Abstract
Placental inflammation is associated with several pregnancy disorders. Inflammation is limited by anti-inflammatory and proresolving mechanisms, the latter partly mediated by resolvins and protectins derived from omega-3 polyunsaturated fatty acids (n-3PUFA). We examined effects of dietary n-3PUFAs on levels of resolvins, protectins, and lipoxygenase (ALOX) enzymes in the rat placenta. Rats consumed standard (Std) or high n-3PUFA (Hn3) diets from day 1 of pregnancy; tissues were collected on day 17 or 22 (term = day 23). Maternal Hn3 diet increased resolvin and protectin precursors, 18R/S-HEPE (P < 0.001), and 17R/S-HDHA (P < 0.01) at both days. Resolvins (17R-RvD1 and RvD1) increased at day 22 (P < 0.001) after Hn3 consumption, coincident with higher Alox15b and Alox5 mRNA expression, while RvD2 increased at both days (P < 0.05). Protectins, PD1, and 10S,17S-DiHDHA increased over late gestation (P < 0.001), coincident with higher Alox15 mRNA expression (P < 0.001) and further increased with Hn3 diet (P < 0.05). Maternal systemic and placental proinflammatory mediators were not suppressed by Hn3 diet; systemic IL1β, placental Il1β, and Il6 mRNA expression increased marginally with Hn3 at day 22 (P < 0.001), while Ptgs1 (Cox1) expression increased both days (P < 0.05). Our data indicate that maternal n-3PUFA supplementation enhances expression of enzymes in the n-3PUFA metabolic pathway and increases placental levels of resolvins and protectins.
Keywords: n-3PUFA, inflammation, pregnancy, lipoxygenase
Fatty acids are important biological constituents that serve a range of structural, energetic, and signaling roles (1). The developing fetus requires substantial amounts of fatty acids to support rapid cellular growth and activity, and among these, the omega-3 (n-3) polyunsaturated fatty acids (PUFAs) are particularly important (2). Although subject to some controversy, maternal dietary supplementation with n-3PUFAs has demonstrated beneficial effects on human pregnancy outcomes, including increased gestation length (3), reduced risk of pregnancy complications (4–6), and increased fetal growth (7). We have recently shown that dietary supplementation with n-3PUFAs in pregnant rats enhanced placental and fetal growth in association with reduced placental oxidative stress (8). Omega-3 PUFAs are involved in several physiological pathways that may account for such beneficial effects. For example, n-3PUFAs are ligands for the peroxisome proliferator-activated receptors, transcription factors involved in gene regulation of anti-inflammatory, metabolic, and developmental processes (9). The anti-inflammatory properties of n-3PUFAs are particularly well documented, whereby they interrupt pro-inflammatory eicosanoid generation through competition for active sites of prostaglandin-endoperoxide synthase 2 (PTGS; also known as cyclooxygenase) and lipoxygenase (ALOX) enzymes (10); they also play a role in inhibiting cytokine production, cell adhesion molecule expression, nitric oxide synthesis, membrane fluidity and function, and NF-κB activity (11).
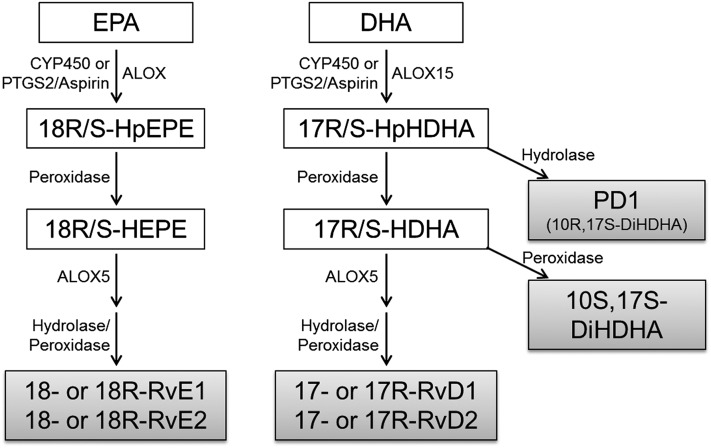
Serhan et al. (12) have described a family of potent anti-inflammatory and pro-resolving lipid mediators derived from n-3PUFAs named “resolvins” and “protectins.” These fatty acid-derived mediators drive an active process to resolve inflammation, and they are derived directly from eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 20:6n-3) by a series of biosynthetic steps involving ALOX enzymes and CYP450 or PTGS2/aspirin (13) (Fig. 1). Resolvins can be generated from either EPA (E-series resolvins; RvE) or DHA (D-series resolvins; RvD), whereas protectins are derived only from DHA (14). The potent pro-resolving and anti-inflammatory effects of resolvins and protectins were initially identified by Serhan and colleagues (15, 16) and have since been confirmed by many in vivo and in vitro studies (for reviews, see Refs. 13 and 17). Evidence that basal levels of these fatty acid-derived mediators can be enhanced by increasing substrate supply via dietary supplementation with n-3PUFAs is limited, although this has been demonstrated for rodent liver (18) and bone marrow (19) and in human blood (20, 21).
