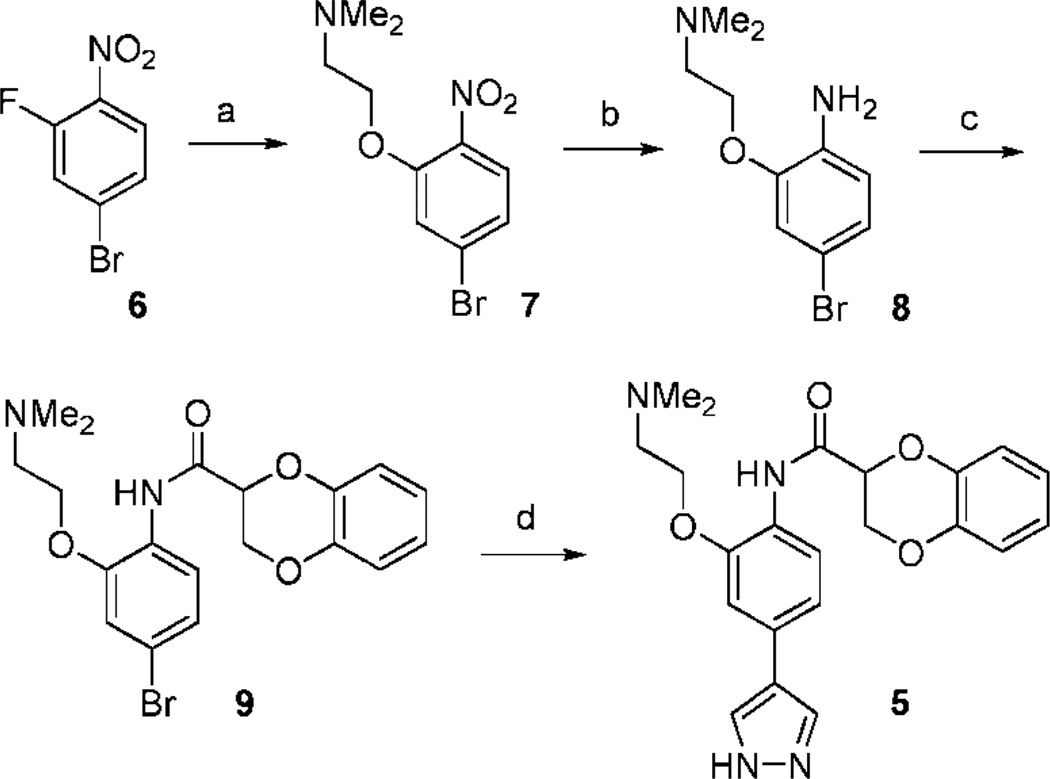
The synthesis of 5 (Scheme 1) began from 2-fluoro-4-bromonitrobenzene (6). Nucleophilic substitution was used to introduce the side chain dimethylaminoethoxy group to provide the nitrobenzene derivative (7). This intermediate was then reduced with SnCl2 to aryl amine (8). Amide formation on the newly formed amino group was accomplished in DMF using HATU as the coupling reagent in the presence of DIEA to give the bromide derivative (9). A Suzuki coupling reaction using Pd[P(Ph)3]4 as the catalyst in the presence of K2CO3 was then applied to provide 5. Similar procedures were also used for the preparation of compounds 2, 3, and 4.
Scheme 1.
Synthesis of 5a
a Reagents and conditions: (a) NaH, THF, 2-(dimethyl-amino)ethanol, rt, 92%; (b) SnCl2, ethanol, 70 °C, 91%; (c) benzodioxane-2-carboxylic acid, HATU/DIEA, DMF, rt, 75%; (d) 4-1H-pyrazoleboronic acid, pinacol ester, Pd[P(Ph)3]4, K2CO3, dioxane/H2O, 95 °C, 35%.
