Abstract
Ubxd8, a multidomain protein sensor for long-chain unsaturated fatty acids (FAs), plays a crucial role to maintain cellular homeostasis of FAs. Ubxd8 polymerizes upon interaction with long-chain unsaturated FAs, but the molecular mechanism involved in this polymerization remains unclear. Here we report that the UAS domain of Ubxd8 mediates this polymerization. We show that a positively charged surface area in the domain is required for the reaction. Mutations changing the positively charged residues in this area to glutamates prevented long-chain unsaturated FAs from inducing oligomerization of Ubxd8. Consequently, the mutant protein no longer responded to regulation by long-chain unsaturated FAs in cultured cells. Long-chain unsaturated FAs also induced polymerization of Fas-associated factor 1 (FAF1), the only other mammalian protein that contains a UAS domain homologous to that of Ubxd8. These results provide further insights into protein-FA interactions by identifying the UAS domain as a motif interacting with long-chain unsaturated FAs.
Keywords: Fas-associated factor 1, Insig-1, protein degradation
Fatty acids (FA) are crucial nutrients for cell survival, yet their overaccumulation is toxic to cells. Thus, cells develop multiple pathways to maintain their homeostasis. One of the pathways is feedback inhibition in FA synthesis. We have previously identified Ubxd8 as a key regulator for the reaction (1, 2). In cells depleted of FAs, Ubxd8 facilitates proteasomal degradation of Insig-1, a membrane protein of the endoplasmic reticulum (ER), through its interaction with the protein (1). Depletion of Insig-1 triggers proteolytic activation of sterol-regulatory element binding protein (SREBP)-1, a transcription factor synthesized as a membrane-bound precursor (1, 3). This activation allows the NH2-terminal domain of SREBP-1 to activate all genes required for FA synthesis (4). Excessive long-chain unsaturated FAs block the interaction between Ubxd8 and Insig-1, leading to stabilization of Insig-1 (1). Consequently, proteolytic activation of SREBP-1 is inhibited, and transcription of genes involved in FA synthesis declines.
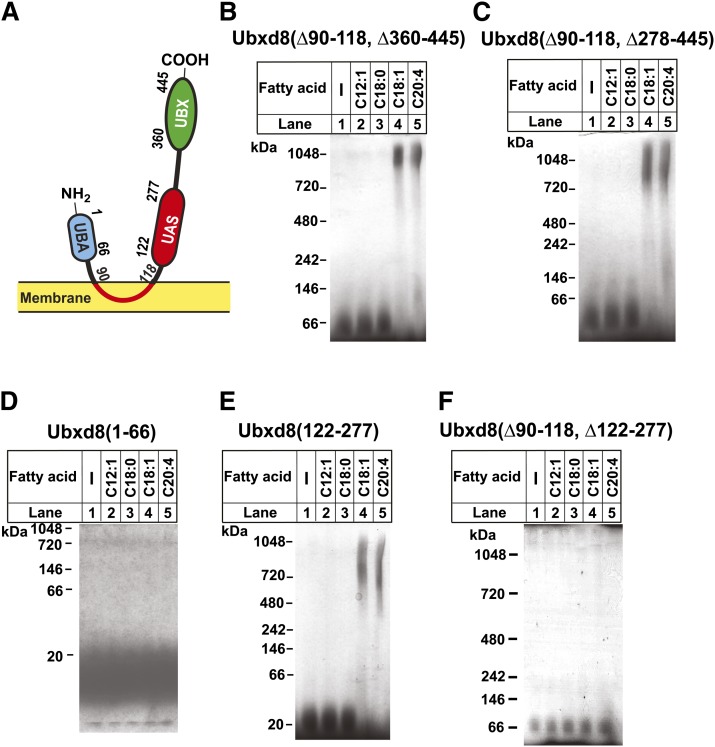
Ubxd8 belongs to a family of proteins that contain a UBX domain (Fig. 1A) (5), which interacts with p97 (6), a protein required for proteasomal degradation of ER-associated membrane proteins (7). In addition to the UBX domain, Ubxd8 also contains a UBA domain, which is known to bind polyubiquitin chains (6), and a UAS domain with unknown function (Fig. 1A). Ubxd8 is inserted into membranes via a stretch of hydrophobic amino acid residues located between the UBA and UAS domain (amino acid residues 90–118) that forms a hairpin loop in membranes (Fig. 1A) (2). Surprisingly, deletion of the membrane localization domain from Ubxd8 did not affect interaction between Ubxd8 and Insig-1 in cells deprived of FAs, and this interaction was still inhibited by long-chain unsaturated FAs (2). Thus, Ubxd8(Δ90–118) became a facile tool to study the interaction of Ubxd8 with FAs in vitro because the assay can be performed in the absence of detergents that frequently interfere with the in vitro-binding assays with FAs. This analysis revealed that long-chain unsaturated FAs stimulated polymerization of purified recombinant Ubxd8(Δ90–118) (2). This effect was specific to long-chain unsaturated FAs as long-chain saturated or median-chain unsaturated FAs failed to produce the same effect (2). This specificity matches the specificity of FAs to stabilize Insig-1 in cultured cells (1, 2). Thus, the in vitro characterization performed with Ubxd8(Δ90–118) is likely to be a good indication of the full-length Ubxd8 protein inside cells.
Fig. 1.
Long-chain unsaturated FAs induce polymerization of purified UAS domain in Ubxd8. (A) Schematic diagram of the domain structure of Ubxd8. (B–F) Indicated purified proteins were incubated with 100 μM of indicated FAs, subjected to blue native PAGE, and visualized with Coomassie blue staining.
In the current study, we used the approach of bimolecular fluorescence complementation to provide direct evidence that long-chain unsaturated FAs also induce polymerization of full-length Ubxd8 in cultured cells. We demonstrate that this polymerization is mediated through the UAS domain present in Ubxd8. Moreover, we show that long-chain unsaturated FAs also specifically stimulate polymerization of Fas-associated factor 1 (FAF1), another mammalian protein that contains a UAS domain. These observations suggest that proteins containing UAS domains may subject to regulation by FAs.