Fig. 1.
Resolvin and protectin generation pathways. EPA is converted to E-series resolvin precursor, 18-HEPE of the R- and S-configurations (18R/S-HEPE), by either CYP450, PTGS2/Aspirin, or ALOX activity followed by peroxidase activity. ALOX5 then initiates RvE generation. DHA is converted to the D-series resolvins and protectins precursor, 17-HpDHA of the R- and S-configurations (17R/S-HpDHA), by either ALOX15 (type A and/or B), CYP450, or PTGS2/Aspirin. This is then converted to protectin D1 (PD1) via hydroxylase, or 17R/S-HDHA via peroxidase activity. The S-configuration (17S-HDHA) is then converted to the protectin 10S,17S-DiHDHA via peroxidase, or alternatively, ALOX5 initiates RvD generation. (Modified from Ref. 12).
The aims of this study were to measure resolvins, protectins, and their precursors in the placental labyrinth zone (LZ) of the rat. The LZ was chosen because it is the site of maternal-fetal exchange, and as such, enhancing the capacity of this placental zone to resolve inflammation could significantly impact fetal growth outcomes. To assess whether the beneficial effects of n-3PUFAs on fetal growth and placental oxidative state are associated with increased levels of resolvins and protectins, we investigated the impact of maternal dietary supplementation with n-3PUFAs on placental levels of these specialized pro-resolving lipid mediators, as well as maternal and placental inflammatory markers. Since levels of resolvins and protectins changed significantly with maternal diet and/or gestational age, we also measured mRNA expression of the ALOX enzymes involved in their production by real-time qPCR. Measurements were made on days 17 and 22 of gestation (term = day 23) to encompass the major period of fetal growth.
MATERIALS AND METHODS
Animals and diets
Nulliparous albino Wistar rats, 8 to 12 weeks old, were obtained from the Animal Resources Centre (Murdoch, Australia) and maintained under controlled conditions as described previously (22). Rats were mated overnight, with day 1 of pregnancy designated as the day on which spermatozoa were present in a vaginal smear. On day 1 of pregnancy, mothers were placed on either a standard (Std) or high n-3PUFA (Hn3) isocaloric semipure diet (Specialty Feeds, Glenn Forrest, Australia). Both diets included 5% total fat; Std diet contained 0.8% of total fatty acids as n-3PUFAs (<0.02% as EPA and <0.02% as DHA), while the Hn3 diet contained 33.2% (5.4% as EPA and 23.8% as DHA). Detailed fatty acid composition of the diets was described by Jones et al. (8). All procedures involving animals were conducted under approval by the Animal Ethics Committee of the University of Western Australia.
Tissue collection
Rats were anesthetized with isoflurane/nitrous oxide at either day 17 or 22 of gestation. Three fetus-placenta pairs were obtained from the mid-region of each uterine horn (total of six per mother) and weighed. Each placenta was then dissected into junctional and labyrinth (LZ) zones, which were weighed individually and snap frozen in liquid nitrogen. Measurement of genes that are differentially expressed between placental zones confirms adequate separation by this method (22). A blood sample was obtained from the dorsal aorta and mixed with 10:1 (v/v) 0.6 M ethylenediaminetetraacetic acid and centrifuged at 13,000 g for 6 min to obtain plasma. All tissues and plasma samples were snap frozen in liquid nitrogen and stored at −80°C until further analysis. Fetal sex was determined by PCR amplification of the Sry gene in day 17 fetuses as previously described (8) and at day 22 by measuring anogenital distance (23).