The in vitro drug metabolism and in vivo pharmaco-kinetic properties of these ROCK-II inhibitors were evaluated (Table 2). Four human CYP-450 enzymes (2C9, 2D6, 3A4, and 1A2) were selected for routine screening. The HTS hit compound 1 strongly inhibited all four enzymes. Replacement of the thiazole group with an unsubstituted phenyl ring (compounds 2 and 3) significantly reduced CYP inhibitions. Addition of a dimethylaminoethoxy group (5) to the central phenyl ring eliminated the 3A4 inhibition seen in the original hit 1. Overall, none of these compounds possessed good in vitro drug metabolism and in vivo pharmacokinetic properties desired for systemic applications. The inhibitors had either low human liver microsomal stability (HLMS), high clearance (Cl), or low AUC (i.v.) and Cmax (p.o.). In addition, all of the compounds had none or very low oral bioavailability (%F). However, as required in a soft drug approach, these properties are desirable for the topical application of a drug for treating glaucoma, which is one of the promising applications for ROCK inhibitors.
Table 2.
Data for Pharmacokinetics in Vitro and in Vivo
| in vivo rat PKc (i.v., 1 mg/kg; p.o., 2 mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| cmpd | CYP-450sa % inh. at 10 µM |
HLMS at 2 mg/mL T1/2 (min)b |
Cl (i.v.) mL/min/kg |
T1/2 (iv) h |
Vd (i.v.) L/kg |
AUC (i.v.) µM-h |
Cmax (p.o.) µM |
F (%) |
| 1 | 96/94/80/90 | 18 | 181 | 0.4 | 3.7 | 0.3 | 0.0 | 0 |
| 2 | 64/33/51/23 | 6 | 161 | 0.5 | 5.3 | 0.3 | 0.0 | 0 |
| 3 | 87/40/1/20 | 20 | 18 | 2.2 | 1.9 | 2.8 | 0.02 | 0.3 |
| 4 | 93/79/36/92 | 15 | 11 | 2.6 | 1.0 | 4.3 | 0.12 | 0.4 |
| 5 | 80/75/0/46 | 35 | 61 | 0.8 | 3.0 | 0.7 | 0.05 | 7.0 |
CYP-450s: 2C9/2D6/3A4/1A2.
HLMS: human liver microsomal stability.
Data reported is the mean of 3 determinations, and the standard error is within 30% of the mean except for Cmax, which is within 50%.
In addition to selectivity studies against the kinases listed in Table 1, 5 was also profiled against a large panel of kinases and other nonkinase but therapeutically relevant enzymes and receptors. In the profiling versus 353 kinases (Ambit screen26) in single-point inhibition using 3 µM of 5 (Supporting Information), 5 was discovered to hit only 5 other kinases with percentage inhibition greater than 50%: Akt3, Clk1, Clk2, Clk4, and Lats2. All other kinases showed either no or very low (<10%) inhibition. An off-target hit rate of only 1.4% (5 out of 352) is believed to be one among the few best known ATP-competitive kinase inhibitors.26 In the profiling studies against a panel of 70 nonkinase enzymes and receptors, 5 inhibited only three adrenergic receptors: α1a, α1b, and α2a with 53%, 68%, and 76% inhibition, respectively, at 3 µM inhibitor concentration, which indicates IC50 values in the 1 µM range or ~300 times higher than the IC50 against ROCK-II in cell-based assays. Furthermore, inhibition of the human-ether-a-go-go (hERG) was only 31% at 3 µM (Supporting Information). All these counter screening results demonstrated that 5 is not only a potent compound but also a highly selective ROCK-II inhibitor.