EXPERIMENTAL PROCEDURES
Materials
We obtained FAs from Nu-Chek-Prep, Inc.; FA-free BSA from Roche Applied Science; MG132 and Nonidet P-40 alternative (NP-40) from Calbiochem; monoclonal anti-T7 from Novagen; horseradish peroxidase-conjugated donkey anti-mouse and anti-rabbit IgGs (affinity-purified) from Jackson ImmunoResearch Laboratories; and Ni-NTA agarose from Qiagen. Hybridoma cells producing IgG-9E10, a mouse monoclonal antibody against Myc tag, were obtained from the American Type Culture Collection. A rabbit polyclonal antibody against human Ubxd8 was generated by immunizing rabbits with human Ubxd8(Δ90–118). Delipidated fetal calf serum (FCS) was prepared from newborn calf serum by n-butyl alcohol and isopropyl ether extraction method (8). All fatty acids added into culture media were conjugated to BSA (8). For in vitro assays, FAs dissolved in ethanol were added into the in vitro assays. The final concentration of ethanol in the reaction mixture was 1% for these assays.
Plasmid constructs
The following plasmids were described in the indicated reference: pCMV-Myc-Ubxd8 encoding human Ubxd8 with five tandem copies of a c-Myc tag at its NH2-terminus under control of the CMV promoter (1); pCMV-Insig1-T7 encoding human Insig-1 followed by three tandem copies of a T7 epitope tag under control of the CMV promoter (9); and pAcHLT-Ubxd8(Δ90–118) used for producing Ubxd8(Δ90–118) in sf9 cells through recombinant baculovirus (2). pAcHLT-Ubxd8(Δ90–118, Δ360–445), pAcHLT-Ubxd8(Δ90–118, Δ278–445), pAcHLT-Ubxd8(122–277), pAcHLT-Ubxd8(1–66), pAcHLT-FAF1 and pAcHLT-FAF1(325–491) were generated to produce indicated fragments of Ubxd8 or FAF1 in sf9 cells through recombinant baculovirus as previously described (2). pCMV-myc-Ubxd8-VenusN encodes human Ubxd8 tagged at the NH2-terminus with five tandem copies of a c-Myc tag fused at the COOH-terminus with the NH2-terminal fragment of the Venus protein under control of the CMV promoter and a neomycin-resistant gene. pCMV-flag-Ubxd8-VenusC encodes NH2-terminal flag epitope-tagged human Ubxd8 fused at the COOH-terminus with the COOH-terminal fragment of the Venus protein under control of the CMV promoter and a hygromycin-resistant gene. The NH2- and COOH-terminal fragments of the Venus gene were amplified through PCR from pBiFC-VN155(I152L) and pBiFC-VC155 (Addgene), respectively. pBiFC bFos-VC155 encoding bFos fused with the COOH-terminal fragment of the Venus protein, pBiFC bJun-VN155(I152L) encoding bJun fused with the NH2-terminal fragment of the Venus protein, and pBiFC bFos(Δzip)-VC155 encoding a mutant bFos, in which the bZIP domain was deleted, fused with the NH2-terminal fragment of the Venus protein were all acquired from Addgene. Mutations in Ubxd8 were generated through a site-directed mutagenesis kit (Stratagene) according to the manufacture's protocol.
Cell culture
All cells were maintained at 37°C in 8.8% CO2. SRD-13A cells are a clone of mutant CHO cells deficient in Scap (10). They were maintained in medium A (1:1 mixture of Ham's F-12 medium and DMEM, 100 units/ml penicillin, 100 µg/ml streptomycin sulfate) supplemented with 5% (v/v) FCS, 5 µg/ml cholesterol, 1 mM sodium mevalonate, and 20 µM sodium oleate. SRD-13A/pUbxd8-Venus cells were generated by stably transfecting pCMV-myc-Ubxd8-VenusN and pCMV-flag-Ubxd8-VenusC into SRD-13A cells. The cells were maintained in medium used to culture SRD-13A cells supplemented with 2.5 mg/ml hygromycin and G418.
Transient transfection
On day 0, SRD-13A cells were set up for experiments at 4.5 × 105 cells per 60 mm dish. On day 1, cells were transiently transfected with the indicated plasmids with FuGENE6 reagent (Roche Applied Science) according to the manufacturer's protocol. Conditions of incubation after the transfection are described in the figure legends.
Immunoblot analysis
Aliquots of the lysate were subjected to SDS-PAGE followed by immunoblot analysis. Antibodies used in the current studies were IgG-9E10 (1 μg/ml), a monoclonal anti-T7 (0.4 μg/ml), a polyclonal anti-Ubxd8 (1 μg/ml), a polyclonal anti-actin (1:2000 dilution). Horseradish peroxidase-conjugated donkey anti-mouse and anti-rabbit IgGs (0.2 μg/ml) were used as the secondary antibody in all immunoblot analysis. Bound antibodies were visualized by chemiluminescence using the SuperSignal substrate system (Pierce) according to the manufacturer's instructions.
Immunoprecipitation
Coimmunoprecipitation experiments to determine the interaction between Ubxd8 and Insig-1 were performed as previously described (1). Briefly, the pooled cell pellets from three 60 mm dishes were lysed in buffer A (25 mM Tris-HCl, pH 7.2, 150 mM NaCl, 0.1% NP-40, 10 µg/ml leupeptin, 5 µg/ml pepstatin A, 10 µg/ml aprotinin, and 25 µg/ml N-acetylleucinal-leucinal-norleucinal), and incubated with 30 µl Anti-c-Myc Affinity Gel (Sigma) to immunoprecipitate transfected Ubxd8. Aliquots of pellet and supernatant fractions of the reaction were subject to SDS-PAGE followed by immunoblot analysis as described in the figure legend.
Live cell fluorescent microscopy
On day 0, SRD-13A/pUbxd8-Venus cells were set up at 6 × 104 cells per 35 mm glass-bottom dish. On day 1, cells were switched to medium A supplemented with 5% delipidated FCS. On day 2, fluorescent images were acquired with EX 492/EM 535 nm filter of Deltavision RT microscope in a 37°C chamber before and after incubation for 6 h with the indicated FAs in the same medium. ImageJ (http://rsbweb.nih.gov/ij) was used for quantification of fluorescent intensity. Relative fluorescent intensity was calculated by dividing the intensity after the FA treatment by that before the treatment. The statistical analysis was performed with one tailed paired t-test.
