Abstract
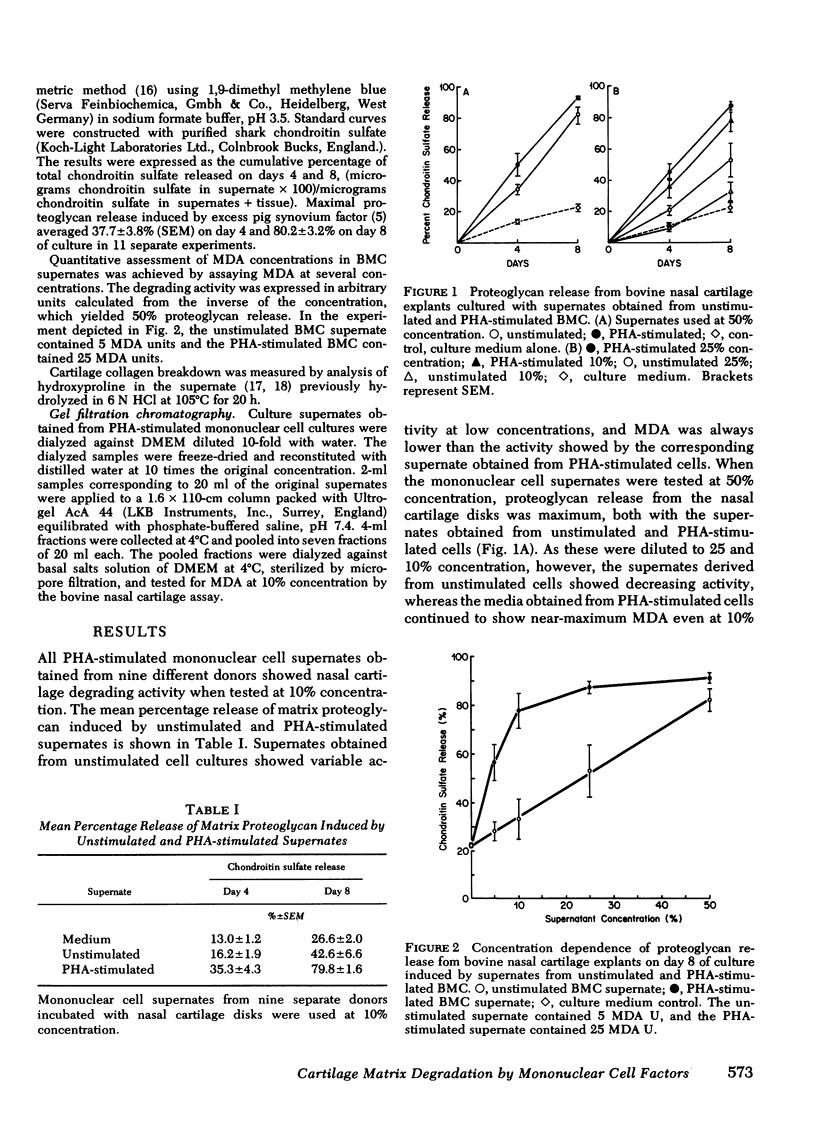
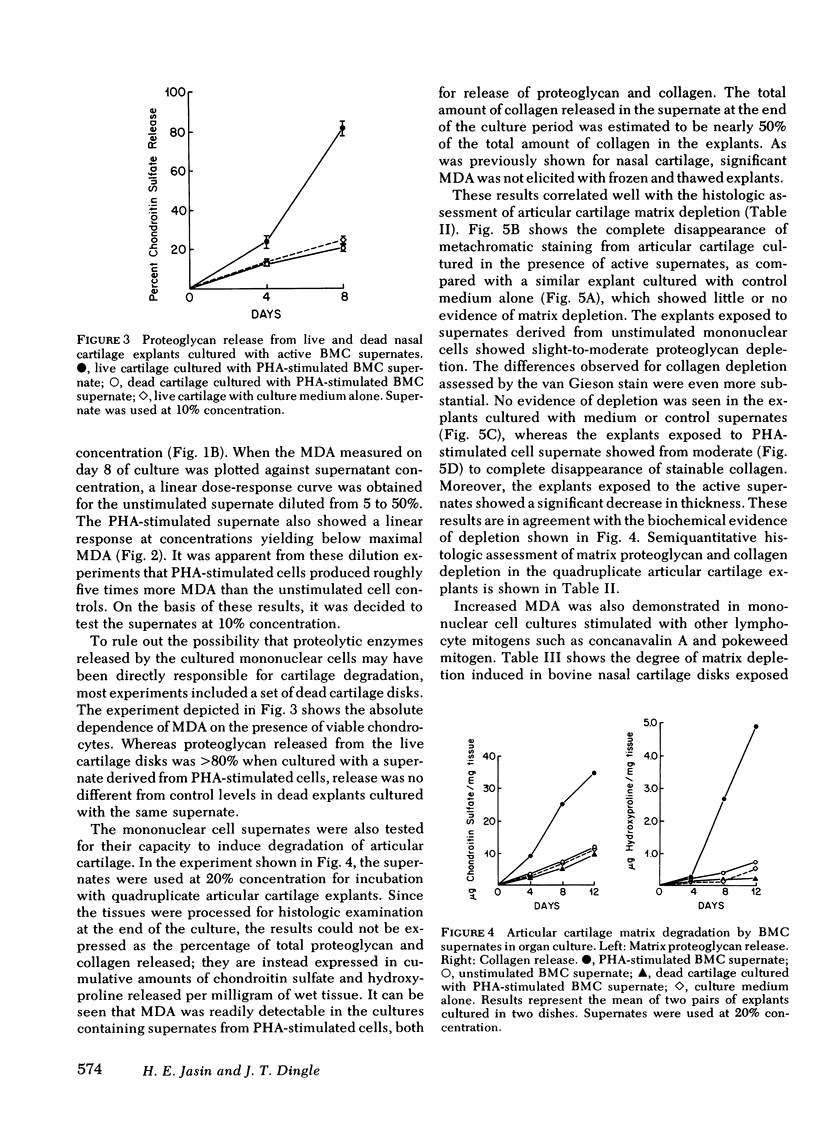
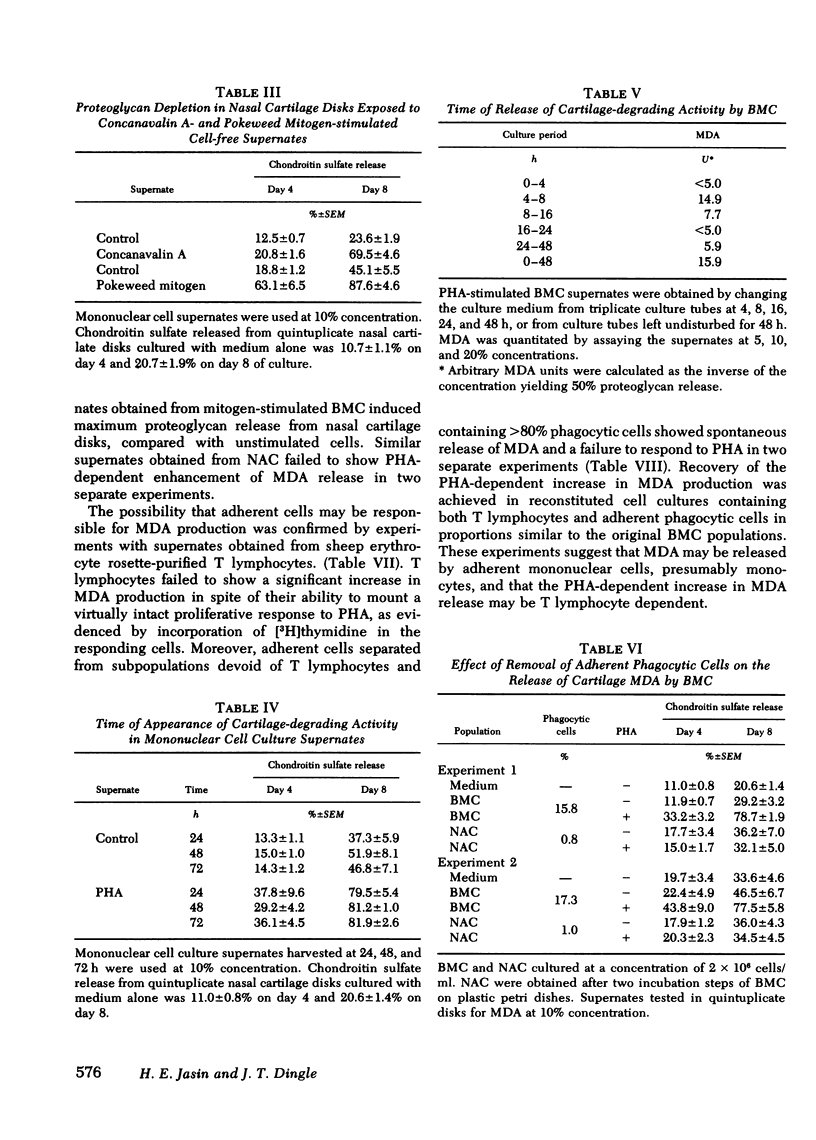
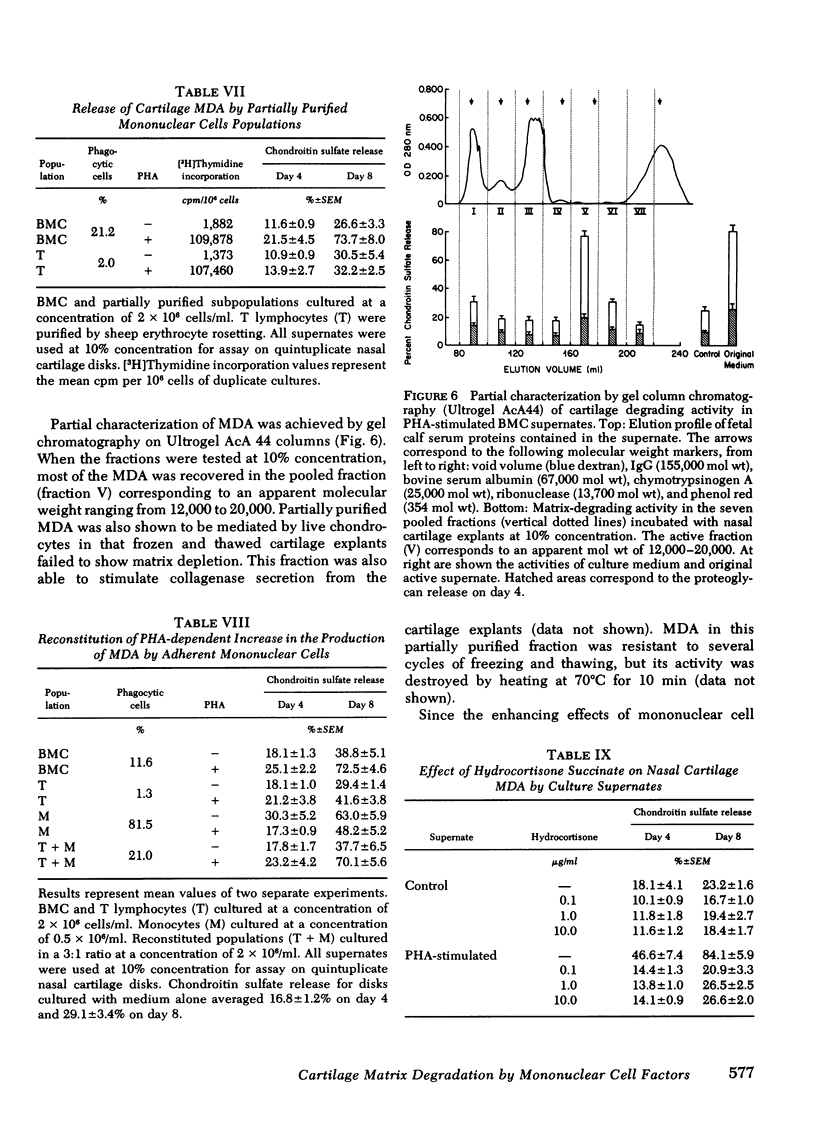
Human blood mononuclear cells (BMC) in short-term culture secrete one or more factors that induce degradation of matrix proteoglycan and collagen in cartilage explants in organ culture. Induction of matrix degradation took place both in nasal septum and articular cartilage explants in the presence of the mononuclear cell supernates. Cartilage degradation in this system was absolutely dependent on the presence of live chondrocytes. Matrix depletion did not occur in dead cartilage explants cultured with active supernates. Supernates obtained from unstimulated BMC showed variable cartilage matrix degrading activity (MDA). BMC stimulated with phytohemagglutinin (PHA) showed increased MDA, which in one dilution experiment was found to be five times higher than that in the unstimulated control supernate. Concanavalin A and pokeweed mitogen were also shown to stimulate release of MDA. Time experiments showed that most of the degrading activity was released by the mononuclear cells during the first day of culture. The cellular origin of MDA was investigated with the aid of partially purified BMC subpopulations. Removal of adherent cells resulted in a decrease of MDA release. Purified T lymphocytes failed to show enhanced MDA release in spite of their ability to mount a virtually intact proliferative response to PHA. Purified adherent cells also failed to show enhanced PHA-dependent MDA release. Nevertheless, restoration of PHA-dependent MDA release took place