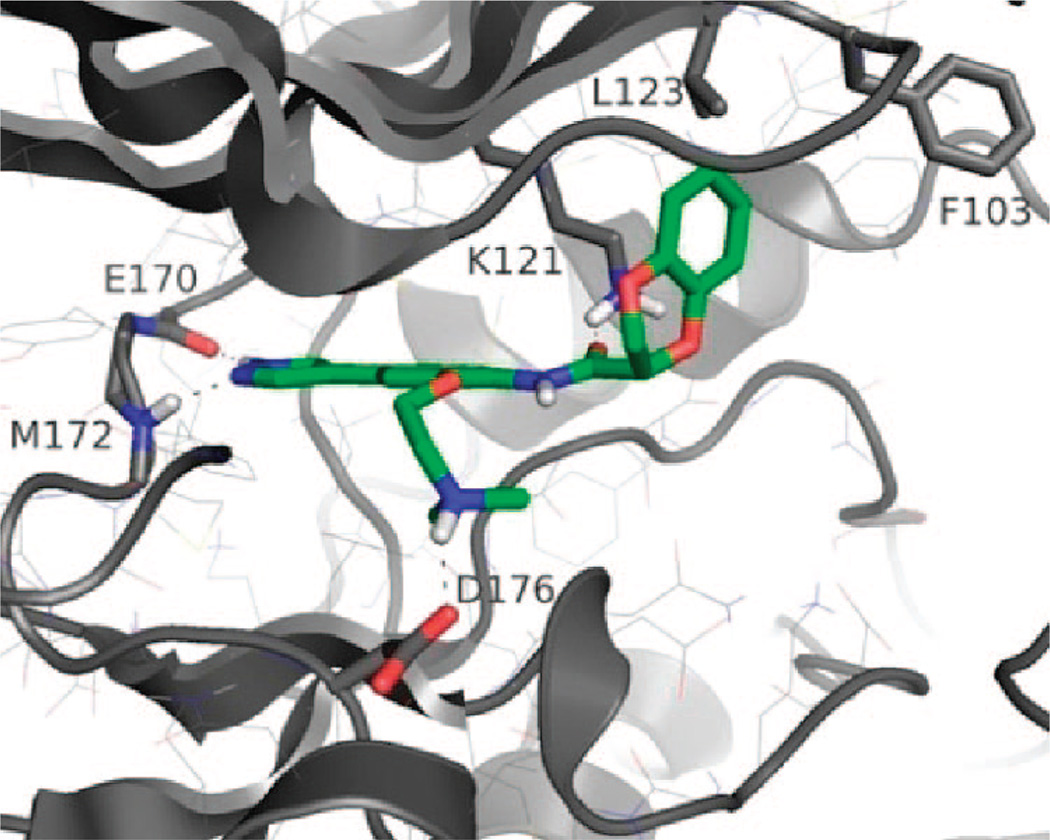
Cocrystal structures of ROCK with ligands Fasudil and Y-27632 have previously been reported.27,28 Our docking results of 5 to ROCK-II (Supporting Information) explain well the observed SAR. There are several key binding elements that contribute to the high potency and selectivity of 5. The best scoring pose (Figure 1) indicated that the pyrazole headgroup binds to the hinge region of the enzyme through two H-bonds: one between the NH of Met-172 and the N of the pyrazole ring and another between the backbone C=O of Glu-170 and the NH of the pyrazole ring. In the phosphate binding region, the amide C=O of 5 forms a hydrogen bond with the side chain NH of Lys-121. A very important H-bonding interaction occurs in the ribose binding region between the protonated tertiary amine of the dimethylaminoethoxy group of 5 and the Asp-176 carboxylate side chain. This interaction is probably the reason for the higher selectivity of 5 compared to that of compounds 1–4 (Table 1) in which this H-bond is absent. This specific interaction was observed in the crystal structure of the ROCK-II-Fasudil complex but was not seen in the cocrystal structure of Y-27632 with ROCK-II (bovine) and ROCK-I (human). Thus, we have combined the binding motifs of both Fasudil and Y-27632 into 5.
Figure 1.
Structure of compound 5 (green) docked in the catalytic domain of the ROCK-II homology model (gray).
