Abstract
We have established that docosahexaenoic acid (DHA), the major polyunsaturated fatty acid in the retina, promotes survival of rat retina photoreceptors during early development in vitro and upon oxidative stress by activating the ERK/MAPK signaling pathway. Here we have investigated whether DHA turns on this pathway through activation of retinoid X receptors (RXRs) or by inducing tyrosine kinase (Trk) receptor activation. We also evaluated whether DHA release from phospholipids was required for its protective effect. Addition of RXR antagonists (HX531, PA452) to rat retinal neuronal cultures inhibited DHA protection during early development in vitro and upon oxidative stress induced with Paraquat or H2O2. In contrast, the Trk inhibitor K252a did not affect DHA prevention of photoreceptor apoptosis. These results imply that activation of RXRs was required for DHA protection whereas Trk receptors were not involved in this protection. Pretreatment with 4-bromoenol lactone, a phospholipase A2 inhibitor, blocked DHA prevention of oxidative stress-induced apoptosis of photoreceptors. It is noteworthy that RXR agonists (HX630, PA024) also rescued photoreceptors from H2O2-induced apoptosis. These results provide the first evidence that activation of RXRs prevents photoreceptor apoptosis and suggest that DHA is first released from phospholipids and then activates RXRs to promote the survival of photoreceptors.
Keywords: apoptosis, oxidative damage, photoreceptor survival, RXR agonists
Photoreceptor cell death, which leads to vision loss, occurs in several retinal neurodegenerative diseases, such as retinitis pigmentosa and age-related macular degeneration. Photoreceptor loss in these pathologies occurs by apoptosis and oxidative stress, and lack of trophic factors is implicated in the activation of the apoptotic mechanisms (1). Identifying the trophic factors and intracellular pathways activated to prevent this apoptosis is crucial for designing procedures to rescue photoreceptors in these diseases.
Previous research from our laboratory has demonstrated that docosahexaenoic acid (DHA), the major n-3 polyunsaturated fatty acid (PUFA) in the retina, acts as a trophic factor for photoreceptor cells. DHA promotes differentiation and postpones apoptosis of photoreceptors, which otherwise occurs in the absence of trophic factors during their early development in culture (2–4); it also effectively prevents photoreceptor apoptosis due to oxidative stress induced by Paraquat (PQ) (5) and H2O2 (6). This antiapoptotic effect is highly specific for DHA, because other saturated, monoenoic, or polyunsaturated fatty acids cannot stop photoreceptor death (2). DHA has several other protective effects in the retina, reducing neovascularization and inflammatory processes and promoting survival in several cell types (7–13). Furthermore, n-3 fatty acid deficiency alters recovery of the rod photoresponse in rhesus monkeys (14).
An understanding of the molecular signaling pathways triggered by survival molecules like DHA is critical to design potential protective therapies and eventually provide an effective treatment for neurodegenerative diseases. Our work has shown that DHA activates the ERK/MAPK signaling pathway in photoreceptors to promote their survival and differentiation, stimulating the expression of antiapoptotic proteins such as Bcl-2 and preserving mitochondrial membrane potential (5, 15). In addition, it decreases the intracellular levels of proapoptotic lipid signals such as ceramide (16). Activation of the ERK/MAPK pathway is also involved in the effects of peptidic trophic factors for photoreceptors, like FGF2 and ciliary neurotrophic factor (17, 18). However, which upstream molecular mechanisms led to its activation by a fatty acid was an intriguing question. As a PUFA, DHA might modify the biophysical properties of neuronal membranes and thus indirectly induce the dimerization and subsequent activation of the tyrosine kinase (Trk)-like membrane receptors, which would in turn activate the ERK/MAPK pathway. Alternatively, DHA might have a direct effect as a receptor ligand; PUFAs, including DHA, are transcriptional regulators acting through multiple interactions with different nuclear receptors (19–21) and transcription factors (22–24). DHA is an endogenous ligand for retinoid X receptors (RXRs) in mouse brain (19, 25). These receptors are members of the steroid/thyroid receptor superfamily, and are believed to bind to DNA response elements only after the formation of either RXR:RXR homodimers or heterodimers with other members of this receptor family, including retinoic acid receptors (26). RXR signaling is involved in nervous system development (27) and is essential for normal development (28). DHA binding to RXRs to activate the ERK/MAPK pathway, among other survival/differentiation pathways, might provide an explanation for DHA pleiotropic effects.
Another pending question is whether DHA acts as a free fatty acid or is esterified to phospholipids to carry out its biological functions. Although the retina, particularly the photoreceptors, is highly enriched in DHA-containing phospholipids (29), the amount of unesterified (free) DHA is negligible under basal conditions in the retina and brain (30–34). However, DHA might be released from phospholipids by the activation of a specific phospholipase A2 (35).
In this work we investigated the mechanisms involved in DHA protection upstream ERK/MAPK in different experimental models of retinal degeneration in vitro, either induced by oxidative damage or by trophic factor deprivation during early development in vitro. Our results support that RXR activation is essential for DHA protection of photoreceptors and that its release and subsequent action as free DHA is required for this protection. In addition, our data suggest that activation of RXRs has an intrinsically neuroprotective effect, promoting photoreceptor survival independently of the agonist involved in their activation.
MATERIALS AND METHODS
Materials
One to two day old albino Wistar rats bred in our own colony were used in all the experiments. All procedures concerning animal use were carried out in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and following the guidelines of the Institutional Committee for the Care of Laboratory Animals from the Universidad Nacional del Sur (Argentina).
Plastic 35 mm diameter culture dishes and multi-chambered slides (NUNC) were from Inter Med (Naperville, IL). Dulbecco's modified Eagle's medium (DMEM) (GIBCO) was from Life Technologies (Grand Island, NY). Trypsin, trypsin inhibitor, transferrin, hydrocortisone, putrescine, insulin, polyornithine, selenium, gentamycin, 4,6-diamidino-2-phenylindole (DAPI), fluorescein-conjugated secondary antibodies, paraformaldehyde, PQ, bovine serum albumin (BSA), and monoclonal anti-syntaxin clone HPC-1 syntaxin were from Sigma (St. Louis, MO). Monoclonal antibodies for pan-RXR (sc-774) and secondary antibody, goat anti-mouse IgG-HRP, and goat anti-rabbit IgG-HRP were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Secondary antibodies, Cy2-conjugated-goat anti-mouse, and Cy5-conjugated-goat anti-rabbit were from Molecular Probes (Eugene, OR). Monoclonal Rho4D2 was a generous gift from Dr. R. Molday (University of South Columbia). DHA was from Nu-Chek (Elysian, MN). MitoTracker (CMXRos) was from Molecular Probes. Terminal deoxynucleotidyl transferase (TdT), recombinant, 5-bromo-2′deoxyuridine 5′-triphosphate (BrdUTP), and TdT buffer were from Molecular Probes and Invitrogen (Argentina), respectively. H2O2 was from Merck (Argentina). 4-Bromoenol lactone (BEL) was from Santa Cruz Biotechnology, Inc. and generously provided by Dr. Gabriela Salvador (Universidad Nacional del Sur, Argentina). RXR noncommercial pan-agonists (HX630 and PA024) and pan-antagonists (HX531 and PA452) were generous gifts from Dr. Kagechika (Tokyo Medical and Dental University, Japan). All other reagents used were analytical grade.