in reconstituted cell populations containing both T lymphocytes and monocytes. These experiments suggest that MDA may be released by adherent mononuclear cells, presumably monocytes, and that the PHA-dependent increase in MDA release may be mediated by T lymphocytes. Partial characterization of MDA by gel chromatography showed one active fraction corresponding to an apparent molecular weight ranging from 12,000 to 20,000. The fraction was also shown to degrade cartilage matrix only in the presence of live chondrocytes. These results demonstrate that factors released by human BMC mediate degradation of matrix proteoglycan and collagen in intact cartilage explants through chondrocyte activation. This pathogenic mechanism may play a role in in vivo cartilage destruction in chronic inflammatory joint diseases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brackertz D., Hagmann J., Kueppers F. Proteinase inhibitors in rheumatoid arthritis. Ann Rheum Dis. 1975 Jun;34(3):225–230. doi: 10.1136/ard.34.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Graham R., Russell G., Krane S. M. Collagenase production by rheumatoid synovial cells: stimulation by a human lymphocyte factor. Science. 1977 Jan 14;195(4274):181–183. doi: 10.1126/science.188134. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Passwell J. H., Schneeberger E. E., Krane S. M. Interactions among rheumatoid synovial cells and monocyte-macrophages: production of collagenase-stimulating factor by human monocytes exposed to concanavalin A or immunoglobulin Fc fragments. J Immunol. 1980 Apr;124(4):1712–1720. [PubMed] [Google Scholar]