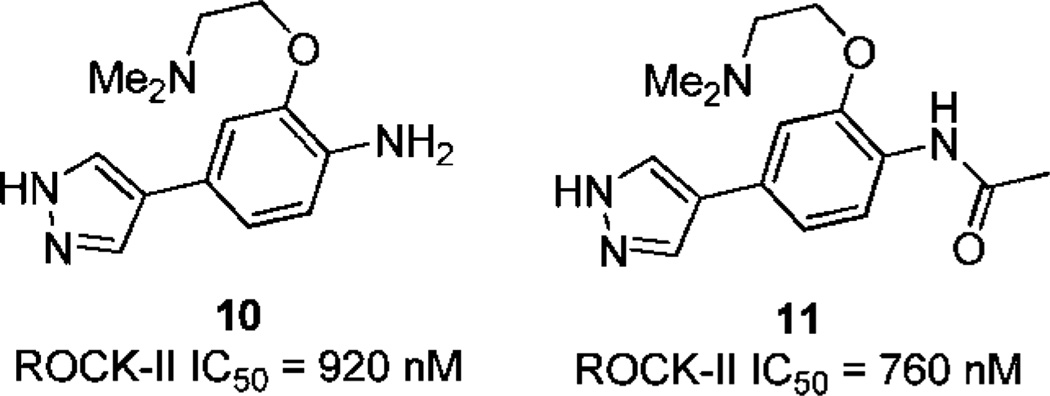
It is important to point out that there is a hydrophobic pocket under the P-loop in this enzyme–ligand complex (Supporting Information), and the benzodioxane phenyl ring of 5 is immersed inside this pocket. It is believed that the hydrophobic interaction of the benzodioxane phenyl ring with the hydrophobic surface of this pocket is the dominating factor that contributes to the high potency of 5. To demonstrate the significance of this interaction, two compounds (10 and 11) without the benzodioxane moiety (Figure 2) were synthesized and evaluated. The binding of 10 and 11 to ROCK-II is much weaker (IC50 = 920 nM and 760 nM, respectively, compared to 3 nM in 5). This hydrophobic interaction further explains why the very simple structures 1, 3, and 4 have high affinity for ROCK-II.
Figure 2.
Compounds 10 and 11 and their ROCK-II potency.
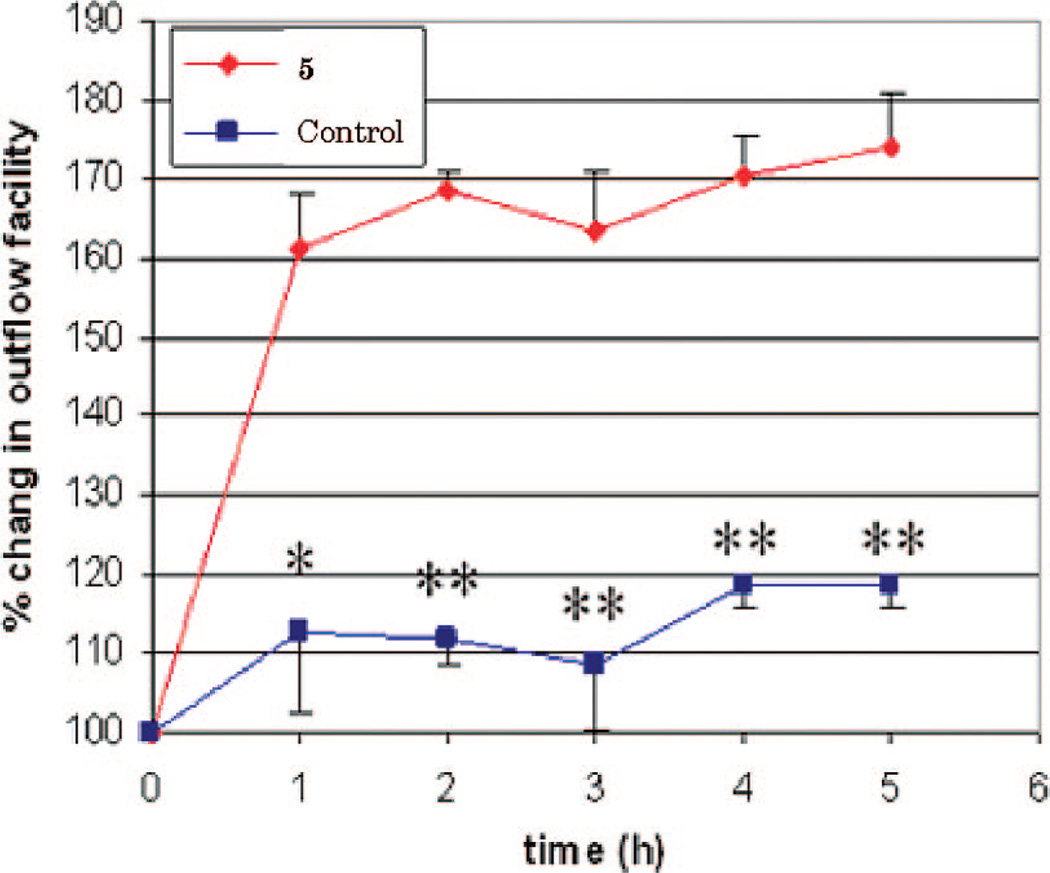
The effect of 5 on ex vivo aqueous humor outflow facility from the trabecular meshwork (TM) of porcine eyes is shown in Figure 3. The results show that continuous exposure of 25 µM 5 increases the outflow facility by 60% at 1 h perfusion, increasing to 70–80% for the 2–5 h time points (p < 0.01). This 70–80% increase in outflow is the maximal response that can be attained and is achieved at doses 2–4-fold lower than needed for Y-27632,29,30 a well studied ROCK inhibitor. The 2–4-fold lower dose of 5 utilized to increase aqueous humor outflow compared to Y-27632 is more modest than one might expect given the difference in biochemical- and cell-based potency between the two compounds and may be due to compound accessibility to the different regions (trabecular meshwork, juxtacanilicular (JCT) area, and Schlemm’s canal) of the aqueous outflow pathway. Since lower doses of 5 were not tested, it may be that lower doses of 5 would also be maximally