Blue native PAGE analysis of purified proteins
Proteins were purified, incubated with indicated FAs, and analyzed by blue native PAGE as previously described (2). Briefly, wild-type or mutant Ubxd8 and FAF1 proteins were expressed in sf9 cells and purified to homogeneity with Ni2+-affinity followed by size exclusion chromatography using Superdex 200 (10/300GL) column (GE Healthcare). Purified proteins (0.7 µg) were incubated with FAs (added as stock solutions dissolved in ethanol) in buffer B (25 mM Tris-HCl, pH 7.2, 0.15 M NaCl, 1 mM DTT) at room temperature for 5 min (final volume, 18 µl). After receiving 2 µl of a 10× loading buffer (5 mM Bis-Tris, pH 7.0, 60% glycerol, 0.5 µg/ml Coomassie G250, and 10 mg/ml 6-aminohexanoic acid), the samples were subjected to 4–12% blue native gel electrophoresis for 3 h at 4°C.
Circular dichroism
Purified proteins (1.3 µM) in 0.5 ml buffer B were measured for circular dichroism (CD) spectrum. The spectra were recorded at wavelength ranging 198–250 nm by a J-815 CD spectrometer (JASCO Inc.).
Structural modeling of the UAS domain in Ubxd8
The structure model of the UAS domain in Ubxd8 was generated by using the NMR structure of the FAF1-UAS domain (PDB ID: 2EC4_A) as the template with programs HHPred (11) and Modeler (12) incorporated at the MPI Bioinformatics Toolkit web server (13).
RESULTS
To identify the region in Ubxd8 that is required for long-chain unsaturated FAs to induce polymerization of the protein, we made various deletion mutants in Ubxd8(Δ90–118) fused with a His6-tag at the NH2 terminus, purified the recombinant protein expressed in sf9 cells to homogeneity using Ni2+-affinity followed by size exclusion chromatography, and analyzed the effect of FAs on polymerization of the proteins through blue native PAGE, a technique that allows detection of protein complexes in their native state (2, 14). Oleate (C18:1) and arachidonate (C20:4), two classes of long-chain unsaturated FA, were able to induce polymerization of Ubxd8(Δ90–118) in which the UBX domain (amino acid residues 360–445) (Fig. 1A) located at the COOH-terminal end of the protein was deleted (Fig. 1B, lanes 4 and 5). Similar to Ubxd8(Δ90–118), this polymerization was specific to long-chain unsaturated FAs as medium-chain unsaturated FA dodecenoate (C12:1) and long-chain saturated FA stearate (C18:0) failed to produce the same effect (Fig. 1B, lanes 2 and 3). This polymerization remained intact when a stretch of 82 amino acids located between the UAS and UBX domain (amino acid residues 278–359) was also deleted (Fig. 1C). These results suggest that the remaining two domains in Ubxd8, namely, the UBA and UAS domains (Fig. 1A), may be required for this polymerization. While long-chain unsaturated FAs failed to induce oligomerization of the purified UBA domain (amino acid residues 1–66) (Fig. 1D), they were effective in stimulating polymerization of the purified UAS domain (amino acid residues 122–277) (Fig. 1E). Consistent with these observations, deletion of the UAS domain from Ubxd8(Δ90–118) significantly inhibited unsaturated FA-induced polymerization of the protein (Fig. 1F).
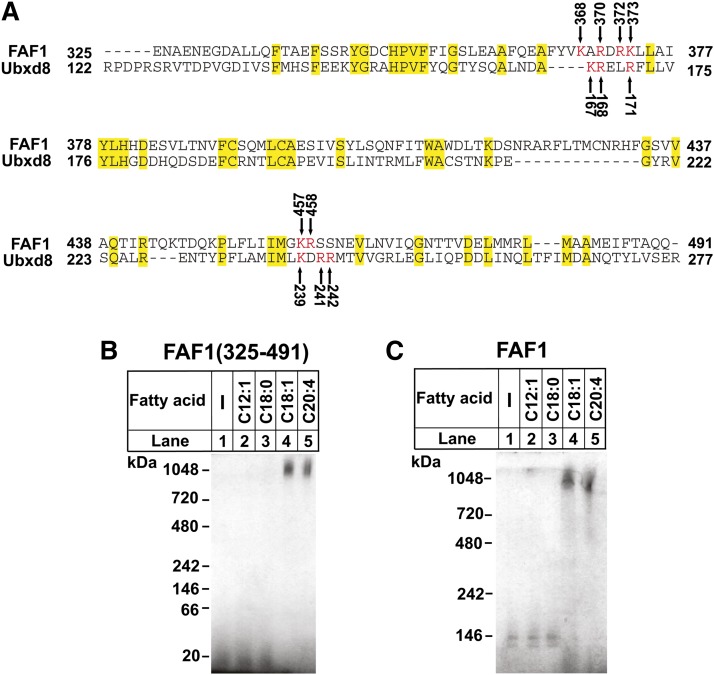
If UAS domain is a motif that polymerizes upon interaction with long-chain unsaturated FAs, proteins other than Ubxd8 that contain a UAS domain should also polymerize in response to these FAs. Database search revealed that FAF1, a cytosolic protein (15), was the only protein expressed in mammalian cells containing a UAS domain homologous to that of Ubxd8. The UAS domain in human FAF1 is 27% identical to that of human Ubxd8 (Fig. 2A). We thus expressed the UAS domain of FAF1 and the full-length FAF1 protein in sf9 cells and purified them to homogeneity. Blue native PAGE analysis indicated that long-chain unsaturated FAs also specifically induced polymerization of the UAS domain of FAF1 [FAF1 (325–491)] (Fig. 2B) as well as the full-length FAF1 protein (Fig. 2C).
Fig. 2.
Long-chain unsaturated FAs induce polymerization of purified FAF1 through its UAS domain. (A) Sequence alignment of UAS domain between human Ubxd8 and FAF1 was performed through the BLAST analysis of the Non-redundant Protein Sequence database in the National Center for Biotechnology Information. Identical residues were shaded in yellow. Lysines and arginine residues in both proteins constituting the conserved positively charged surface (see Fig. 3A) are numbered and highlighted in red. (B and C) Blue native PAGE analysis of indicated purified protein was performed as described in Fig. 1B.