Retinal cultures
Pure retinal cultures were obtained following procedures previously established (2, 3, 36). Photoreceptors and amacrine neurons were the two major cell types present in the cultures and were identified by their morphology using phase contrast microscopy and by immunocytochemistry, using the monoclonal antibodies syntaxin (HPC-1) and Rho4D2, which selectively react with amacrine and photoreceptor neurons, respectively (37–39). Photoreceptors were identified by at least three of the following criteria: 1) a small round cell body (3–5 μm), usually darker than that of amacrine neurons; 2) a single neurite, usually ending in a conspicuous synaptic “spherule” 3) they display sometimes a connecting cilium at the opposite end, but fail to develop their characteristic outer segments; 4) they are labeled by Rho4D2 antibody; and 5) they show opsin expression diffusely distributed over their cell body. Amacrine neurons are larger than photoreceptors (7–20 μm) and have multiple neurites. Almost all amacrine neurons show HPC-1 immunoreactivity starting at early stages of development, and this immunoreactivity is retained even after undergoing degenerative changes that alter their morphological appearance (40).
DHA supplementation
DHA (6.7 μM), complexed with BSA was added at day 1 in vitro (2). Cultures supplemented with the same volume and concentration of BSA were used as controls (BSA controls).
PQ treatment
PQ (48 μM final concentration in the incubation medium, in a calcium- and magnesium-free solution) was added to 3 day cultures (5). Neurons were then incubated for 24 h before fixation.
H2O2 treatment
Treatment of the cultures with H2O2 was done essentially according to Chucair et al. (41), with slight modifications. Briefly, cells were treated at day 3 in culture with 10 μM H2O2 for 30 min at 36°C; the medium was then removed, replaced with fresh neuronal medium, and the cultures were returned to the incubator for 5.5 h before fixation.
Addition of HX531 and PA452
Cultures were treated with two different RXR pan-antagonists, HX531 and PA452 (42–44), at different concentrations: 0.1, 1, and 10 μM or 1 and 10 μM, respectively. These antagonists were added to the cultures at day 1, and DHA or BSA was added 1 h later; the cultures were then either treated or not treated at day 3 with PQ and the cells were fixed 24 h later. A 1 μM concentration of each RXR pan-antagonist was used in further experiments, because a lower HX531 concentration showed little effect on apoptosis and higher HX531 and PA542 concentrations were toxic to neurons.
Addition of HX630 and PA024
Cultures were treated with either of two different RXR pan-agonists, HX630 and PA024 (44–46), at different concentrations: 10, 100, and 1,000 nM or 1, 10, and 100 nM, respectively. Agonists were added at day 1 and cultures were then either treated or not treated at day 3 with H2O2; 6 h later the cells were fixed. A 100 nM HX630 and 10 nM PA024 concentration was used in further experiments, because these were the lowest concentrations with protective effects on photoreceptor apoptosis.
Addition of K252a
To evaluate whether DHA activated Trk receptors, cultures were treated at day 1 with the Trk receptor inhibitor K252a (47, 48) at different concentrations (200, 300, 400, 800, and 1,000 nM); cultures were supplemented with BSA or DHA 1 h later and were fixed at day 6. As a positive control, we evaluated the inhibition of insulin activation of Trk receptors in amacrine neurons by treating cultures with K252a at 200, 300, and 400 nM at day 1; cultures were supplemented with insulin or vehicle 1 h later and cells were fixed at day 6.
Addition of BEL
To determine whether DHA release from phospholipids by a calcium-independent phospholipase A2 (iPLA2) was required for DHA neuroprotective effect, we inhibited iPLA2 with the suicide substrate BEL, an irreversible inhibitor of this enzyme (35, 49, 50). Day 1 retinal neurons were supplemented with BSA or DHA for 24 h; culture medium was then replaced by fresh medium and at day 3 and cells were incubated with BEL (5 μM) for 30 min and finally either treated or not treated with H2O2 for 6 h.
Immunocytochemical methods
Cultures were fixed for at least 1 h with 2% paraformaldehyde in PBS, followed by permeation with Triton X-100 (0.1%) for 15 min. After blocking with PBS (5% BSA), samples were incubated with the appropriate primary antibody prepared in PBS (3% BSA). After washing with PBS, the samples were incubated with secondary Cy2-conjugated-goat anti-mouse or Cy5-conjugated-goat anti-rabbit antibody. To evaluate mitochondrial function, cultures were incubated for 30 min before fixing with the fluorescent probe MitoTracker (0.1 μg/ml). The samples were examined using a Leica TCS SP2 AOBS confocal laser microscope. Neuronal cell types were identified with specific monoclonal antibodies as described above.
Cultures were analyzed by phase contrast and epifluorescence microscopy using a Nikon Eclipse E600 microscope with a C-C phase contrast turret condenser and a Y-FL Epi-Fluorescence attachment and a laser scanning confocal microscope (Leica DMIRE2) with a 63× water objective; images were collected and processed with LCS software (Leica) and Photoshop 8.0 (Adobe Systems, San Jose, CA).
Western blot analysis
Proteins (20 μg) dissolved in 4× Laemmli sample buffer were seeded in SDS polyacrylamide gels (10% acrylamide) and electrotransferred to PVDF membranes. After blocking with 5% nonfat milk in TBST buffer (50 mM Tris pH 7.2–7.4, 200 mM NaCl, 0.1% Tween-20), the membranes were incubated overnight with a pan-RXR antibody in TBST plus 3% nonfat milk. After washing, membranes were incubated with a horse radish peroxidase-conjugated secondary antibody in TBST plus 3% nonfat milk. Finally, the blots were developed by ECL with the use of Kodak BioMax Light film and digitalized with a GS-700 imaging densitometer (Bio-Rad, Hercules, CA). ARPE-19 cells were used as a positive control for RXR expression.
Cell viability and apoptosis
Cell death was determined by quantifying cells labeled with propidium iodide (PI), 0.5 μg/ml in culture, after 30 min incubation (51).
Apoptosis was determined by two different methods: DAPI and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Nuclei integrity was evaluated after staining cell nuclei with DAPI, a fluorescent dye that binds to DNA. Briefly, cells were permeated with Triton X-100, washed with PBS, and incubated with DAPI for 20 min. Cells were considered to be apoptotic when they showed either fragmented or condensed (pycnotic) nuclei, a characteristic feature of apoptotic cell death. The amount of apoptotic photoreceptors or amacrine cells was determined in cultures double-labeled with DAPI and with either Rho4D2 or HPC-1, to unambiguously identify cells as either photoreceptors or amacrine neurons, respectively, and thus establish the total number of each cell type. The percentage of apoptotic photoreceptors or amacrine neurons was calculated, taking into account the percentage of Rho4D2-labeled cells or HPC-1-labeled cells, respectively.
For TUNEL assay, the cells were fixed at day 4 with 2% paraformaldehyde for 15 min and then stored in 70% ethanol for 48 h at −20°C. Before labeling, cells were washed twice with PBS for 5 min each at room temperature. Samples were preincubated with TdT buffer for 15 min and then incubated with the TdT reaction mixture (0.05 mM BrdUTP, 0.3 U/μl TdT in TdT buffer) at 37°C in a humidified atmosphere for 1 h. The reaction was stopped by 15 min incubation with stop buffer (300 mM NaCl, 30 mM sodium citrate, pH 7.4) at room temperature. Negative controls were prepared by omitting TdT. BrdU was detected with an anti-bromodeoxyuridine (anti-BrdU) monoclonal antibody, following a standard immunocytochemical technique.
Statistical analysis
The results represent the average of at least three separate experiments (± SD), unless specifically indicated, and each experiment was performed in triplicate. For cytochemical studies, 10 fields per sample were analyzed in each case. Statistical significance was determined by either Student's t-test or two-way ANOVA followed by a Tukey's test.