effective in increasing aqueous humor outflow and thus be more reflective of the intrinsic potency differences between 5 and Y-27632. These studies are currently ongoing in our laboratories. These results underscore the important role ROCK plays in aqueous humor outflow and illustrate the promise of this class of compounds for potential intraocular pressure lowering in glaucoma.
Figure 3.
Time course for the aqueous humor outflow facility by 25 µM 5 perfused in enucleated porcine eyes (N = 3; average ± SE; *p < 0.05; **p < 0.01).
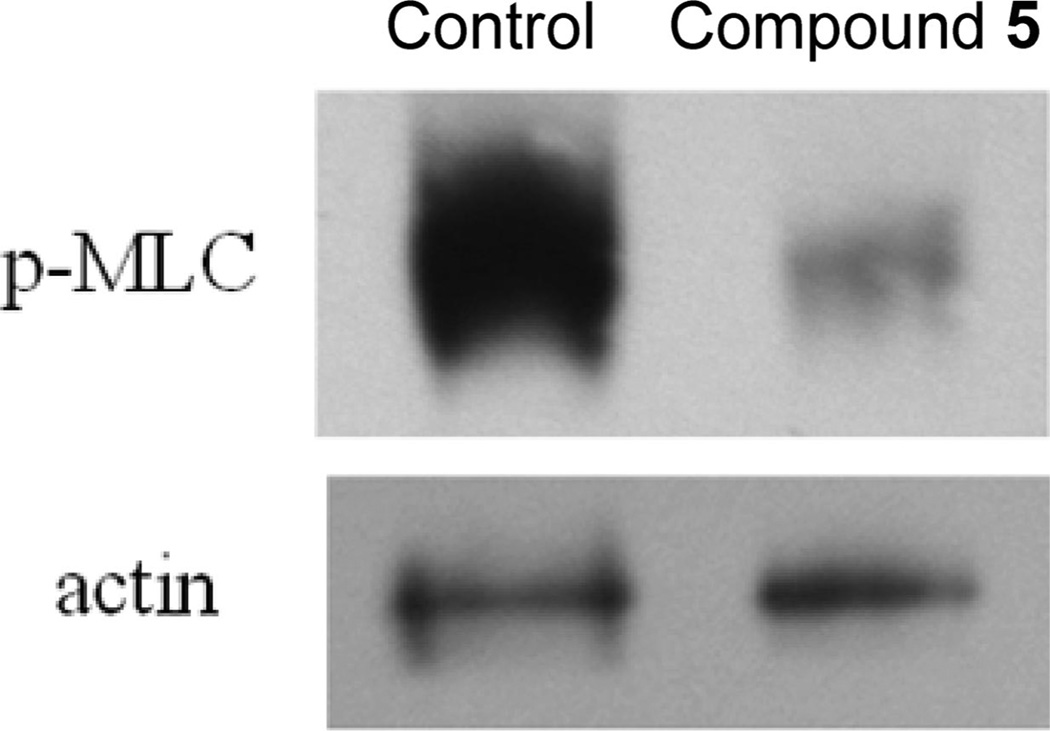
In an effort to ensure that 5 acted through inhibition of ROCK ex vivo, we monitored the phosphorylation state of myosin light chain in trabecular meshwork from porcine eyes. Figure 4 presents the Western blot analysis for p-MLC in TM tissue isolated from porcine eyes that were perfused with 25 µM 5 at 15 mmHg for 5 h. The results show a strong cross-reactive band for p-MLC in the control lane indicating large amounts of p-MLC. TM tissue derived from the eyes perfused with 25 µM 5 shows a very faint band for p-MLC suggesting greater than 90% inhibition of Rho kinase in the TM tissue at this dose. Actin immuno-cross-reactivity for both samples showed equal loading of protein between wells. These data strongly support the notion that selective ROCK inhibitors from this scaffold are potent modulators of myosin light chain phosphorylation in TM tissue and correlate nicely with the increased outflow facility seen in these very same porcine eyes (Figure 3).
Figure 4.
Western blot analysis of p-MLC in the TM tissue derived from the porcine eyes untreated (control) and perfused with 25 µM 5.