Quantitation of resolvins and protectins
The following fatty acid-derived mediators were quantitated as previously described (24) in female LZ samples: 18R/S-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid (18R/S-HEPE); 17S-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid (17R/S-HDHA); 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (RvD1); 7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E19Z-docosahexaenoicacid (17R-RvD1); 7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid (RvD2); 10R,17S-dihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid (protectin D1, PD1); and 10S,17S-dihydroxy-4Z,7Z,11E,13Z,15E,19Z-docosahexaenoicacid (10S,17S-DiHDHA). Stereochemical assignment of RvD1 and 17R-RvD1 (25); RvD2 (26); and PD1 and 10S,17S-DiHDHA (27) have been described previously. The PD1 standard was kindly provided as a gift by Professor Charles N. Serhan (Harvard Medical School, Boston, MA). Briefly, samples were homogenized in 15% methanol (v/v) using a Polytron PT1200B homogenizer (Kinematica, Switzerland). Leukotriene B4-d4 (80 ng; Cayman Chemicals, MI) was added as the internal standard. Samples were then acidified to pH 3 with 0.25 M HCl and applied to solid-phase extraction cartridges (Bond Elut C18 500 mg; Agilent Technologies, Vic, Australia) and washed in 15 ml 15% methanol, 15 ml double-distilled H2O, and 15 ml hexane. Resolvins and protectins were eluted with 10 ml methyl formate, dried under nitrogen, and then reconstituted in 100 µl 5 mmol/l ammonium acetate (pH = 9)/methanol (50/50; v/v) for analysis by liquid chromatography-tandem mass spectrometry as described previously (24).
We previously reported that male and female LZ exhibit similar gestational age and dietary responses with regard to growth outcomes, fatty acid profiles, and oxidative states (8). Therefore, resolvin and protectin analyses were limited to female placentas.
RNA sample preparation
Total RNA was isolated from placental LZ samples using Tri-Reagent (Molecular Resources Centre, Cincinnati, OH) as per the manufacturer's instructions. Total RNA (1 μg) was used as a template for cDNA synthesis by murine Moloney leukemia virus Reverse Transcriptase RNase H Point Mutant and random hexamer primers (Promega, Madison, WI) as per the manufacturer's instructions. The resultant cDNAs were purified using the Ultraclean PCR Cleanup kit (MoBio Industries, Solana Beach, CA).
Real-time RT-PCR
Analyses of mRNA expression levels for the lipoxygenase enzymes Alox15 (also known as 12/15-Lox), Alox15b, Alox5, and Alox5 activating protein (Alox5ap); for the pro-inflammatory mediator genes tumor necrosis factor-α (Tnfα), interleukin-1β (Il1β), interleukin-6 (Il6), Ptgs1, and Ptgs2; and for the reference genes Ppia, Sdha, and Ywhaz were performed by real time RT-PCR on the Rotorgene 6000 (Corbett Industries, Sydney, Australia) using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Primer pairs for all genes of interest (Table 1) were designed using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast) (28), with the exception of Il1β, which was purchased (QT00181657; Qiagen, Melbourne, Australia). Each of the selected primer pairs were positioned to span introns to ensure no product was amplified from genomic DNA, and the resulting amplicons were sequenced to confirm specificity. Standard curves for each amplicon were generated with 10-fold serial dilutions of gel-extracted (QIAEX II, Qiagen, Melbourne, Australia) PCR products using the Rotorgene 6000 software. All samples were normalized against Ppia, Sdha, and Ywhaz using the GeNorm algorithm (29).
TABLE 1.
Primers and PCR conditions used to measure placental expression of the inflammatory genes, lipoxygenase enzymes, and reference genes by real time RT-PCR
| Gene | Forward/Reverse Primer Sequence | Annealing Temp.(°C) | Amplicon Size (bp) | MgCl2 (mM) |
| Alox15 | F 5′ CACCGGAGACTCCAAGTACG 3′ | 60 | 153 | 3 |
| R 5′ AGTGGCCCAAGGTATCCTGA 3′ | ||||
| Alox15b | F 5′ CCCTGTTATCAGTGGCTGGA 3′ | 60 | 190 | 3 |
| R 5′ TCACGGTCTCATGGTCAAGG 3′ | ||||
| Alox5 | F 5′ TGGCATCTAGGTGCAGTGTG 3′ | 60 | 133 | 3 |
| R 5′ CCTCCAGGTTCTTGCGGAAT 3′ | ||||
| Alox5ap | F 5′ GAGAAGCTTCCAGAGGACGG 3′ | 60 | 154 | 3 |
| R 5′ ATACATCAGCCCAGCGAAGG 3′ | ||||
| Tnfα | F 5′ TACTGAACTTCGGGGTGATTGGTCC 3′ | 60 | 295 | 3 |
| R 5′ CAGCCTTGTCCCTTGAAGAGAACC 3′ | ||||
| Il6 | F 5′ TCCGCAAGAGACTTCCAGCCAGT 3′ | 60 | 148 | 2 |
| R 5′ AGCCTCCGACTTGTGAAGTGGT 3′ | ||||
| Ptgs1 | F 5′ TGCCCTCTGTACCCAAAGAC 3′ | 60 | 104 | 2 |
| R 5′ CTCCCTTCTCAGCAGCAATC 3′ | ||||
| Ptgs2 | F 5′ GAAGGGACACCCTTTCACAT 3′ | 59 | 178 | 4 |
| R 5′ TGGGGAGACCATGGTAGAAC 3′ | ||||
| Ppia | F 5′ AGCATACAGGTCCTGGCATC 3′ | 62 | 127 | 3 |
| R 5′ TTCACCTTCCCAAAGACCAC 3′ | ||||
| Sdha | F 5′ TGGGGCGACTCGTGGCTTTC 3′ | 60 | 134 | 2 |
| R 5′ CCCCGCCTGCACCTACAACC 3′ | ||||
| Ywhaz | F 5′ GACGGAGCTGAGGGACATCTGC 3′ | 60 | 75 | 2 |
| R 5′ GGCTGCGAAGCATTGGGGATCA 3′ |
Pro-inflammatory cytokine quantitation
Levels of the pro-inflammatory cytokines TNFα, IL1β, and IL6 were measured in maternal aortic plasma using a MILLIPLEX xMAP Kit; Rat Cytokine/Chemokine (Cat# RCYTO-80K; Merck Millipore, MA). Samples were centrifuged at 13,000 g for 5 min prior to analysis. The assay was performed as per manufacturer's instructions using a CS1000 Autoplex Anaylzer (PerkinElmer, WA), running Luminex xPONENT software (Luminex).