RESULTS
RXRs are expressed in photoreceptors
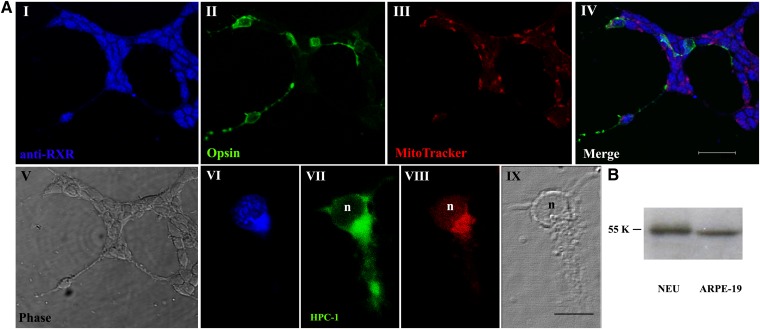
We first investigated whether retinal neurons growing in culture expressed RXRs. Confocal microscopy analysis of 6 day retinal cultures showed that both photoreceptors and amacrine neurons expressed these receptors. Triple immunofluorescence detection with a pan-RXR antibody that recognizes the three RXR isoforms (Fig. 1AI in blue), a photoreceptor marker (Rho4D2) (Fig. 1AII in green), and a mitochondrial marker (Fig. 1AIII in red) evidenced that in photoreceptors RXRs were localized in the nucleus (Fig. 1AIV merge). Instead, amacrine neurons labeled with the HPC-1 antibody (Fig. 1AVII) showed mainly a cytoplasmic RXR expression and only a sparse nuclear distribution (Fig. 1AI, VI). Expression of RXRs in neuronal cultures was confirmed by Western blot analysis (Fig. 1B).
Fig. 1.
Expression of RXRs in retina photoreceptors in vitro. A: Phase (V and IX) and fluorescence (I–IV, VI–VIII) micrographs of 6 day retina neuronal cultures immunolabeled with pan-RXR antibody (blue staining in I, IV, and VI), Rho4D2 (II, green), MitoTracker (III and VIII, red staining), merge (IV), and HPC-1 (VII, green staining). Note that opsin (+) cells (photoreceptors) express RXRs mainly in their nuclei. In contrast, HPC-1 (+) cells (amacrine neurons) express RXRs mainly in the cytoplasm, with only scarce aggregates in their nuclei (n). The scale bar in I–V represents 20 μm and in VI–IX, 10 μm. B: Proteins obtained from lysates from 3 day neurons (NEU) and from human retinal pigment epithelial cells (ARPE-19, used as a positive control) were analyzed by Western blot with a pan-RXR antibody.
RXR antagonists blocked DHA protective effects on photoreceptor degeneration in vitro
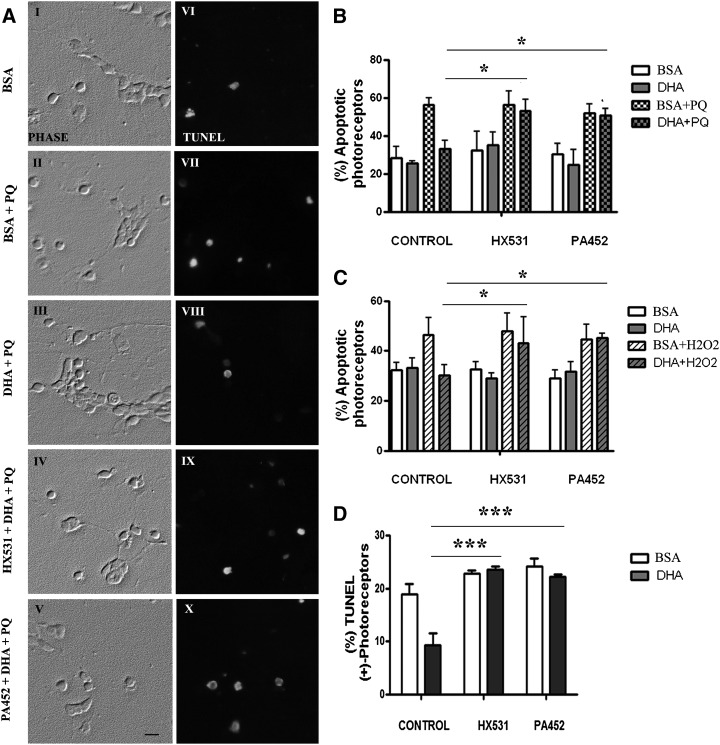
We have established that DHA protects retina photoreceptors from apoptosis induced by oxidative stress or trophic factor deprivation. To investigate whether DHA exerted its protective effect through activation of RXRs, retinal neurons were either treated or not treated (control) with the RXR pan-antagonists, HX531 or PA452, prior to the addition of BSA or DHA, and then exposed to oxidative damage or cultured for 6 days without trophic factors (BSA control) or with DHA. As previously shown (5), generation of oxidative damage with PQ triggered apoptosis in retina neurons, increasing the number of TUNEL-positive cells (Fig. 2AVI, VII) and inducing a 2-fold increase in photoreceptor apoptosis compared with BSA controls (BSA) (P < 0.001) (Fig. 2B). DHA supplementation protected photoreceptors (Fig. 2AVIII) (5, 15, 16), reducing the percentage of photoreceptors with fragmented or pycnotic nuclei from 56% to nearly 35% (P < 0.001) (Fig. 2B). However, when cultures were pretreated with RXR antagonists, PA452 or HX531, before DHA addition, the number of TUNEL-positive cells (Fig. 2AX, IX) and the percentage of apoptotic photoreceptors were similar to those found in PQ-treated cultures lacking DHA (P < 0.05) (Fig. 2B).
Fig. 2.
Effect of RXR antagonists on DHA prevention of photoreceptor apoptosis. A: Phase (left) and fluorescence (right) micrographs showing TUNEL in 4 day cultures without (I, VI; BSA) or with PQ (II, VII; BSA+PQ) treatment, and supplemented with DHA, without (III, VIII) or with pretreatment with RXR antagonists HX531 (IV, IX) and PA452 (V, X) before PQ addition. The scale bar represents 10 μm. B: Day 1 retinal neurons were preincubated with vehicle (control) or with either RXR antagonist for 1 h, and then supplemented without (BSA) or with DHA (DHA). The cultures were finally treated or not treated at day 3 with PQ for 24 h. The percentage of apoptotic photoreceptors was determined by analyzing nuclear fragmentation with DAPI. C: Retinal neurons were preincubated with vehicle (control) or with the RXR antagonist for 1 h, then supplemented without (BSA) or with DHA (DHA) and finally treated or not treated with H2O2 for 5.5 h at day 3. The percentage of apoptotic photoreceptors was determined with DAPI. D: Retinal neurons were cultured for 6 days without (BSA) or with DHA (DHA) in cultures incubated without (control) or with the RXR antagonists (1 μM HX531 or 1 μM PA452). The percentage of apoptotic photoreceptors was determined by TUNEL assay. Each value represents the mean of three experiments ± SD. *P < 0.05, ***P < 0.001.
Similar results were obtained when cultures were exposed to oxidative damage with H2O2. As previously demonstrated (41), H2O2 increased photoreceptor apoptosis from about 30% in BSA controls (BSA) to about 50% in H2O2-treated cultures (P < 0.05), and DHA prevented this increase (Fig. 2C). Pretreating cultures with RXR antagonists inhibited DHA protection, because the percentage of apoptotic photoreceptors after H2O2 treatment was similar in DHA-supplemented and in DHA-lacking cultures (Fig. 2C).