In summary, we have identified a new class of potent and highly selective ROCK-II inhibitors. These novel compounds possess a pyrazole group that can function as the hinge binding moiety and a side chain with H-bonding capability that can enhance the inhibitor’s selectivity. Compound 5 is very potent in both enzyme and cell-based assays with single digit IC50 values. Pharmacology studies showed that 5 was efficacious in both increasing aqueous humor outflow and in target modulation–reduction of p-MLC. Further optimizations of this scaffold, including the synthesis and evaluation of each enantiomer of 5, and more pharmacology studies are underway in our laboratories and will be reported in due course.
Supplementary Material
Acknowledgment
We thank Dr. Michael Chalmers for the high resolution mass spectra, Ambit Bioscience for the kinase panel screening, and MDS for the nonkinase enzyme and receptor screening. We are also grateful to Professor Patrick Griffin and Professor William Roush for their support. Y. Feng thanks Dr. Yen Ting Chen for reading the manuscript and for the helpful discussion. This work was supported in part by NIH grant R01-EY018590 (P.V.R.).
Footnotes
Abbreviations: Fasudil, 5-(1,4-diazepan-1-ylsulfonyl)isoquinoline; H-1152P, (S)-4-methyl-5-(2-methyl-1,4-diazepan-1-ylsulfonyl)isoquinoline; Y-27632, (R)-4-(1-aminoethyl)-N-(pyridin-4-yl)cyclohexanecarboxamide; Y-39983, (R)-4-(1-aminoethyl)-N-(1H-pyrrolo[2,3-b]pyridin-4-yl)benzamide; AUC, pharmacokinetic area under curve; Cl, pharmacokinetic clearance; Cmax, pharmacokinetic maximum concentration; F, oral bioavailability; HLMS, human liver microsomal stability; hERG, human ether-a-go-go; HTS, high-throughput screening; ppMLC, bis-phospho myosin light chain; TM, trabecular meshwork; Vd, volume of distribution.
Supporting Information Available: More docking information for 5; procedures for biologic testing; and experimental procedures and spectra data for compounds 2, 3, 4, 5, 10, and 11. This material is available free of charge via the Internet at http://pubs.acs.org/instruct/jmcmar.pdf.