Statistical analysis
All analyses were conducted using Genstat version 14 (VSN International Ltd., Hemel Hempstead, UK). Where data were not normally distributed (based on residuals plot analyses), values were log transformed prior to statistical analysis. In all instances, “n” refers to the number of litters analyzed. Variation in placental gene expression was assessed by three-way ANOVA, with variation attributed to maternal diet, gestational age, and fetal sex. Differences in placental levels of resolvins and protectins and in plasma levels of pro-inflammatory cytokines were assessed by two-way ANOVA, with variation attributed to maternal diet and gestational age. For all ANOVAs, when significant interaction between sources of variation was seen, separate comparisons were conducted by two-way ANOVA or unpaired t-test as appropriate. Where the F test reached statistical significance (P < 0.05), subsequent post hoc analyses were performed using least-significant-difference (LSD) tests (30).
RESULTS
In this same cohort of animals, we have previously demonstrated increased fetal (6%) and placental (12%) weights at day 22 with Hn3 diet, the latter attributable primarily to growth of the LZ. Furthermore, levels of EPA (1.8- to 3-fold) and DHA (3.2- to 4-fold) were elevated in the LZ with Hn3 diet (8).
Placental levels of resolvins and protectins
The E-series and D-series precursors, 18R/S-HEPE and 17R/S-HDHA, respectively, were both detected in the placental LZ (Fig. 2). In Std diet-fed animals, 18R/S-HEPE levels were unaltered from day 17 to day 22 of gestation (Fig. 2A), while 17R/S-HDHA increased (5.2-fold, P < 0.001) over the same period (Fig. 2B). Consumption of the Hn3 diet increased levels of these precursors at both gestational days; 18R/S-HEPE levels were 12-fold higher (P < 0.001) at day 17 and 54-fold higher (P < 0.001) at day 22 (Fig. 2A), while the increases in 17R/S-HDHA concentrations were far more modest (1.5- to 2.3-fold, overall P < 0.01; Fig. 2B).
Fig. 2.
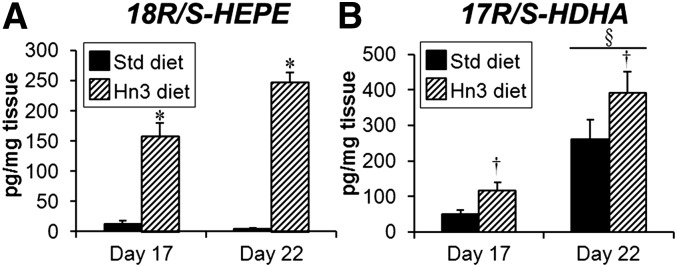
Levels of female labyrinth zone resolvin/protectin precursors (A) 18R/S-HEPE and (B) 17R/S-HDHA at days 17 and 22 of pregnancy. Mothers were fed either a standard or high n-3PUFA diet from day 1. Values are mean ± SEM (n = 5–8 per group). *P < 0.001 compared with corresponding Std diet (unpaired t-test); †P < 0.01 overall compared with Std diet (two-way ANOVA); §P < 0.001 compared with day 17 (two-way ANOVA).