In the absence of trophic factors, photoreceptors develop normally for 3–4 days in culture and then start degenerating through an apoptotic pathway that is postponed by DHA (2, 4, 15). To find out whether the activation of RXRs was involved in this protective effect of DHA, cultures were pretreated with RXR antagonists and then either supplemented or not supplemented with DHA. As previously reported, in day 6 BSA controls (BSA) the percentage of TUNEL-positive photoreceptors (Fig. 2D) amounted to 19.4%, and DHA supplementation reduced it to about 9% (P < 0.01) (15). RXR antagonists blocked this reduction, increasing TUNEL-positive photoreceptors to about the same percentage found in DHA-lacking cultures (Fig. 2D).
These results demonstrate that activation of RXRs was essential for DHA rescue of photoreceptors subjected to oxidative stress and during development in vitro.
RXR agonists rescued cultured photoreceptors from apoptosis induced by oxidative stress
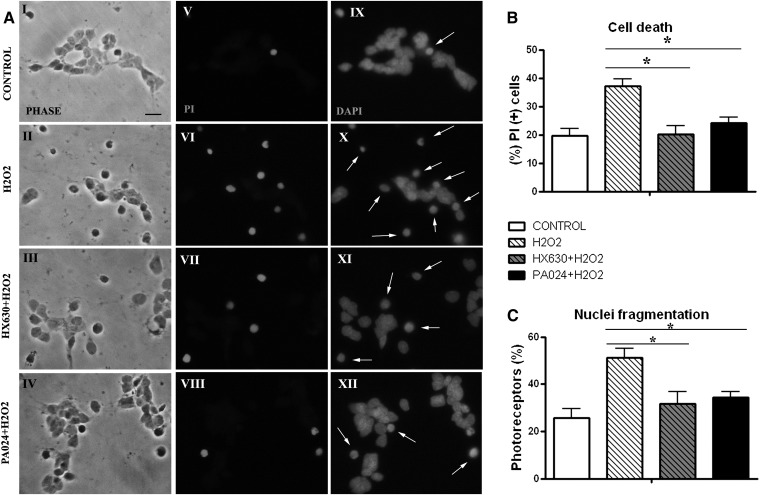
To evaluate whether activation of RXRs had a neuroprotective effect by itself, we treated the cultures with two RXR agonists, HX630 or PA024, before addition of H2O2. As previously reported, at day 3 in vitro only 20% of photoreceptors showed PI labeling (Fig. 3AV, B), an indicator of cell death. Generation of oxidative damage with H2O2 induced a 2-fold increase in PI labeling and increased the number of apoptotic photoreceptors from about 25% in control cultures to almost 50% in H2O2-treated cultures (P < 0.05) (Fig. 3C). In these cultures, photoreceptors had shrunken cell bodies, fragmented neurites, lost their characteristic morphology (Fig. 3AII), and showed fragmented or pycnotic nuclei (arrows in Fig. 3AX). Both RXR agonists efficiently prevented photoreceptor death after H2O2 treatment (Fig. 3AVII, VIII, XI, XII; B, C), decreasing the percentage of PI-labeled photoreceptors and of photoreceptors with fragmented nuclei. PA024 was much more effective than HX630, because it had a similar protective effect at a 10-fold lower concentration.
Fig. 3.
RXR agonists prevented photoreceptor death upon oxidative stress. Retinal neurons without (control) or with RXR agonists, 100 nM HX630 or 10 nM PA024, added at day 1, were treated or not treated with H2O2 at day 3. A: Micrographs show: left, phase contrast; middle, PI (+) cells; right, nuclei labeling with DAPI. Arrows indicate photoreceptors showing fragmented or pycnotic nuclei. The scale bar represents 10 μm. B: The percentage of dead cells was determined by PI labeling and (C) of apoptotic photoreceptors with DAPI. Bars are means ± SD. *Statistical significance was calculated using two-way ANOVA test for each graphic. At the 0.05 level, each RXR agonist+H2O2 mean was significantly different compared with H2O2. Mean values were compared using Tukey statistics (P < 0.05).
These results support that activation of RXRs, either by well-established agonists or by DHA, has a protective effect on photoreceptors.
Trk receptors were not involved in the protective effect of DHA
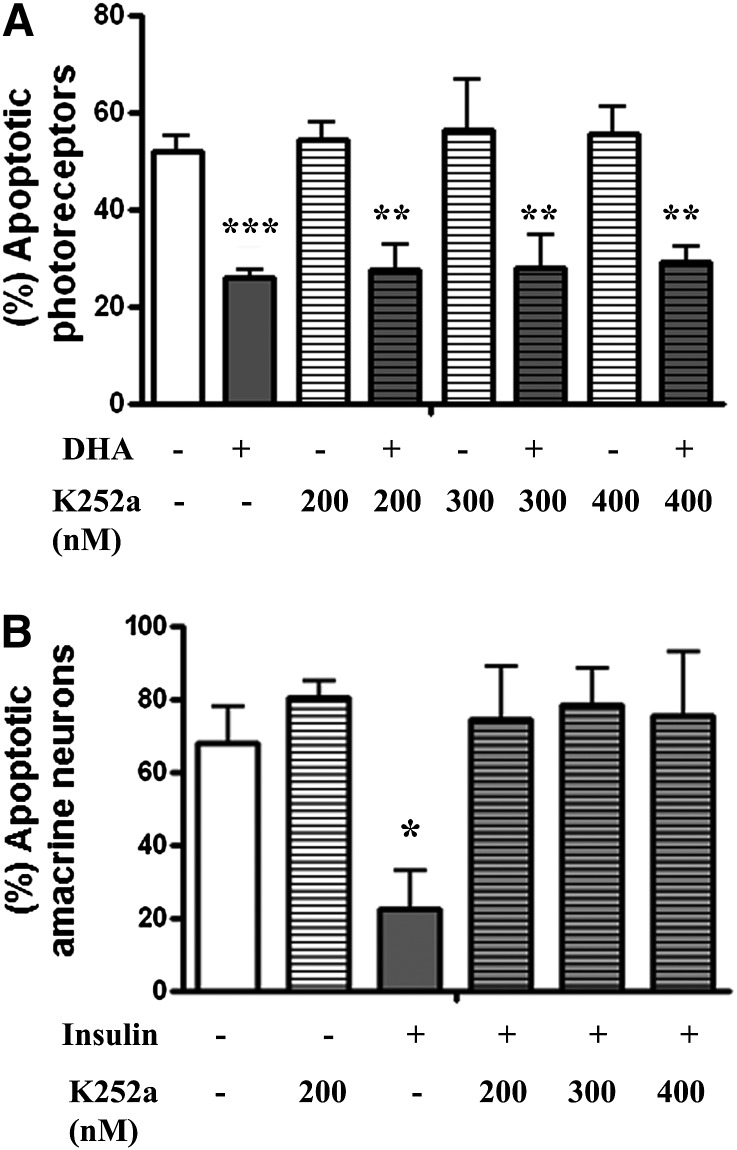
To establish whether activation of Trk receptors was also involved in the protective effect of DHA during in vitro development of photoreceptors, we treated the cultures with a Trk receptor inhibitor, K252a, before DHA supplementation. Analysis of photoreceptor nuclear integrity after 6 days in culture showed a high percentage of apoptotic photoreceptors in BSA controls whereas DHA effectively decreased this percentage (P < 0.001), preventing photoreceptor apoptosis (Fig. 4A). Addition of K252a at different concentrations (200, 300, and 400 nM) did not impair the protective effect of DHA (Fig. 4A); higher K252a concentrations (800 nM) were toxic (not shown). As a control, we analyzed the effect of K252a on amacrine neurons, which depend on insulin signaling through a Trk receptor to activate the PI3K pathway and thus prevent apoptosis (40). By day 6, the percentage of apoptotic amacrine cells in insulin-lacking cultures was about 78% (Fig. 4B) and was reduced to nearly 22% in insulin-supplemented cultures (P < 0.05) (Fig. 4B). Treatment with ≥200 nM K252a significantly increased (P < 0.05) the percentage of apoptotic amacrine cells, regardless of the presence of insulin (Fig. 4B). These results confirm that K252a effectively inhibited Trk receptors and imply that these receptors were not involved in DHA protection of photoreceptors.