References
- 1.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 2.Lingor P, Tonges L, Pieper N, Bermel C, Barski E, Planchamp V, Bahr M. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- 3.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat. Rev. Drug Discovery. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 4.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masumoto A, Masahiro M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitors fasudil in patients with vasospastic angina. Circulation. 2002:1545–1547. doi: 10.1161/hc1002.105938. n/a. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Hamid IA. Can smooth muscle represent a useful target for the treatment of rapid ejaculation. Drug Discovery Today. 2005;10:1459–1466. doi: 10.1016/S1359-6446(05)03596-8. [DOI] [PubMed] [Google Scholar]
- 7.Rao VP, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs. 2007;21:167–177. doi: 10.2165/00063030-200721030-00004. [DOI] [PubMed] [Google Scholar]
- 8.Tokushige H, Inatani M, Nemoto S, Sakaki H, Katayama K, Uehata M, Tanihara H. Effects of topical administration of Y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest. Ophthalmol. Visual Sci. 2007;48:3216–2322. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Kume H, Oguma T, Makino Y, Ito Y, Shimokata K. Mast cell tryptase causes homologous desensitization of beta-adrenoceptors by Ca2+ sensitization in tracheal smooth muscle. Clin. Exp. Allergy. 2008;38:135–144. doi: 10.1111/j.1365-2222.2007.02879.x. [DOI] [PubMed] [Google Scholar]
- 10.Cicha I, Goppelt-Struebe M, Muehlich S, Yilmaz A, Raaz D, Daniel WG, Garlichs CD. Pharmacological inhibition of RhoA signaling prevents connective tissue growth factor induction in endothelial cells exposed to non-uniform shear stress. Atherosclerosis. 2008;196:136–145. doi: 10.1016/j.atherosclerosis.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2006;290:C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin L, Morishige K, Takahashi T, Hashimoto K, Ogata S, Tsutsumi S, Takata K, Ohta T, Kawagoe J, Takahashi K, Kurachi H. Fasudil inhibits vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Mol. Cancer Ther. 2007;6:1517–1525. doi: 10.1158/1535-7163.MCT-06-0689. [DOI] [PubMed] [Google Scholar]
- 13.Nishikimi T, Akimoto K, Wang X, Mori Y, Tadokoro K, Ishikawa Y, Shimokawa H, Ono H, Matsuoka H. Fasudil, a Rho-kinase inhibitor, attenuates glomerulosclerosis in Dahl salt-sensitive rats. J. Hypertens. 2004;22:1787–1796. doi: 10.1097/00004872-200409000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline)sulfonyl]-homopi-perazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol. Ther. 2002;93:225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 15.Arita M, Saito t, Okuda H, Sato H, Uehata M. 4-Amino(alkyl) cyclohexane-1-carboxamide Compound and Use Thereof. US5478838. 1995 [Google Scholar]
- 16.Takanashi S, Naito Y, Tanaka H, Uehata M, Katayama K. Amide Compounds and Use Thereof. WO01068607. 2001 [Google Scholar]
- 17.Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch. Ophthalmol. 2008;128:309–315. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Cameron MD, Frackowiak B, Griffin E, Lin L, Ruiz C, Schroter T, LoGrasso P. Structure-activity relationships, and drug metabolism and pharmacokinetic properties for indazole piperazine and indazole piperidine inhibitors of ROCK-II. Bioorg. Med. Chem. Lett. 2007;17:2355–2360. doi: 10.1016/j.bmcl.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 19.Goodman KB, Cui H, Dowdell SE, Gaitanopoulos DE, Ivy RL, Sehon CA, Stavenger RA, Wang GZ, Viet AQ, Xu W, Ye G, Semus SF, Evans C, Fries HE, Jolivette LJ, Kirkpatrick RB, Dul E, Khandekar SS, Yi T, Jung dK, Wright LL, Smith GK, Behm DJ, Bentley R, Doe CP, Hu E, Lee D. Development of dihydropyridone indazole amides as selective Rho-kinase inhibitors. J. Med. Chem. 2007;50:6–9. doi: 10.1021/jm0609014. [DOI] [PubMed] [Google Scholar]