- Deshmukh-Phadke K., Lawrence M., Nanda S. Synthesis of collagenase and neutral proteases by articular chondrocytes: stimulation by a macrophage-derived factor. Biochem Biophys Res Commun. 1978 Nov 14;85(1):490–496. doi: 10.1016/s0006-291x(78)80068-0. [DOI] [PubMed] [Google Scholar]
- Deshmukh-Phadke K., Nanda S., Lee K. Macrophage factor that induces neutral protease secretion by normal rabbit chondrocytes. Studies of some properties and effects on metabolism of chondrocytes. Eur J Biochem. 1980 Feb;104(1):175–180. doi: 10.1111/j.1432-1033.1980.tb04413.x. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Dingle T. T. The site of cartilage matrix degradation. Biochem J. 1980 Aug 15;190(2):431–438. doi: 10.1042/bj1900431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Horsfield P., Fell H. B., Barratt M. E. Breakdown of proteoglycan and collagen induced in pig articular cartilage in organ culture. Ann Rheum Dis. 1975 Aug;34(4):303–311. doi: 10.1136/ard.34.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Saklatvala J., Hembry R., Tyler J., Fell H. B., Jubb R. A cartilage catabolic factor from synovium. Biochem J. 1979 Oct 15;184(1):177–180. doi: 10.1042/bj1840177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Vater C. A., Mainardi C. L., Werb Z. Cellular control of collagen breakdown in rheumatoid arthritis. Agents Actions. 1978 Jan;8(1-2):36–42. doi: 10.1007/BF01972399. [DOI] [PubMed] [Google Scholar]