Fig. 4.
Inhibition of Trks did not affect DHA prevention of photoreceptor death during development in vitro. A: Retinal neurons were cultured for 6 days without (−) or with DHA (+) in cultures treated without (−) or with (+) different concentrations of K252a, a Trk inhibitor. The percentage of apoptotic photoreceptors was determined with DAPI. B: Retinal neurons were cultured for 6 days without (control) or with insulin in cultures incubated either without (−) or with (+) different concentrations of K252a. The percentage of apoptotic amacrine neurons was determined with DAPI. Bars represent means ± SD. Statistically significant differences compared with control: *P < 0.05, **P < 0.01, ***P < 0.001.
An iPLA2-specific inhibitor blocked DHA protective effect on photoreceptor apoptosis induced by H2O2
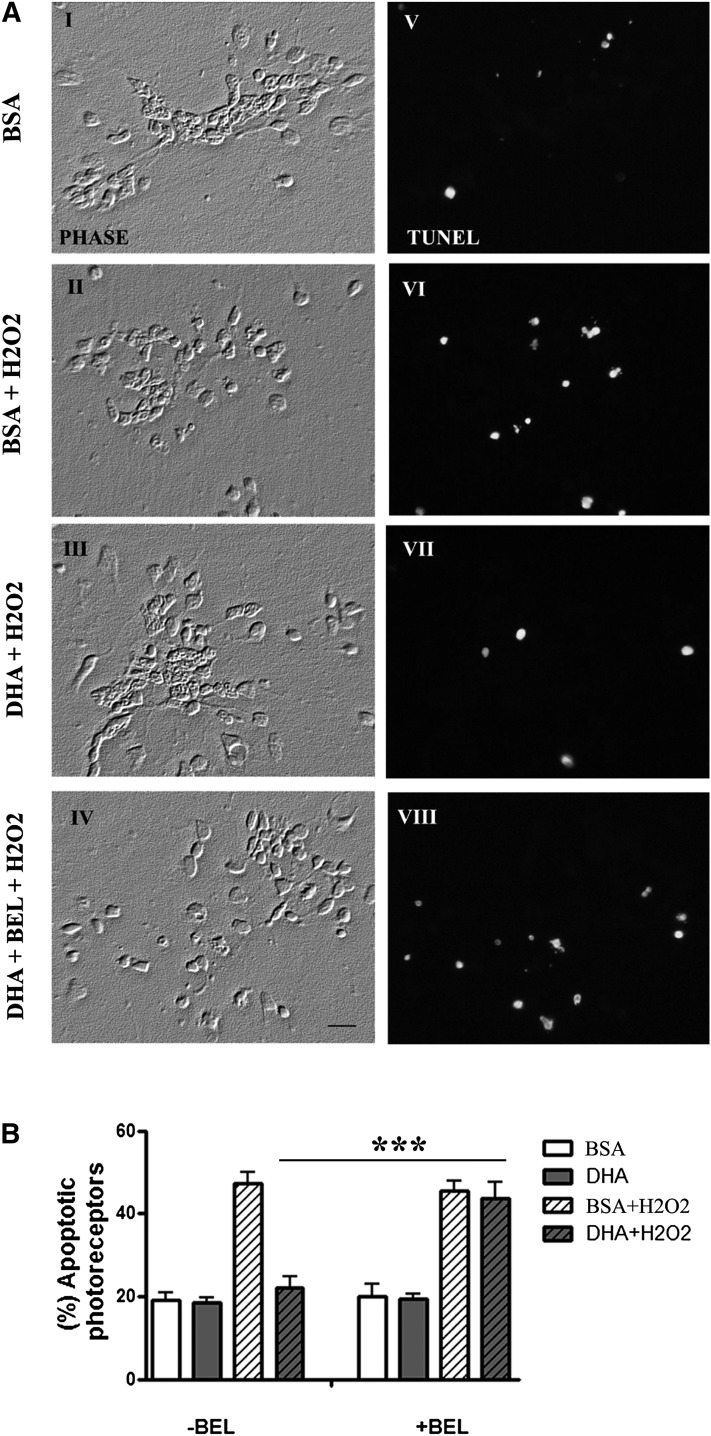
We then investigated whether DHA remains acylated to phospholipids to exert its protection or acts as a free fatty acid, either by being preserved as such in photoreceptor membranes or through its release from membrane phospholipids. To evaluate this, we blocked iPLA2, the main phospholipase involved in DHA release from phospholipids, with a specific inhibitor, BEL, before subjecting neuronal cultures to H2O2-oxidative stress. In the absence of BEL, DHA effectively prevented photoreceptor apoptosis induced by H2O2 treatment, reducing the number of TUNEL-positive cells (Fig. 5AVII) and the percentage of apoptotic photoreceptors almost to BSA control values (Fig. 5B). Addition of BEL completely inhibited this protection, leading to a marked increase in the amount of TUNEL-positive cells and doubling the percentage of photoreceptors with fragmented nuclei in spite of DHA presence (P < 0.001) (Fig. 5AVIII, B). This suggests that DHA has to be released from membrane phospholipids to prevent photoreceptor apoptosis.
Fig. 5.
Inhibition of iPLA2 blocked DHA protection of photoreceptors from oxidative stress-induced apoptosis. Retinal neurons, supplemented without (BSA) or with DHA (DHA) at day 1 for 24 h were preincubated or not preincubated (control) with iPLA2 inhibitor BEL (5 μM) at day 3 for 30 min and finally treated or not treated with H2O2 for 5.5 h at day 3. A: Micrographs show: left column, phase contrast; right column, TUNEL (+) cells. The scale bar represents 10 μm. B: The percentage of apoptotic photoreceptors in the different experimental conditions was determined with DAPI. Bars represent means ± SD. ***P < 0.001.
DISCUSSION
This work provides the first evidence, to our knowledge, that activation of nuclear RXRs is essential for the protective effect of DHA, and supports that, by itself, the activation of RXRs, even by agonists other than DHA, has a protective effect on photoreceptors, preventing apoptosis due to induction of oxidative stress. Our results also demonstrate that DHA has to be released from membrane phospholipids to elicit photoreceptor survival.
Our previous work has established that DHA promotes the differentiation and the survival of retina photoreceptors both during development in vitro and upon oxidative damage (3–5) through the activation of the ERK/MAPK signaling pathway (15). As DHA has been established as a natural ligand for RXRs (19), we explored whether these nuclear receptors might be involved in DHA effects. Immunochemical and Western blot analyses of rat retina neuronal cultures revealed RXRs were expressed in photoreceptors and showed an almost exclusive nuclear localization. In contrast, RXRs were heavily concentrated in the cytoplasm in amacrine neurons and only as sparse aggregates in the nuclei. This is consistent with previous findings in rodent and chick retinas, showing RXRs had specific and dynamic patterns of distribution in ocular tissues during development in vivo (52). In mouse retina, one of RXR isoforms, RXRγ, is expressed in postmitotic cones and participates in regulating cone development (53). The varying cellular localization of RXRs might correspond with different roles in amacrine and photoreceptor neurons.