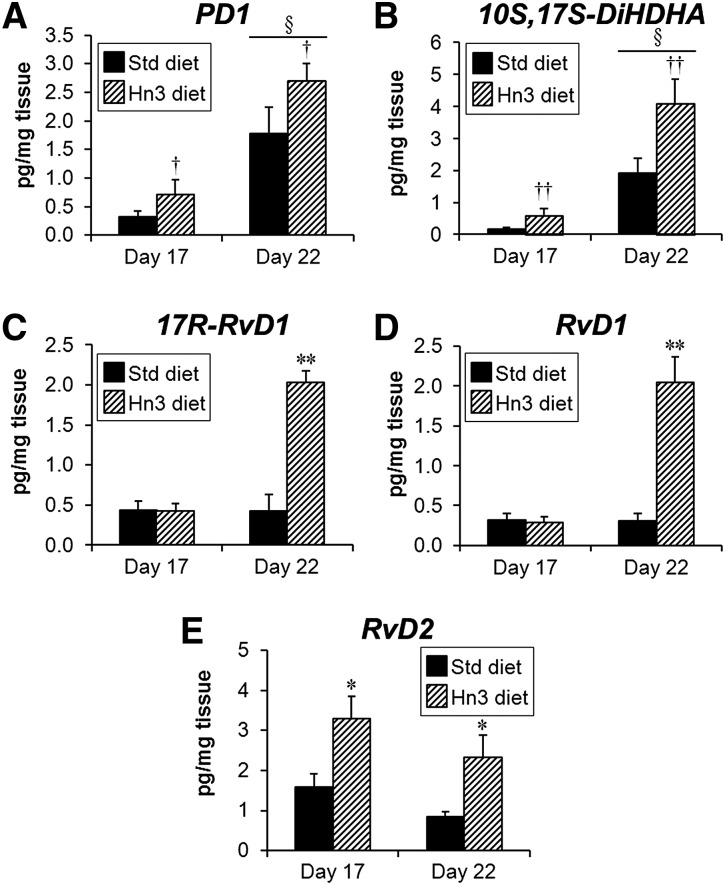
Placental LZ levels of the two protectins, PD1 and 10S,17S-DiHDHA, were similarly affected by gestational age and diet (Fig. 3A, B). Both PD1 and 10S,17S-DiHDHA concentrations increased from day 17 to day 22 of gestation (5.4- and 11-fold, respectively; P < 0.001). While the Hn3 diet increased levels of both, this effect was greater for 10S,17S-DiHDHA (2.1- to 3.4-fold, overall P < 0.01; Fig. 3B) compared with PD1 (1.5- to 2.2-fold, overall P < 0.05; Fig. 3A).
Fig. 3.
Levels of female labyrinth zone protectins (A) PD1 and (B) 10S,17S-DiHDHA, and resolvins (C) 17R-RvD1, (D) RvD1, and (E) RvD2 at days 17 and 22 of pregnancy. Mothers were fed either a standard or high n-3PUFA diet from day 1. Values are mean ± SEM (n = 5–8 per group). **P < 0.001 and *P < 0.05 compared with corresponding Std diet (unpaired t-test or two-way ANOVA, LSD test); ††P < 0.01 and †P < 0.05 overall compared with Std diet (two-way ANOVA); §P < 0.001 compared with day 17 (two-way ANOVA).
Levels of both 17R-RvD1 and RvD1 were unaltered from day 17 to day 22 of gestation and were not affected by the Hn3 diet at day 17 (Fig. 3C, D). At day 22, however, levels of both 17R-RvD1 and RvD1 were significantly increased (4.7- and 6.7-fold, respectively; P < 0.001; Fig. 3C, D) by the Hn3 diet. Placental levels of RvD2 were unaltered from day 17 to day 22, but increased with Hn3 diet (day 17: 2.1-fold, P < 0.05; day 22: 2.7-fold, P < 0.05; Fig. 3E).
Placental expression of lipoxygenase enzymes
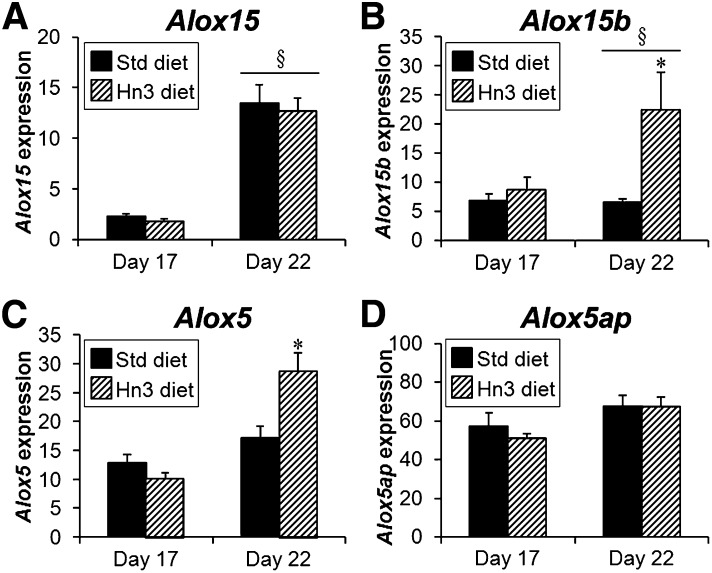
Gene expression of Alox15 was overall higher in LZ of female fetuses than LZ of male fetuses at day 22 of pregnancy (44 to 71% higher; P < 0.01); however, gestational and dietary changes were similar between sexes. For all other genes, fetal sex did not affect placental mRNA expression, so male and female data were pooled for further analyses.