- Huybrechts-Godin G., Hauser P., Vaes G. Macrophage-fibroblast interactions in collagenase production and cartilage degradation. Biochem J. 1979 Dec 15;184(3):643–650. doi: 10.1042/bj1840643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JASIN H. E., FINK C. W., WISE W., ZIFF M. Relationship between urinary hydroxyproline and growth. J Clin Invest. 1962 Oct;41:1928–1935. doi: 10.1172/JCI104650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of lymphoid cells in the rheumatoid synovial membrane. Arthritis Rheum. 1973 Jul-Aug;16(4):471–486. doi: 10.1002/art.1780160407. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane S. M. IV. Joint erosion in rheumatoid arthritis. Arthritis Rheum. 1974 May-Jun;17(3):306–312. doi: 10.1002/art.1780170316. [DOI] [PubMed] [Google Scholar]
- Lavie G., Zucker-Franklin D., Franklin E. C. Degradation of serum amyloid A protein by surface-associated enzymes of human blood monocytes. J Exp Med. 1978 Oct 1;148(4):1020–1031. doi: 10.1084/jem.148.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Farrar J. J. Revised nomenclature for antigen-nonspecific T-cell proliferation and helper factors. Cell Immunol. 1979 Dec;48(2):433–436. doi: 10.1016/0008-8749(79)90139-4. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Reynolds J. J., Murphy G., Sellers A., Cartwright E. A new factor that may control collagen resorption. Lancet. 1977 Aug 13;2(8033):333–335. doi: 10.1016/s0140-6736(77)91490-8. [DOI] [PubMed] [Google Scholar]
- Ridge S. C., Oronsky A. L., Kerwar S. S. Induction of the synthesis of latent collagenase and latent neutral protease in chondrocytes by a factor synthesized by activated macrophages. Arthritis Rheum. 1980 Apr;23(4):448–454. doi: 10.1002/art.1780230407. [DOI] [PubMed] [Google Scholar]