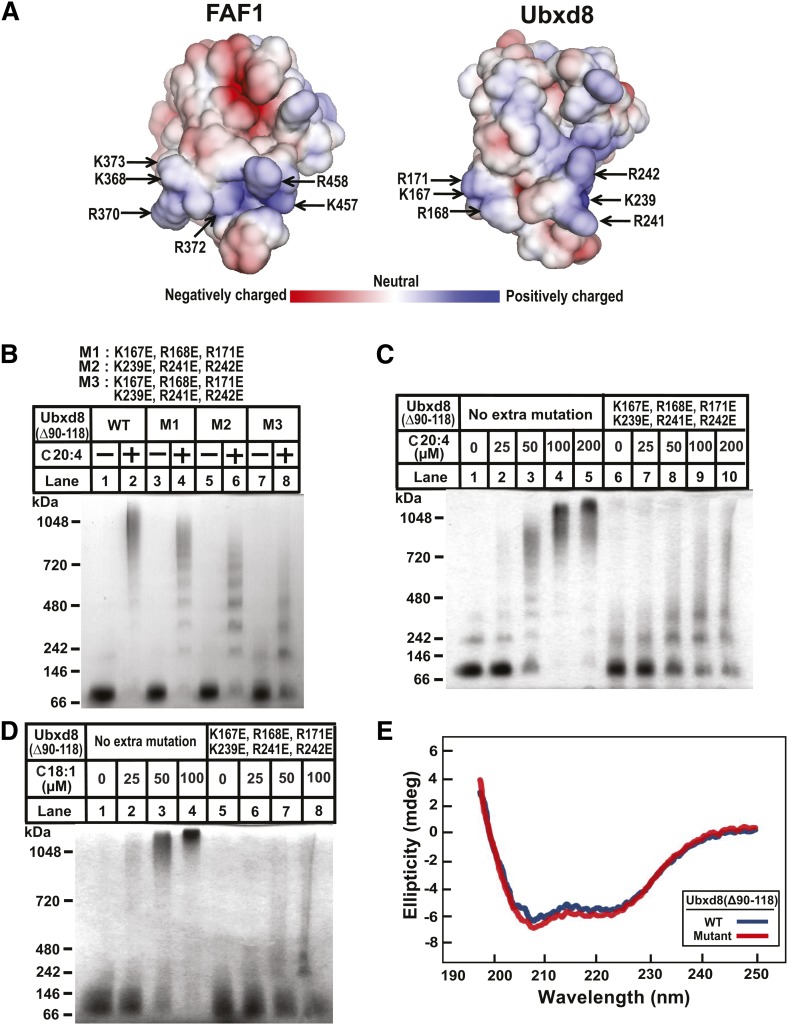
While the structure of the UAS domain in Ubxd8 remains unsolved, the structure of the same domain in FAF1 has been determined through nuclear magnetic resonance (PDB ID: 2EC4_A). According to the structure, there is a surface area highly enriched in positively charged residues (Fig. 3A, left panel). The structural model built on the homology of the UAS domain between Ubxd8 and FAF1 (Fig. 2A) predicts that the same positively charged area also exists in Ubxd8 and that this area is made by two adjacent loops, each of which contains three positively charged residues (i.e., K167, R168, and R171 in one loop, and K239, R241, and R242 in the other loop) (Fig. 3A, right panel). Since the negatively charged carboxyl group is required for long-chain unsaturated FAs to interact with purified Ubxd8(Δ90–118) (2), the positively charged residues in this area may be important for the interaction. To test this hypothesis, we mutated these lysines and arginines to negatively charged glutamates, and then analyzed the effect of the mutations on long-chain unsaturated FA-induced polymerization of Ubxd8(Δ90–118). Arachidonate was less effective in inducing polymerization of Ubxd8(Δ90–118, K167E, R168E, R171E) and Ubxd8(Δ90–118, K239E, R241E, R242E) compared with that of Ubxd8(Δ90–118), but the effect was rather modest (Fig. 3B, lanes 1–6). This polymerization was more pronouncedly inhibited by combined mutations of all six positively charged residues to glutamates (Fig. 3B, lanes 7 and 8). To further determine the effect of the mutation on long-chain unsaturated FA-induced oligomerization of Ubxd8(Δ90–118), we incubated Ubxd8(Δ90–118) and Ubxd8(Δ90–118, K167E, R168E, R171E, K239E, R241E, R242E) with various concentrations of oleate or arachidonate. Replacing all of the positively charged residues in the surface patch to glutamates not only inhibited polymerization induced by arachidonate (Fig. 3C) but also that induced by oleate (Fig. 3D). Ubxd8(Δ90–118, K167E, R168E, R171E, K239E, R241E, R242E) displayed a CD spectrum identical to that of Ubxd8(Δ90–118), an observation suggesting that the mutation did not alter the global folding of the protein (Fig. 3E).
Fig. 3.
Effects of the mutations in the UAS domain on polymerization of purified Ubxd8(Δ90–118). (A) The structure of the UAS domain of FAF1 (left, PDB code 2EC4) and that of Ubxd8 predicted by molecular modeling (right). Lysine and arginine residues on the conserved positively charged surface are highlighted. Red and blue denotes negatively and positively charged residues, respectively. (B–D) Blue native PAGE analysis of indicated purified proteins incubated with 100 µM arachidonate (B), or indicated concentration of arachidonate (C) or oleate (D) was performed as described in Fig. 1B. (E) CD spectrum of indicated purified proteins.