In Std diet-fed animals, gene expression of Alox15, a lipoxygenase enzyme that drives the initial step in resolvin and protectin formation, increased from day 17 to day 22 of pregnancy (male: 4-fold; female: 5.7-fold, both P < 0.001; Fig. 4A), whereas Alox15b remained similar between gestational days (Fig. 4B). Those enzymes responsible for the final stages of resolvin production, Alox5 and Alox5ap, were not different between gestational days (Fig. 4C, D). Maternal Hn3 diet increased LZ mRNA expression of Alox15b and Alox5 at day 22 (by 3.4- and 1.9-fold, respectively; P < 0.001), while expression of Alox15 and Alox5ap were unaffected by Hn3 diet.
Fig. 4.
Labyrinth zone expression of (A) Alox15 (female only), (B) Alox15b, (C) Alox5, and (D) Alox5ap mRNAs at days 17 and 22 of pregnancy with fetal sex pooled, except for Alox15. Mothers were fed either a standard or high n-3PUFA diet from day 1. Values are mean ± SEM (n = 7–8 per group) adjusted by GeNorm algorithm. *P < 0.001 compared with corresponding Std diet group (two-way ANOVA); §P < 0.001 compared with day 17 (three-way or two-way ANOVA).
Placental expression of pro-inflammatory mediators
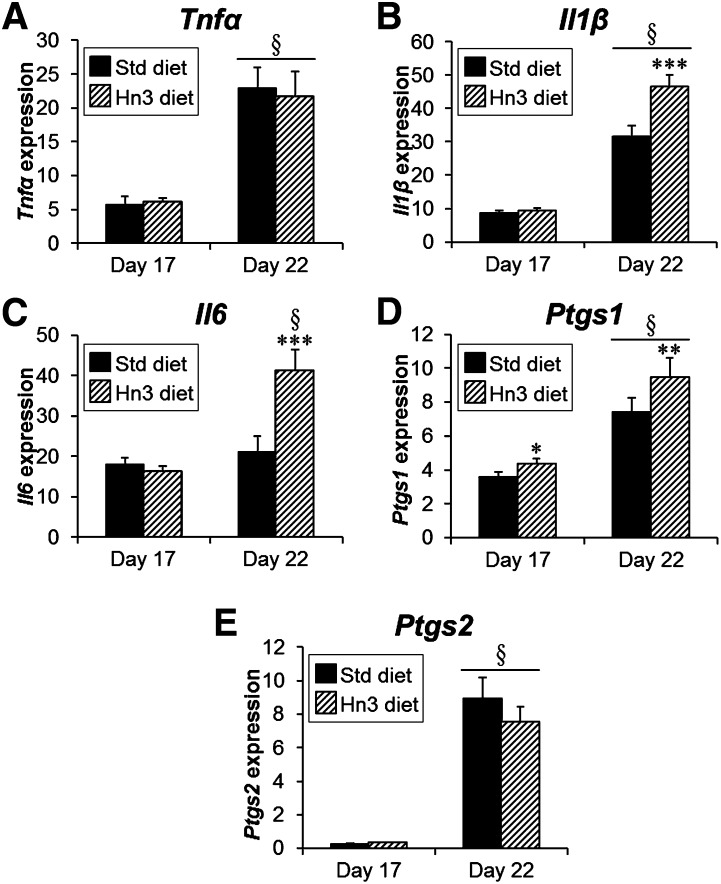
LZ mRNA expression of Tnfα, Il1β, Ptgs1, and Ptgs2 increased from day 17 to day 22 of rat pregnancy (Tnfα: 4-fold, P < 0.001; Il1β: 3.4-fold, P < 0.001; Ptgs1: 95%, P < 0.001; and Ptgs2: 31-fold, P < 0.001), while Il6 remained consistent between gestational days (Fig. 5). Surprisingly, mRNA expression of Il1β and Il6 was higher with Hn3 dietary intake at day 22 (2.2- and 1.6-fold, respectively; P < 0.001), and Ptgs1 was higher at both day 17 and day 22 (by 22%, P < 0.05, and 27%, P < 0.01, respectively).