- Sellers A., Cartwright E., Murphy G., Reynolds J. J. Evidence that latent collagenases are enzyme-inhibitor complexes. Biochem J. 1977 May 1;163(2):303–307. doi: 10.1042/bj1630303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J., Burleigh M. C. The degradation of articular collagen by neutrophil proteinases. Biochim Biophys Acta. 1977 Aug 11;483(2):386–397. doi: 10.1016/0005-2744(77)90066-3. [DOI] [PubMed] [Google Scholar]
- Stastny P., Rosenthal M., Andreis M., Ziff M. Lymphokines in the rheumatoid joint. Arthritis Rheum. 1975 May-Jun;18(3):237–243. doi: 10.1002/art.1780180307. [DOI] [PubMed] [Google Scholar]
- Steinberg J., Sledge C. B., Noble J., Stirrat C. R. A tissue-culture model of cartilage breakdown in rheumatoid arthritis. Quantitative aspects of proteoglycan release. Biochem J. 1979 May 15;180(2):403–412. doi: 10.1042/bj1800403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplits M. S., Crissman J. D., Herman J. H. Histologic assessment of lymphokine-mediated suppression of chondrocyte glycosaminoglycan synthesis. Arthritis Rheum. 1979 Jan;22(1):66–70. doi: 10.1002/art.1780220110. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. The release of collagenase as an inactive proenzyme by bone explants in culture. Biochem J. 1972 Jan;126(2):275–289. doi: 10.1042/bj1260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater C. A., Mainardi C. L., Harris E. D., Jr Activation in vitro of rheumatoid synovial collagenase from cell cultures. J Clin Invest. 1978 Nov;62(5):987–992. doi: 10.1172/JCI109228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by lymphokine-activated macrophages. Science. 1975 Jan 24;187(4173):261–263. doi: 10.1126/science.163038. [DOI] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]