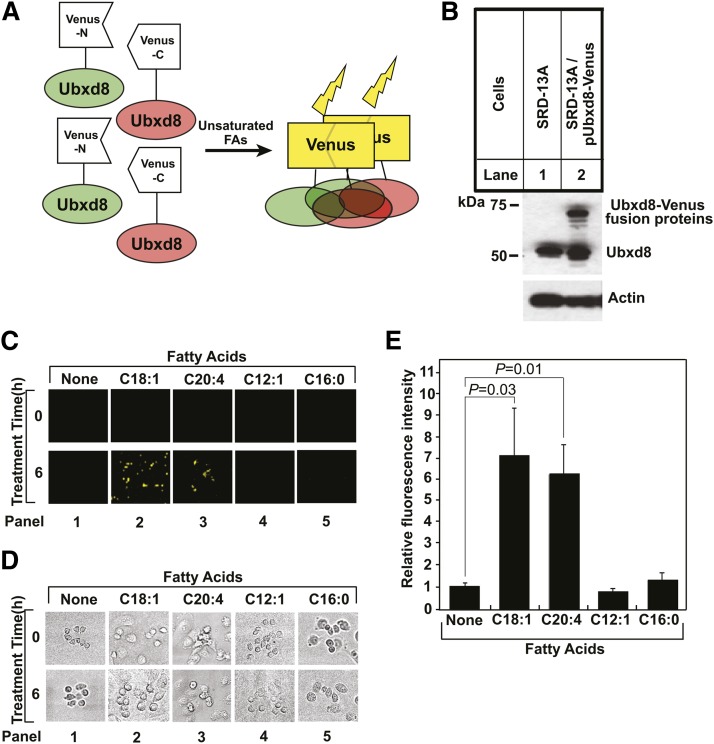
We then determined the effect of the mutations on long-chain unsaturated FA-induced polymerization of full-length Ubxd8 in cultured cells. To perform the experiments, we had to first develop an assay to measure such polymerization. We chose the approach of bimolecular fluorescence complementation (16) to investigate long-chain unsaturated FA-induced polymerization of Ubxd8. For this purpose, we generated two plasmids encoding full-length Ubxd8 fused at its COOH-terminus with either NH2-terminal or COOH-terminal half of the Venus protein, a variant of yellow fluorescent protein (16) (Fig. 4A). Cells in which Ubxd8 is not polymerized should not be fluorescent because the NH2- and COOH-terminal halves of the Venus protein are separated from each other (Fig. 4A). Polymerization of Ubxd8 should bring the NH2- and COOH-terminal halves of the Venus protein to close proximity to reconstitute the fluorescent activity of the Venus protein (Fig. 4A). To use this approach to determine long-chain unsaturated FA-induced polymerization of Ubxd8, we stably transfected SRD-13A cells with these two plasmids and selected a clone of the cells (SRD-13A/pUbxd8-Venus) in which the Ubxd8 fusion proteins were expressed at an amount no more than that of endogenous Ubxd8 (Fig. 4B). We chose SRD-13A cells because they are mutant CHO cells auxotrophic for unsaturated FAs that can be easily depleted of these FAs by incubating them in medium free of FAs (2, 10). These cells were barely fluorescent when they were incubated in the absence of FAs (Fig. 4C, D, panel 1). Supplementation of the incubation medium with long-chain unsaturated Fas, such as oleate (C18:1) or arachidonate (C20:4), dramatically enhanced the fluorescent intensity of the cells (Fig. 4C, D, panels 2 and 3). Quantification of the fluorescent signal in these images showed that these long-chain unsaturated FAs increased the fluorescent intensity by 6- to 7-fold (Fig. 4E). This effect was specific to long-chain unsaturated FAs, as medium-chain unsaturated FA C12:1 and saturated FA palmitate (C16:0) failed to produce the same result (Fig. 4C, D, panels 4 and 5, and Fig. 4E).
Fig. 4.
Long-chain unsaturated FAs induce polymerization of full-length Ubxd8 in cells. (A) Principle of using bimolecular fluorescence complementation to analyze long-chain unsaturated FA-induced polymerization of Ubxd8. (B) Immunoblot analysis of indicated cells. Ubxd8 fused with either NH2 or COOH-terminal fragment of the Venus protein migrated at the same position on SDS-PAGE. These proteins are thus labeled as Ubxd8-Venus fusion proteins. (C and D) Fluorescent (C) and bright field images (D) of SRD-13A/pUbxd8-Venus cells before and after 6 h treatment with 100 µM of indicated FAs. (E) Relative fluorescent intensity calculated by dividing fluorescent intensity after the FA treatment by that before the treatment shown in (C). Results were reported as mean ± SE from three independent experiments.
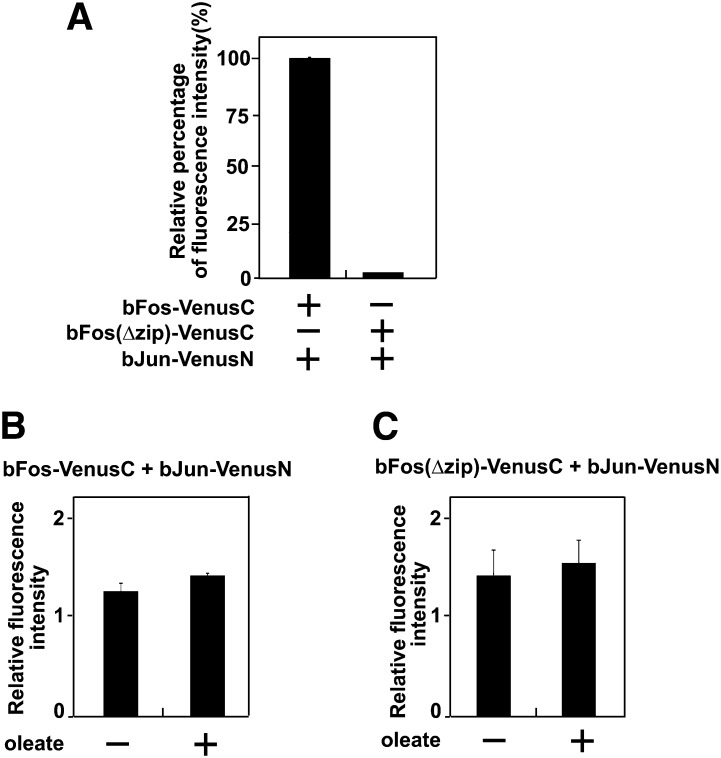
As a control, we also examined the effect of long-chain unsaturated FAs on interaction between bFos and bJun analyzed through the same bimolecular fluorescence complementation approach (16). SRD-13A cells were transfected with a plasmid encoding NH2-terminal half of the Venus protein fused with bJun (bJun-VenusN) and a plasmid encoding COOH-terminal half of the Venus protein fused with either wild-type bFos (bFos-VenusC) or a mutant version of the protein in which the bzip domain required for interaction with bJun was deleted [bFos(Δzip)-VenusC] (16). The fluorescent intensity of the cells cotransfected with the plasmid encoding the mutant bFos fusion protein was only 2% of that cotransfected with the plasmid encoding the wild-type bFos fusion protein (Fig. 5A). Treatment with oleate did not affect the fluorescent intensity of the cells expressing the wild-type bFos fusion protein (Fig. 5B). This result suggests that oleate does not enhance fluorescent intensity of the reconstituted Venus protein. Oleate also had no effect on fluorescent intensity of the cells expressing the mutant bFos fusion protein (Fig. 5C). This observation suggests that oleate does not induce reconstitution of the Venus protein nonspecifically.
Fig. 5.
Oleate does not affect bimolecular fluorescence complementation between bFos and bJun. (A) Fluorescent intensity of SRD-13A cells transiently transfected with indicated plasmids. The fluorescent intensity of the cells transfected with the plasmids encoding bJun-VenusN and bFos-VenusC is set to 100%. (B and C) The effect of oleate on relative fluorescent intensity of the cells transfected with the indicated plasmids was quantified as described in Fig. 4E.