We then investigated whether these nuclear receptors were involved in DHA protection of photoreceptors upstream of ERK/MAPK activation. Addition of RXR antagonists revealed that they completely blocked the protective effect of DHA from apoptosis induced with two different oxidants, PQ and H2O2. DHA also acts as a natural RXR agonist in other cell types. In a human placental choriocarcinoma cell line, BeWo cells for instance, RXR antagonists PA451 and HX531 inhibited DHA induction of adipose differentiation-related protein (Adrp) mRNA and ADRP protein accumulation (44), suggesting that DHA activated RXRs to increase ADRP levels. We have previously demonstrated that DHA activates defense mechanisms to prevent apoptosis induced both by oxidative stress and the absence of trophic factors at early stages of development and turns on a program to advance differentiation of photoreceptors (54). Our present findings demonstrate that DHA requires the activation of RXRs to promote the survival of photoreceptors. The evidence that establishes DHA as a potent ligand for RXRs, which induces their robust activation (19, 25), supports the hypothesis that DHA might directly bind and activate RXRs to promote photoreceptor survival. Alternatively, DHA-derived metabolites, such as neuroprotectin D1, which acts as an activator for peroxisome proliferator-activated receptor γ (55), might also act as ligands for RXRs, activating downstream survival pathways in photoreceptors. Although further research is required to establish whether DHA directly activates RXRs, DHA signaling through RXRs appears to be central for its pleiotropic effects in these neurons.
An alternative or complementary pathway for DHA signaling might be its indirect activation of Trk receptors. GM1 ganglioside, a lipid molecule as well, has the potential to protect injured and aged central neurons, as some neurotrophins do, through modulation of Trk and Erk phosphorylation and activity in the brain (56–58). However, in our studies DHA protection of photoreceptors was unaffected by selectively inhibiting Trk receptors, implying that Trk receptors were not involved in the antiapoptotic effect of DHA. As a whole, our results support that DHA promotes photoreceptor survival mainly through the activation of RXRs, which in turn might trigger the ERK/MAPK signaling pathway leading to photoreceptor rescue. DHA signaling through RXRs appears to be pivotal for orchestrating survival mechanisms in photoreceptors.
Phospholipids are the main storage sites for PUFAs in mammals, and their release is tightly controlled by phospholipase A2 (59). To take part in RXR activation, DHA might remain as a free fatty acid in photoreceptor membranes or be stored in membrane phospholipids to be deacylated when required. DHA accumulation in membrane phospholipids, chiefly phosphatidylserine, has been shown to be responsible for the protective effect of DHA in Neuro2A cells (60). DHA is extraordinarily enriched in the retina; in rat retina it increases during development from about 5% to 25% of the fatty acids esterified in phospholipids between postnatal days 2 to 30 (2), and it is particularly concentrated in the outer segments of photoreceptors (29). When added to neuronal cultures, DHA is efficiently taken up and esterified in phospholipids to reach the concentration found in adult rat retinas, with minor amounts remaining as free DHA (2, 61). The release of DHA, and thus the size of this free fatty acid pool, is tightly controlled, mainly by iPLA2 (35). Inhibiting iPLA2 in retinal neurons supplemented with DHA before inducing oxidative damage completely blocked the antiapoptotic effect of DHA on photoreceptors. This result suggests that DHA is stored in phospholipids and has to be released from them to trigger the mechanisms leading to photoreceptor protection. Ischemia and seizures elicit a rapid release of DHA in brain (31, 62); likewise, and as occurs with arachidonic acid (63), oxidative damage might elicit the activation of iPLA2 and its subsequent release of DHA, which would then lead to the activation of RXRs to promote photoreceptor survival.
However, DHA release might turn out to be a double-edged sword. A massive release of DHA would have catastrophic consequences for retina integrity. DHA acts in a narrow window of concentrations in cultured retina neurons, and over 10 μM is deleterious for photoreceptors (2). DHA is peroxidized during neurodegenerative diseases of the retina and its products are toxic to neurons (64), depleting cellular detoxification systems. It is noteworthy that despite its sensitivity to peroxidation, DHA protects photoreceptors in culture from oxidative stress-induced apoptosis (5), supporting the relevance of a precisely regulated release. These data underscore the importance of an adequate chemical state and concentration of DHA when administrated in clinical trials of retina pathologies and suggests the convenience of using agents more stable than DHA which mimic its protective effect and reduce the deleterious effects of bioactive subproducts for therapeutical purposes. The requirement for RXR activation in DHA neuroprotection prompted us to investigate whether activation of these nuclear receptors might have an intrinsic protective effect for photoreceptors. Our results demonstrated that two synthetic RXR agonists effectively prevented photoreceptor apoptosis induced by oxidative stress. This implies that agonists other than DHA can turn on survival mechanisms in photoreceptors through activation of RXRs, involving these receptors for the first time, to our knowledge, in photoreceptor in vitro neuroprotection. Given the differences in complexity between the in vitro system used here and the retina in vivo regarding cell differentiation, structural organization, and interactions between different cells and cell types, further research is required to find out whether activation of RXRs has the same protective effect on photoreceptors in vivo. This finding would be of potential clinical significance; diverse therapeutic protocols currently treat several diseases with RXR synthetic agonists (65–68), which have already been tested for their safety and might eventually be useful for treating retinal neurodegenerative diseases. In this context, the finding that PA024 had a more potent protective effect on photoreceptors than HX630 emphasizes the need to screen different synthetic agonists to select the most effective one.
In conclusion, our work suggests a novel pathway for DHA effects in photoreceptors, which involves its initial release from phospholipids upon cellular stress followed by its activation of RXRs to promote photoreceptor survival. In addition, the finding that protection of photoreceptors can also be achieved through activation of RXRs by synthetic ligands introduces versatility into the control of the survival of these cells and may provide new potential tools for treating diseases involving the death of photoreceptors.
Acknowledgments
The authors thank Beatriz De los Santos and Edgardo Buzzi for excellent technical assistance.
Footnotes
Abbreviations:
- BEL
- 4-bromoenol lactone
- BrdUTP
- 5-bromo-2′deoxyuridine 5′-triphosphate
- DAPI
- 4,6-diamidino-2-phenylindole
- DHA
- docosahexaenoic acid
- iPLA2
- calcium-independent phospholipase A2
- PI
- propidium iodide
- PQ
- Paraquat
- RXR
- retinoid X receptor
- TdT
- terminal deoxynucleotidyl transferase
- Trk
- tyrosine kinase
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick end labeling
This work was supported by funds from: Secretaría de Ciencia y Tecnología, Universidad Nacional del Sur (Grants 24-B190 and 24/B163 to L.E.P. and N.P.R.); Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 6529 to L.E.P. and N.P.R.); Agencia Nacional Para la Ciencia y Tecnología (ANPCYT) (PICT-2006-00711 to L.E.P.); and Innovative Grant PABSELA SCRT08-Fundación Crimson-Partners Harvard Medical International (to O.L.G.).