- 20.Takami A, Iwakubo M, Okada Y, Kawata T, Odai H, Takahashi N, Shindo K, Kimura K, Tagami Y, Miyake M, Fukushima K, Inagaki M, Amano M, Kaibuchi K, Iijima H. Design and synthesis of Rho kinase inhibitors (I) Bioorg. Med. Chem. 2004;12:2115–2137. doi: 10.1016/j.bmc.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Zhu G-D, Gandhi VB, Gong J, Thomas S, Woods KW, Song X, Li T, Diebold Rb, Luo Y, Liu X, Guan R, Klinghofer V, John EF, Bouska J, Olson A, Marsh KC, Stoll VS, Mamo M, Polakowski J, Campbell TJ, Martin RL, Gintant GA, Penning TD, Li Q, Rosenberg SH, Giranda VL. Synthesis of potent, selective, and orally bioavailable indazole-pyridine series of protein kinase B/Akt inhibitors with reduced hypotension. J. Med. Chem. 2007;50:2990–3003. doi: 10.1021/jm0701019. [DOI] [PubMed] [Google Scholar]
- 22.Iwakubo M, Takami A, Okada Y, Kawata T, Tagami Y, Ohashi H, Sato M, Sugiyama T, Fukushima K, Iijima H. Design and synthesis of Rho kinase inhibitors. Bioorg. Med. Chem. 2007;15:350–367. doi: 10.1016/j.bmc.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 23.Stavenger RA, Cui H, Dowdell SE, Franz RG, Gaitanopoulos DE, Goodman KB, Hilfiker MA, Ivy RL, Leber JD, Marino JP, Jr, Oh HJ, Viet AQ, Xu W, Ye G, Zhang D, Zhao Y, Jolivette LJ, Head MS, Semus SF, Elkins PA, Kirkpatrick RB, Dul E, Khandekar SS, Yi T, Jung DK, Wright LL, Smith GK, Behm DJ, Doe CP, Bentley R, Chen ZX, Hu E, Lee D. Discovery of aminofurazan-azabenzimidazoles as inhibitors of Rho-kinase with high kinase selectivity and antihypertensive activity. J. Med. Chem. 2007;50:2–5. doi: 10.1021/jm060873p. [DOI] [PubMed] [Google Scholar]
- 24.Schröter T, Minond D, Weiser A, Dao C, Habel J, Spicer T, Chase P, Baillargeon P, Scampavia L, Schurer S, Chung C, mader C, Southern M, Tsinoremas N, LoGrasso P, Hodder P. Comparison of miniaturized time-resolved fluorescence resonance energy transfer and enzyme-coupled luciferase high-throughput screening assays to discover inhibitors of Rho-kinase II (ROCK-II) J. Biomol. Screen. 2008;13:40–53. doi: 10.1177/1087057107310806. [DOI] [PubMed] [Google Scholar]
- 25.Schröter T, Griffin E, Weiser A, Feng Y, LoGrasso P. Detection of myosin light chain phosphorylation - A cell-based assay for screening Rho-kinase inhibitors. Biochem. Biophys. Res. Commun. 2008;374:356–360. doi: 10.1016/j.bbrc.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008;6:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi H, Kasa M, Amano M, Kaibuchi K, Hakoshima T. Molecular mechanism for the regulation of rho-kinase by dimerization and its inhibition by fasudil. Structure. 2006;14:589–600. doi: 10.1016/j.str.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi H, Miwa Y, Kasa M, Kitano K, Amano M, Kaibuchi K, Hakoshima T. Structural basis for induced-fit binding of Rho-kinase to the inhibitor Y-27632. J. Biochem. 2006;140:305–311. doi: 10.1093/jb/mvj172. [DOI] [PubMed] [Google Scholar]
- 29.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Visual Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- 30.Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp. Eye Res. 2005;80:197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.