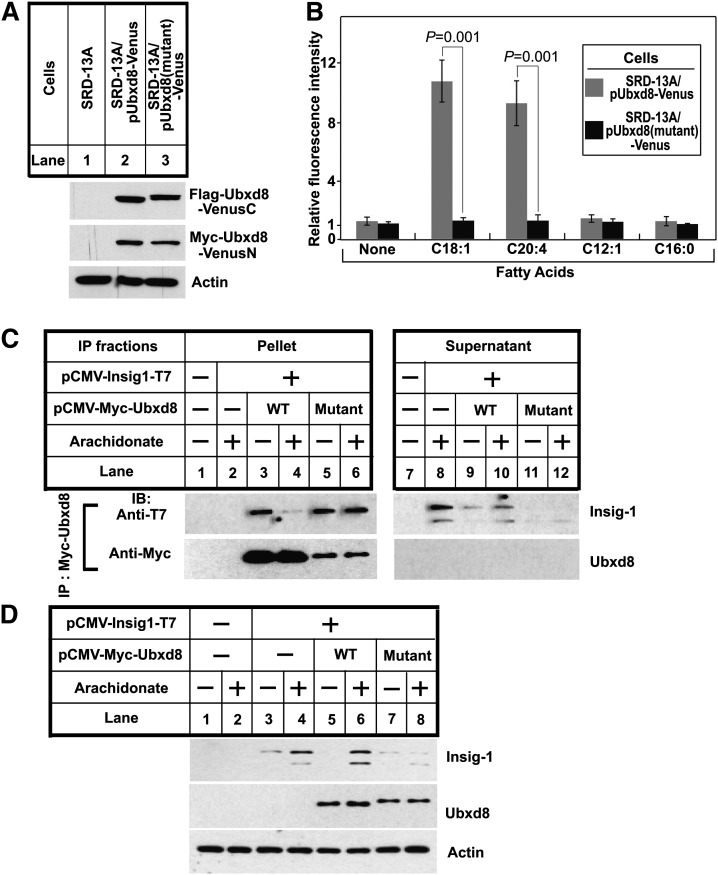
We then made the same mutations that disrupted long-chain unsaturated FA-induced polymerization of Ubxd8(Δ90–118) (K167E, R168E, R171E, K239E, R241E and R242E) in full-length Ubxd8. We stably transfected SRD-13A cells with plasmids encoding the mutant Ubxd8 fused with NH2- or COOH-terminal fragment of the Venus protein, and selected a clone of the cells [SRD-13A/pUbxd8(mutant)-Venus] in which expression of the mutant Ubxd8 fusion proteins was similar to that of wild-type Ubxd8 fusion proteins found in SRD-13A/pUbxd8-Venus cells (Fig. 6A). We determined the effect of the mutation on long-chain unsaturated FA-induced polymerization of the protein through bimolecular fluorescence complementation. While arachidonate and oleate markedly enhanced the fluorescent intensity of the cells transfected with the wild-type Ubxd8-Venus fusion proteins (SRD-13A/pUbxd8-Venus), these FAs failed to increase the fluorescent intensity of the cells transfected with the mutant Ubxd8-Venus fusion proteins [SRD-13A/pUbxd8(mutant)-Venus] (Fig. 6B). This result suggests that long-chain unsaturated FAs are unable to induce polymerization of the mutant Ubxd8 in cultured cells.
Fig. 6.
Effects of the mutations in the UAS domain on functions of Ubxd8 in cells. (A) Immunoblot analysis of Flag epitope-tagged Ubxd8 fused with COOH-terminal fragment of the Venus protein (Flag-Ubxd8-VenusC) and Myc epitope-tagged Ubxd8 fused with NH2-terminal fragment of the Venus protein (Myc-Ubxd8-VenusN) in indicated cells. (B) Quantitative analysis of relative fluorescent intensity of indicated cells treated with indicated FAs was performed as described in Fig. 4E. (C) On day 0, SRD-13A cells were set up at 4.5 × 105 cells/60-mm dish. On day 1, they were transfected with a plasmid encoding T7 epitope-tagged Insig-1 (0.3 µg/dish) and a plasmid encoding wild-type or the mutant Ubxd8 fused with the Myc epitope tag (0.3 µg/dish), followed by incubation in medium A supplemented with 5% delipidated FCS. On day 2, cells were treated with 100 μM arachidonate as indicated in medium A supplemented with 5% delipidated FCS and 30 μM MG132 for 6 h. Detergent lysates of these cells were subjected to immunoprecipitation with anti-Myc to precipitate transfected Ubxd8. Pellets (representing 0.5 dish of cells) and supernatants (representing 0.1 dish of cells) of the immunoprecipitation were subjected to immunoblot analysis. (D) SRD-13A cells transfected with indicated plasmids were treated as described in (C), except that the cells were incubated in the absence of MG132. Cell lysates were subject to immunoblot analysis.
The lack of the response of the mutant Ubxd8 to long-chain unsaturated FAs may be caused by a specific defect in response to these FAs or a global defect in protein folding. If the mutation specifically disrupts the interaction with long-chain unsaturated FAs, the mutant protein should still be able to bind Insig-1 and to stimulate its degradation in FA-depleted cells, but the effect can no longer be inhibited by these FAs. On the other hand, if the mutation affects protein folding, the mutant protein should not be functional so that the protein is not expected to bind or degrade Insig-1. To differentiate these possibilities, we first examined the interaction between Insig-1 and the mutant Ubxd8. For this purpose, we transfected SRD-13A cells with a plasmid encoding Insig-1 together with a plasmid encoding either wild-type or the mutant Ubxd8, treated the cells with the proteasome inhibitor MG132 to block degradation of Insig-1, and determined their interaction through a coimmunoprecipitation experiment. Insig-1 was coimmunoprecipitated with wild-type Ubxd8 in cells depleted of FAs but not those treated with arachidonate (Fig. 6C, lanes 3 and 4). Insig-1 was also coimmunoprecipitated with the mutant Ubxd8, but the amount of Insig-1 coimmunoprecipitated was no longer reduced by treatment with arachidonate (Fig. 6C, lanes 5 and 6).
We then determined the effect of the mutant Ubxd8 on degradation of Insig-1. To this end, we transfected SRD-13A cells with a plasmid encoding Insig-1 with or without cotransfection with a plasmid encoding either wild-type or the mutant Ubxd8. Arachidonate increased the amount of Insig-1 protein (Fig. 6D, lanes 3 and 4). This effect was not altered by cotransfection with a plasmid encoding wild-type Ubxd8 (Fig. 6D, lanes 5 and 6). In contrast to these results, arachidonate was unable to increase the amount of Insig-1 protein in cells cotransfected with a plasmid encoding the mutant Ubxd8 (Fig. 6D, lanes 7 and 8), an observation suggesting that the mutant Ubxd8 stimulated degradation of Insig-1 even in cells treated with arachidonate. The results shown in Fig. 6C, D suggest that the mutation specifically impairs the response of the protein to long-chain unsaturated FAs without affecting the global folding of the protein.