REFERENCES
- 1.Portera-Cailliau C., Sung C. H., Nathans J., Adler R. 1994. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 91: 974–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotstein N. P., Aveldano M. I., Barrantes F. J., Politi L. E. 1996. Docosahexaenoic acid is required for the survival of rat retinal photoreceptors in vitro. J. Neurochem. 66: 1851–1859 [DOI] [PubMed] [Google Scholar]
- 3.Rotstein N. P., Aveldano M. I., Barrantes F. J., Roccamo A. M., Politi L. E. 1997. Apoptosis of retinal photoreceptors during development in vitro: protective effect of docosahexaenoic acid. J. Neurochem. 69: 504–513 [DOI] [PubMed] [Google Scholar]
- 4.Politi L. E., Rotstein N. P., Carri N. G. 2001. Effect of GDNF on neuroblast proliferation and photoreceptor survival: additive protection with docosahexaenoic acid. Invest. Ophthalmol. Vis. Sci. 42: 3008–3015 [PubMed] [Google Scholar]
- 5.Rotstein N. P., Politi L. E., German O. L., Girotti R. 2003. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Invest. Ophthalmol. Vis. Sci. 44: 2252–2259 [DOI] [PubMed] [Google Scholar]
- 6.Rotstein N. P., Agnolazza D. L., Agbaga M-P. G., Anderson R. E. 2011 Activation of antioxidant defense mechanisms by docosahexaenoic acid and eicosapentaenoic acid prevents apoptosis of retina photoreceptors (Abstract). 2011 ARVO Annual Meeting. [Google Scholar]
- 7.Stahl A., Sapieha P., Connor K. M., Sangiovanni J. P., Chen J., Aderman C. M., Willett K. L., Krah N. M., Dennison R. J., Seaward M. R., et al. 2010. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ. Res. 107: 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazan N. G., Molina M. F., Gordon W. C. 2011. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu. Rev. Nutr. 31: 321–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbar M., Calderon F., Wen Z., Kim H. Y. 2005. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA. 102: 10858–10863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee P. K., Marcheselli V. L., Serhan C. N., Bazan N. G. 2004. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 101: 8491–8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L., Hackett S. F., Mincey A., Lai H., Campochiaro P. A. 2006. Effects of different types of oxidative stress in RPE cells. J. Cell. Physiol. 206: 119–125 [DOI] [PubMed] [Google Scholar]
- 12.Connor K. M., Sangiovanni J. P., Lofqvist C., Aderman C. M., Chen J., Higuchi A., Hong S., Pravda E. A., Majchrzak S., Carper D., et al. 2007. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 13: 868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SanGiovanni J. P., Chew E. Y. 2005. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 24: 87–138 [DOI] [PubMed] [Google Scholar]
- 14.Jeffrey B. G., Mitchell D. C., Gibson R. A., Neuringer M. 2002. n-3 fatty acid deficiency alters recovery of the rod photoresponse in rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 43: 2806–2814 [PubMed] [Google Scholar]
- 15.German O. L., Insua M. F., Gentili C., Rotstein N. P., Politi L. E. 2006. Docosahexaenoic acid prevents apoptosis of retina photoreceptors by activating the ERK/MAPK pathway. J. Neurochem. 98: 1507–1520 [DOI] [PubMed] [Google Scholar]
- 16.German O. L., Miranda G. E., Abrahan C. E., Rotstein N. P. 2006. Ceramide is a mediator of apoptosis in retina photoreceptors. Invest. Ophthalmol. Vis. Sci. 47: 1658–1668 [DOI] [PubMed] [Google Scholar]
- 17.Kinkl N., Sahel J., Hicks D. 2001. Alternate FGF2–ERK1/2 signaling pathways in retinal photoreceptor and glial cells in vitro. J. Biol. Chem. 276: 43871–43878 [DOI] [PubMed] [Google Scholar]
- 18.Rhee K. D., Goureau O., Chen S., Yang X. J. 2004. Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J. Neurosci. 24: 9779–9788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Urquiza A. M., Liu S., Sjöberg M., Zetterström R. H., Griffiths W., Sjövall J., Perlmann T. 2000. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 290: 2140–2144 [DOI] [PubMed] [Google Scholar]
- 20.Jump D. B., Thelen A., Ren B., Mater M. 1999. Multiple mechanisms for polyunsaturated fatty acid regulation of hepatic gene transcription. Prostaglandins Leukot. Essent. Fatty Acids. 60: 345–349 [DOI] [PubMed] [Google Scholar]
- 21.Wisely G. B., Miller A. B., Davis R. G., Thornquest A. D., Jr, Johnson R., Spitzer T., Sefler A., Shearer B., Moore J. T., Miller A. B., et al. 2002. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure. 10: 1225–1234 [DOI] [PubMed] [Google Scholar]
- 22.Jump D. B., Botolin D., Wang Y., Xu J., Christian B., Demeure O. 2005. Fatty acid regulation of hepatic gene transcription. J. Nutr. 135: 2503–2506 [DOI] [PubMed] [Google Scholar]
- 23.Jump D. B., Botolin D., Wang Y., Xu J., Demeure O., Christian B. 2008. Docosahexaenoic acid (DHA) and hepatic gene transcription. Chem. Phys. Lipids. 153: 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou J., Tu H., Shan B., Luk A., DeBose-Boyd R. A., Bashmakov Y., Goldstein J. L., Brown M. S. 2001. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA. 98: 6027–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lengqvist J., Mata de Urquiza A. M., Bergman A. C., Willson T. M., Sjovall J., Perlmann T., Griffiths W. J. 2004. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol. Cell. Proteomics. 3: 692–703 [DOI] [PubMed] [Google Scholar]
- 26.Cvekl A., Wang W. L. 2009. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 89: 280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomin L., Johansson C. B., Zetterstrom R. H., Bissonnette R. P., Heyman R. A., Olson L., Lendahl U., Frisen J., Perlmann T. 1998. Retinoid-X receptor signalling in the developing spinal cord. Nature. 395: 398–402 [DOI] [PubMed] [Google Scholar]
- 28.Mascrez B., Mark M., Dierich A., Ghyselinck N. B., Kastner P., Chambon P. 1998. The RXRalpha ligand-dependent activation function 2 (AF-2) is important for mouse development. Development. 125: 4691–4707 [DOI] [PubMed] [Google Scholar]
- 29.Fliesler S. J., Anderson R. E. 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22: 79–131 [DOI] [PubMed] [Google Scholar]
- 30.Aveldaño M. I., Bazán N. G. 1974. Displacement into incubation medium by albumin of highly unsaturated retina free fatty acids arising from membrane lipids. FEBS Lett. 40: 53–56 [DOI] [PubMed] [Google Scholar]
- 31.Aveldaño M. I., Bazán N. G. 1975. Differential lipid deacylation during brain ischemia in a homeotherm and a poikilotherm. Content and composition of free fatty acids and triacylglycerols. Brain Res. 100: 99–110 [DOI] [PubMed] [Google Scholar]
- 32.Horrocks L. A., Farooqui A. A. 1994 NMDA receptor-stimulated release of arachidonic acid: mechanisms for the Bazan effect. In Cell Signal Transduction, Second Messengers, and Protein Phosphorylation in Health and Disease. A. M. Municio and M. T. Miras-Portugal, editors. Plenum Press, New York. 113–128. [Google Scholar]
- 33.Bazan N. G. 2003. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J. Lipid Res. 44: 2221–2233 [DOI] [PubMed] [Google Scholar]
- 34.Sun G. Y., Xu J., Jensen M. D., Simonyi A. 2004. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J. Lipid Res. 45: 205–213 [DOI] [PubMed] [Google Scholar]