Fig. 5.
Labyrinth zone expression of (A) Tnfα, (B) Il1β, (C) Il6, (D) Ptgs1, and (E) Ptgs2 mRNAs at days 17 and 22 of pregnancy with fetal sex pooled. Mothers were fed either a standard or high n-3PUFA diet from day 1. Values are mean ± SEM (n = 7–8 per group) adjusted by GeNorm algorithm. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with corresponding Std diet group (two-way ANOVA); §P < 0.001 compared with day 17 (three-way or two-way ANOVA).
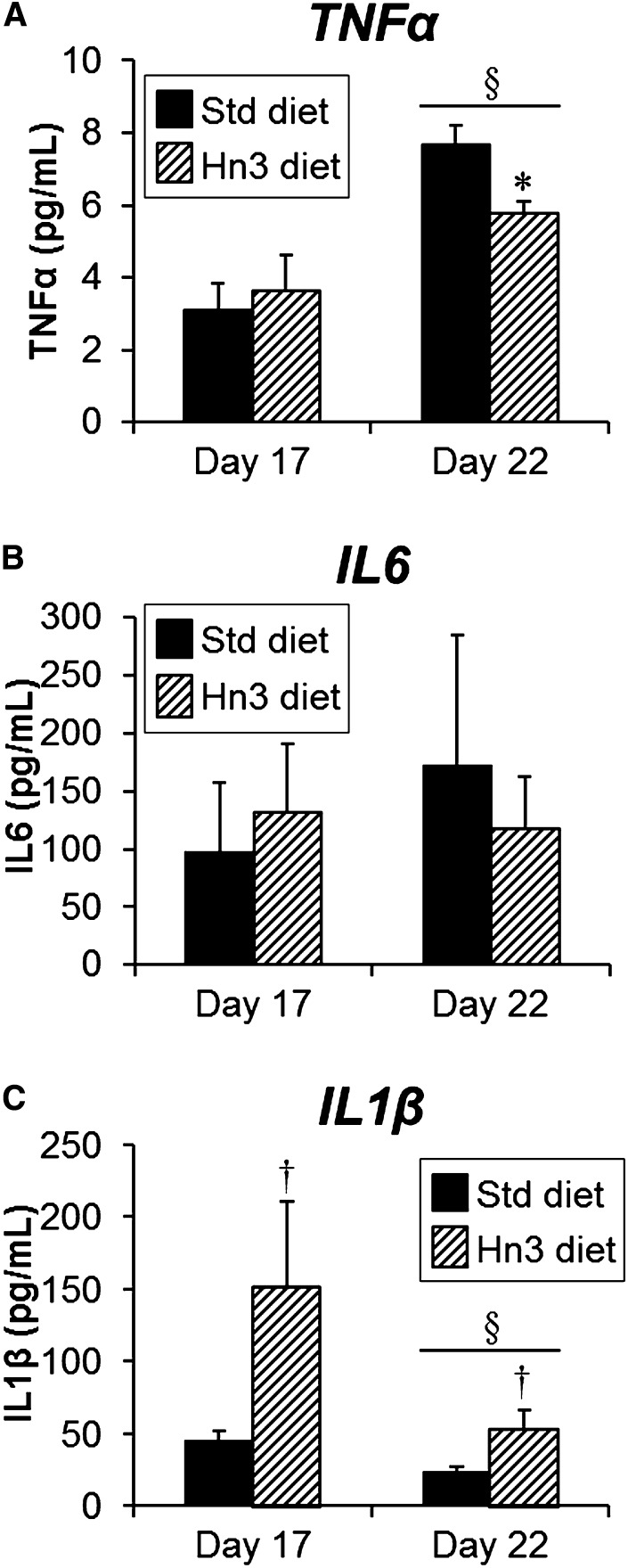
Plasma pro-inflammatory cytokine levels
Maternal systemic levels of TNFα increased from day 17 to day 22 (2.5-fold, P < 0.05; Fig. 6A), whereas levels of IL6 did not change (Fig. 6B). Plasma IL1β concentrations decreased overall (both diets pooled) from day 17 to day 22 (P < 0.05), but this only reached significance by post hoc analysis in the Hn3 group (Fig. 6C). Plasma TNFα levels were unaffected by maternal diet at day 17, but by day 22, levels were reduced by 25% with maternal Hn3 dietary intake (P = 0.01). In contrast, plasma IL1β levels increased with maternal Hn3 dietary intake (2.3- to 3.4-fold, overall P < 0.05), while IL6 levels were unaffected.