DISCUSSION
The current study identifies UAS domain as a motif polymerizing upon interaction with long-chain unsaturated FAs. UAS domain is a divergent member of the large thioredoxin-like protein superfamily (17). However, no other members of this superfamily are known to interact with FAs. We show that a predicted positively charged surface patch composed by six lysine and arginine residues located at two adjacent loops in UAS domain are important for long-chain unsaturated FAs to induce polymerization of the UAS domain. Thus, it is possible that long-chain unsaturated FAs may bind to the surface of the UAS domain. Such binding may render the protein surface more hydrophobic, a condition that may facilitate self-association of the UAS-FA complexes. Alternatively, these positively charged residues may not be required for FA binding but critical for polymerization of the domain upon interaction with the FAs. Obviously, structural analysis of the UAS domain in complex with long-chain unsaturated FAs is required to differentiate these possibilities. However, the heterogeneous sizes of Ubxd8 polymers pose a considerable challenge for such analysis.
A major discovery in the current study is that proteins containing a UAS domain may subject to regulation by long-chain unsaturated FAs. Indeed, Ubxd8 is such a protein. We show that long-chain unsaturated FAs induce polymerization of Ubxd8 to prevent the protein from stimulating degradation of Insig-1. FAF1 is the only other mammalian protein that contains a UAS domain homologous to that of Ubxd8. We show that long-chain unsaturated FAs stimulated polymerization of purified recombinant FAF1, an observation suggesting that the function of FAF1 may also be regulated by these FAs. FAF1 has diverse functions (18), but none of the functions so far has been demonstrated to be regulated by FAs. FAF1 was identified as a regulator for nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (19–21), a transcription factor that activates proinflammatory cytokines (22). It was reported previously that saturated but not unsaturated FAs activated production of proinflammatory cytokines through activation of NF-κB (23–25). These observations suggest that the FA sensor involved in this regulation should tell the difference between saturated and unsaturated FAs. Our in vitro analysis indicates that FAF1 meets this requirement. Thus, future work is required to determine whether FAF1 is involved in FA-mediated activation of NF-κB.
In addition to Ubxd8 and FAF1, Ubxd7 is the only other mammalian protein that contains a domain remotely resembling that of the UAS domain found in Ubxd8 and FAF1 (26). Ubxd7 facilitates degradation of hypoxia-inducible factor 1 alpha (HIF1α) (26), a transcription factor activating genes in response to hypoxia (27). Ubxd7 also functions as an activator for cullin-RING ubiquitin ligases (28). It is currently unknown whether functions of Ubxd7 are regulated by long-chain unsaturated FAs.
Our finding that UAS domain is a sensor for long-chain unsaturated FAs suggests that these free FAs may serve as signaling molecules. It is assumed that free FAs are condensed with CoA immediately following their entry to cells. Consequently, free FAs are believed to be kept in very low concentration so that they may not reach high enough concentration to bind UAS domain-containing proteins in cytosol. This traditional view has been challenged by a recent study in which the amount of free FAs and their CoA conjugates in cells was measured directly. The result shows that the amount of free FAs is 100 times more than that of their CoA conjugates in cultured cells (29). Moreover, free FAs have been reported to be concentrated in membranes in their protonated form, and they are readily released into aqueous environment adjacent to the membranes as FA anions (30). Thus, the local concentration of free FAs at the interface between cytosol and ER membranes may be high enough to trigger polymerization of membrane-anchored Ubxd8. Future studies are required to further delineate the roles of free FAs in cell signaling.
Ubxd8 is known to localize in the ER in FA-depleted cells but transport to the lipid droplets in cells treated with excess FAs as a result of increased synthesis of triglycerides (TG) (31–33). While TG synthesis is not required for Ubxd8 to stabilize Insig-1 in cells treated with unsaturated FAs (1), translocation of Ubxd8 to lipid droplets may be required to execute another function of the protein in maintaining cellular FA homeostasis, namely, regulating TG metabolism (2, 33). We previously reported that Ubxd8 inhibited TG synthesis in FA-depleted cells by inhibiting the activity of diacylglycerol acyltransferase (2), a committed step in TG synthesis (34). A recent report showed that translocation of Ubxd8 to lipid droplets resulted in inhibition of TG lipolysis (33). It will be interesting to determine whether UAS domain-mediated polymerization of Ubxd8 is required for the protein to transport to lipid droplets in cells loaded with FAs.
Acknowledgments
The authors thank Lisa Beatty, Muleya Kapaale, Nimisha Jacob, Hue Dao, and Ijeoma Onwuneme for help with tissue culture; Kate Luby-Phelps, Karen Rothberg, and Abhijit Bugale for help with live cell imaging, and Saada Abdalla for technical assistance.
Footnotes
Abbreviations:
- CD
- circular dichroism
- ER
- endoplasmic reticulum
- FAF1
- Fas-associated factor 1
- FCS
- fetal calf serum
- TG
- triglyceride
This work was supported by National Institutes of Health Grant HL-20948 and Welch Foundation Grant I-1832.