- 35.Strokin M., Sergeeva M., Reiser G. 2003. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+. Br. J. Pharmacol. 139: 1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Politi L. E., Bouzat C., de los Santos E. B., Barrantes F. J. 1996. Heterologous retinal cultured neurons and cell adhesion molecules induce clustering of acetylcholine receptors and polynucleation in mouse muscle BC3H-1 clonal cell line. J. Neurosci. Res. 43: 639–651 [DOI] [PubMed] [Google Scholar]
- 37.Barnstable C. J. 1980. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 286: 231–235 [DOI] [PubMed] [Google Scholar]
- 38.Kljavin I. J., Lagenaur C., Bixby J. L., Reh T. A. 1994. Cell adhesion molecules regulating neurite growth from amacrine and rod photoreceptor cells. J. Neurosci. 14: 5035–5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hicks D., Barnstable C. J. 1987. Different rhodopsin monoclonal antibodies reveal different binding patterns on developing and adult rat retina. J. Histochem. Cytochem. 35: 1317–1328 [DOI] [PubMed] [Google Scholar]
- 40.Politi L. E., Rotstein N. P., Salvador G., Giusto N. M., Insua M. F. 2001. Insulin-like growth factor-I is a potential trophic factor for amacrine cells. J. Neurochem. 76: 1199–1211 [DOI] [PubMed] [Google Scholar]
- 41.Chucair A. J., Rotstein N. P., Sangiovanni J. P., During A., Chew E. Y., Politi L. E. 2007. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Invest. Ophthalmol. Vis. Sci. 48: 5168–5177 [DOI] [PubMed] [Google Scholar]
- 42.Ebisawa M., Umemiya H., Ohta K., Fukasawa H., Kawachi E., Christoffel G., Gronemeyer H., Tsuji M., Hashimoto Y., Shudo K., et al. 1999. Retinoid X receptor-antagonistic diazepinylbenzoic acids. Chem. Pharm. Bull. (Tokyo). 47: 1778–1786 [DOI] [PubMed] [Google Scholar]
- 43.Takahashi B., Ohta K., Kawachi E., Fukasawa H., Hashimoto Y., Kagechika H. 2002. Novel retinoid X receptor antagonists: specific inhibition of retinoid synergism in RXR-RAR heterodimer actions. J. Med. Chem. 45: 3327–3330 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K., Takahashi K., Nishimaki-Mogami T., Kagechika H., Yamamoto M., Itabe H. 2009. Docosahexaenoic acid induces adipose differentiation-related protein through activation of retinoid x receptor in human choriocarcinoma BeWo cells. Biol. Pharm. Bull. 32: 1177–1182 [DOI] [PubMed] [Google Scholar]
- 45.Umemiya H., Fukasawa H., Ebisawa M., Eyrolles L., Kawachi E., Eisenmann G., Gronemeyer H., Hashimoto Y., Shudo K., Kagechika H. 1997. Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the RXR-RAR heterodimers. J. Med. Chem. 40: 4222–4234 [DOI] [PubMed] [Google Scholar]
- 46.Ohta K., Kawachi E., Inoue N., Fukasawa H., Hashimoto Y., Itai A., Kagechika H. 2000. Retinoidal pyrimidinecarboxylic acids. Unexpected diaza-substituent effects in retinobenzoic acids. Chem. Pharm. Bull. (Tokyo). 48: 1504–1513 [DOI] [PubMed] [Google Scholar]
- 47.Berg M. M., Sternberg D. W., Parada L. F., Chao M. V. 1992. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J. Biol. Chem. 267: 13–16 [PubMed] [Google Scholar]
- 48.Skoff A. M., Adler J. E. 2006. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp. Neurol. 197: 430–436 [DOI] [PubMed] [Google Scholar]
- 49.Ackermann E. J., Conde-Frieboes K., Dennis E. A. 1995. Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J. Biol. Chem. 270: 445–450 [DOI] [PubMed] [Google Scholar]
- 50.Strokin M., Chechneva O., Reymann K. G., Reiser G. 2006. Neuroprotection of rat hippocampal slices exposed to oxygen-glucose deprivation by enrichment with docosahexaenoic acid and by inhibition of hydrolysis of docosahexaenoic acid-containing phospholipids by calcium independent phospholipase A2. Neuroscience. 140: 547–553 [DOI] [PubMed] [Google Scholar]
- 51.Jordán J., Galindo M. F., Prehn J. H., Weichselbaum R. R., Beckett M., Ghadge G. D., Roos R. P., Leiden J. M., Miller R. J. 1997. p53 expression induces apoptosis in hippocampal pyramidal neuron cultures. J. Neurosci. 17: 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mori M., Ghyselinck N. B., Chambon P., Mark M. 2001. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest. Ophthalmol. Vis. Sci. 42: 1312–1318 [PubMed] [Google Scholar]
- 53.Roberts M. R., Hendrickson A., McGuire C. R., Reh T. A. 2005. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest. Ophthalmol. Vis. Sci. 46: 2897–2904 [DOI] [PubMed] [Google Scholar]
- 54.Garelli A., Rotstein N. P., Politi L. E. 2006. Docosahexaenoic acid promotes photoreceptor differentiation without altering Crx expression. Invest. Ophthalmol. Vis. Sci. 47: 3017–3027 [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y., Calon F., Julien C., Winkler J. W., Petasis N. A., Lukiw W. J., Bazan N. G. 2011. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer's disease models. PLoS ONE. 6: e15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hadjiconstantinou M., Neff N. H. 1998. GM1 ganglioside: in vivo and in vitro trophic actions on central neurotransmitter systems. J. Neurochem. 70: 1335–1345 [DOI] [PubMed] [Google Scholar]
- 57.Hadjiconstantinou M., Neff N. H. 2000 GM1 ganglioside: commonalities of action with neurotrophins. In Neurobiology of the Neurotrophins. I. Mocchetti, editor. FP Graham Publishing Co, Johnson City, TN. 427–453. [Google Scholar]
- 58.Duchemin A. M., Ren Q., Mo L., Neff N. H., Hadjiconstantinou M. 2002. GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J. Neurochem. 81: 696–707 [DOI] [PubMed] [Google Scholar]
- 59.Capper E. A., Marshall L. A. 2001. Mammalian phospholipases A(2): mediators of inflammation, proliferation and apoptosis. Prog. Lipid Res. 40: 167–197 [DOI] [PubMed] [Google Scholar]
- 60.Kim H. Y., Akbar M., Lau A., Edsall L. 2000. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J. Biol. Chem. 275: 35215–35223 [DOI] [PubMed] [Google Scholar]
- 61.Rotstein N. P., Politi L. E., Aveldano M. I. 1998. Docosahexaenoic acid promotes differentiation of developing photoreceptors in culture. Invest. Ophthalmol. Vis. Sci. 39: 2750–2758 [PubMed] [Google Scholar]
- 62.Bazán N. G., Jr 1970. Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim. Biophys. Acta. 218: 1–10 [DOI] [PubMed] [Google Scholar]
- 63.Balboa M. A., Balsinde J. 2002. Involvement of calcium-independent phospholipase A2 in hydrogen peroxide-induced accumulation of free fatty acids in human U937 cells. J. Biol. Chem. 277: 40384–40389 [DOI] [PubMed] [Google Scholar]
- 64.Long E. K., Murphy T. C., Leiphon L. J., Watt J., Morrow J. D., Milne G. L., Howard J. R., Picklo M. J., Sr 2008. Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J. Neurochem. 105: 714–724 [DOI] [PubMed] [Google Scholar]
- 65.Altucci L., Leibowitz M. D., Ogilvie K. M., de Lera A. R., Gronemeyer H. 2007. RAR and RXR modulation in cancer and metabolic disease. Nat. Rev. Drug Discov. 6: 793–810 [DOI] [PubMed] [Google Scholar]
- 66.Kagechika H., Shudo K. 2005. Synthetic retinoids: recent developments concerning structure and clinical utility. J. Med. Chem. 48: 5875–5883 [DOI] [PubMed] [Google Scholar]
- 67.Pérez E., Bourguet W., Gronemeyer H., de Lera A. R. 2012. Modulation of RXR function through ligand design. Biochim. Biophys. Acta. 1821: 57–69 [DOI] [PubMed] [Google Scholar]
- 68.Cramer P. E., Cirrito J. R., Wesson D. W., Lee C. Y., Karlo J. C., Zinn A. E., Casali B. T., Restivo J. L., Goebel W. D., James M. J., et al. 2012. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 335: 1503–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]