REFERENCES
- 1.Lee J. N., Zhang X., Feramisco J. D., Gong Y., Ye J. 2008. Unsaturated fatty acids inhibit proteasomal degradation of insig-1 at a post-ubiquitination step. J. Biol. Chem. 283: 33772–33783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J. N., Kim H., Yao H., Chen Y., Weng K., Ye J. 2010. Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis. Proc. Natl. Acad. Sci. USA. 107: 21424–21429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang T., Espenshade P. J., Wright M. E., Yabe D., Gong Y., Aebersold R., Goldstein J. L., Brown M. S. 2002. Crucial step in cholesterol homeostasis: sterols promote binding of Scap to Insig-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 110: 489–500 [DOI] [PubMed] [Google Scholar]
- 4.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai Y., Nakada A., Hashida R., Sugita Y., Tanaka T., Tsujimoto G., Matsumoto K., Akasawa A., Saito H., Oshida T. 2002. Cloning and characterization of the highly expressed ETEA gene from blood cells of atopic dermatitis patients. Biochem. Biophys. Res. Commun. 297: 1282–1290 [DOI] [PubMed] [Google Scholar]
- 6.Buchberger A. 2002. From UBA to UBX: new words in the ubiquitin vocabulary. Trends Cell Biol. 12: 216–221 [DOI] [PubMed] [Google Scholar]
- 7.Halawani D., Latterich M. 2006. p97: The cell's molecular purgatory? Mol. Cell. 22: 713–717 [DOI] [PubMed] [Google Scholar]
- 8.Hannah V. C., Ou J., Luong A., Goldstein J. L., Brown M. S. 2001. Unsaturated fatty acids down-regulate SREBP isoforms 1a and 1c by two mechanisms in HEK-293 cells. J. Biol. Chem. 276: 4365–4372 [DOI] [PubMed] [Google Scholar]
- 9.Lee J. N., Gong Y., Zhang X., Ye J. 2006. Proteasomal degradation of ubiquitinated Insig proteins is determined by serine residues flanking ubiquitinated lysines. Proc. Natl. Acad. Sci. USA. 103: 4958–4963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawson R. B., Bose-Boyd R., Goldstein J. L., Brown M. S. 1999. Failure to cleave sterol regulatory element-binding proteins (SREBPs) causes cholesterol auxotrophy in Chinese hamster ovary cells with genetic absence of SREBP cleavage-activating protein. J. Biol. Chem. 274: 28549–28556 [DOI] [PubMed] [Google Scholar]
- 11.Soding J. 2005. Protein homology detection by HMM-HMM comparison. Bioinformatics. 21: 951–960 [DOI] [PubMed] [Google Scholar]
- 12.Sali A., Blundell T. L. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234: 779–815 [DOI] [PubMed] [Google Scholar]
- 13.Biegert A., Mayer C., Remmert M., Sôding J., Lupas A. N. 2006. The MPI Bioinformatics Toolkit for protein sequence analysis. Nucleic Acids Res. 34: W335–W339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittig I., Braun H. P., Schagger H. 2006. Blue native PAGE. Nat. Protoc. 1: 418–428 [DOI] [PubMed] [Google Scholar]
- 15.Song E. J., Yim S. H., Kim E., Kim N. S., Lee K. J. 2005. Human Fas-associated factor 1, interacting with ubiquitinated proteins and valosin-containing protein, is involved in the ubiquitin-proteasome pathway. Mol. Cell. Biol. 25: 2511–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodama Y., Hu C. 2010. An improved bimolecular fluorescence complementation assay with a high signal-to-noise ratio. Biotechniques. 49: 793–805 [DOI] [PubMed] [Google Scholar]
- 17.Marchler-Bauer A., Lu S., Anderson J. B., Chitsaz F., Derbyshire M. K., Weese-Scott C., Fong J. H., Geer L. Y., Geer R. C., Gonzales N. R., et al. 2011. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39: D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menges C. W., Altomare D. A., Testa J. R. 2009. FAS-Associated Factor 1 (FAF1): diverse functions and implications for oncogenesis. Cell Cycle. 8: 2528–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park M. Y., Moon J. h., Lee K. S., Choi H. I., Chung J., Hong H. J., Kim E. 2007. FAF1 suppresses IκB Kinase (IKK) activation by disrupting the IKK complex assembly. J. Biol. Chem. 282: 27572–27577 [DOI] [PubMed] [Google Scholar]
- 20.Park M. Y., Jang H. D., Lee S. Y., Lee K. J., Kim E. 2004. Fas-associated factor-1 inhibits nuclear factor-κB (NF-κB) activity by interfering with nuclear translocation of the RelA (p65) subunit of NF-κB. J. Biol. Chem. 279: 2544–2549 [DOI] [PubMed] [Google Scholar]
- 21.Altomare D. A., Menges C. W., Pei J., Zhang L., Skele-Stump K. L., Carbone M., Kane A. B., Testa J. R. 2009. Activated TNF-α/NF-κB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc. Natl. Acad. Sci. USA. 106: 3420–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sen R. 2011. The origins of NF-kappaB. Nat. Immunol. 12: 686–688 [DOI] [PubMed] [Google Scholar]
- 23.Coll T., Eyre E., Rodriguez-Calvo R., Palomer X., Sanchez R. M., Merlos M., Laguna J. C., Vázquez-Carrera M. 2008. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J. Biol. Chem. 283: 11107–11116 [DOI] [PubMed] [Google Scholar]
- 24.Weigert C., Brodbeck K., Staiger H., Kausch C., Machicao F., Häring H. U., Schleicher E. D. 2004. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-κB. J. Biol. Chem. 279: 23942–23952 [DOI] [PubMed] [Google Scholar]
- 25.Jove M., Planavila A., Laguna J. C., Vázquez-Carrera M. 2005. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor κB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology. 146: 3087–3095 [DOI] [PubMed] [Google Scholar]
- 26.Alexandru G., Graumann J., Smith G. T., Kolawa N. J., Fang R., Deshaies R. J. 2008. UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1α turnover. Cell. 134: 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majmundar A. J., Wong W. J., Simon M. C. 2010. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 40: 294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.den Besten W., Verma R., Kleiger G., Oania R. S., Deshaies R. J. 2012. NEDD8 links cullin-RING ubiquitin ligase function to the p97 pathway. Nat. Struct. Mol. Biol. 19: 511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dennis E. A., Deems R. A., Harkewicz R., Quehenberger O., Brown H. A., Milne S. B., Myers D. S., Glass C. K., Hardiman G., Reichart D., et al. 2010. A mouse macrophage lipidome. J. Biol. Chem. 285: 39976–39985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen G. H., Rasmussen K., Niels-Christiansen L. L., Danielsen E. M. 2011. Dietary free fatty acids form alkaline phosphatase-enriched microdomains in the intestinal brush border membrane. Mol. Membr. Biol. 28: 136–144 [DOI] [PubMed] [Google Scholar]
- 31.Zehmer J. K., Bartz R., Bisel B., Liu P., Seemann J., Anderson R. G. W. 2009. Targeting sequences of UBXD8 and AAM-B reveal that the ER has a direct role in the emergence and regression of lipid droplets. J. Cell Sci. 122: 3694–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farese R. V., Walther T. C. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 139: 855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olzmann J. A., Richter C. M., Kopito R. R. 2013. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proc. Natl. Acad. Sci. USA. 110: 1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yen C. L. E., Stone S. J., Koliwad S., Harris C., Farese R. V., Jr 2008. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49: 2283–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]