Fig. 6.
Maternal aortic plasma levels of (A) TNFα, (B) IL6, and (C) Il1β at days 17 and 22 of pregnancy. Mothers were fed either a standard or high n-3PUFA diet from day 1. Values are mean ± SEM (n = 5–8 per group). *P = 0.01 compared with corresponding Std diet group (unpaired t-test); †P < 0.05 overall compared with Std diet (three-way ANOVA); §P < 0.05 compared with day 17 (two-way ANOVA).
DISCUSSION
This is the first report describing the presence of resolvins and protectins in rat placenta. The major findings were that relatively high levels of the resolvins 17R-RvD1, RvD1, and RvD2, the protectins PD1 and 10S,17S-DiHDHA, and their respective precursors 18R/S-HEPE and 17R/S-HDHA were detected in the rat placental LZ in the final third of gestation, with levels of protectins increasing over this period. Moreover, levels of resolvins, protectins, and their respective pathway precursors were markedly increased by maternal dietary supplementation with n-3PUFAs, whereas markers of inflammation were not suppressed.
All of the measured resolvins and protectins were readily detectable in rat placental LZ, and although current literature is limited, levels appeared to be high relative to other tissues. For example, concentrations of RvD1, RvD2, and PD1 were higher than levels recently reported in human subcutaneous adipose tissue by approximately 60-fold, 170- to 300-fold, and 7- to 37-fold, respectively (31). Furthermore, these fatty acid-derived mediators have shown to exhibit potent pro-resolving activity in nanomolar to picomolar concentrations in a variety of cell types (12); here placental LZ levels appear relatively high, ranging from picomolar (10S,17S-DiHDHA at day 17) to low micromolar (17R/S-HDHA at day 22) concentrations. Our analysis was conducted on tissue homogenates, so extracellular concentrations were not determined; therefore, it is unclear whether biological actions of these fatty acid-derived mediators are confined to local effects within the placenta or may also play a role in other target tissues. Levels of PD1 and 10S,17S-DiHDHA and their precursor 17R/S-HDHA increased over late gestation in the placental LZ of Std diet-fed animals. Consistent with these changes, gene expression of Alox15, one of the enzymes that drive the initial step in resolvin and protectin formation, also increased toward term. In contrast, Alox15b, which is similarly involved in resolvin and protectin formation, did not increase toward term, which may indicate this form of the enzyme did not drive late gestational increases in protectin levels. CYP450 activity also may have contributed to gestational increases of these fatty acid-derived mediators; the relative contribution of ALOX15 and CYP450 to placental resolvin and protectin generation remains to be determined. Increased protectin levels are unlikely to be simply due to greater substrate availability because, although DHA levels were higher in the placental LZ at day 22 compared with day 17 (25%; see Ref. 8), the corresponding increase in protectin levels was considerably greater (5- to 11-fold). High protectin levels in the LZ at late gestation may serve to maintain a healthy inflammatory balance as these signals rise markedly leading up to and during parturition. Such a role may be specific to protectins, since neither the D-series resolvins nor the E-series precursor (18R/S-HEPE) increased in abundance toward term. Accordingly, gene expression of the enzymes responsible for the final stages of resolvin production, Alox5 and Alox5ap, remained unchanged in the LZ, consistent with the stable levels of the resolvins during late gestation.
Excessive inflammation in uteroplacental tissues has been implicated in the development of several pregnancy complications (32), and n-3PUFAs have been proposed as a possible therapeutic intervention. Maternal n-3PUFA supplementation enhanced placental LZ levels of those specialized proresolving mediators and their precursors. Levels of both 18R/S-HEPE and 17R/S-HDHA were significantly increased by the Hn3 diet at both gestational days. The presence of these precursors suggests an overall increase in basal production of resolvins and protectins in the placental LZ with maternal Hn3 dietary intake. The pathway precursors 18R/S-HEPE and 17R/S-HDHA have shown to exert pro-resolving and anti-inflammatory activity (18, 33–35), albeit at a higher concentration than their downstream resolvin and protectin metabolites, raising the possibility that they may contribute to the pro-resolving effects of these lipids in the placenta. Both PD1 and 10S,17S-DiHDHA levels were also increased by the Hn3 diet on days 17 and 22. In contrast, 17R-RvD1 and RvD1 abundance was increased with Hn3 diet only at day 22 of pregnancy. Coincident with these increases, gene expression of Alox15b and Alox5 were stimulated by the Hn3 diet at day 22, suggesting that exposure to dietary n-3PUFAs may enhance enzymatic conversion to pro-resolving mediators, exerting beneficial effects. CYP450 activity also may have contributed to enhanced resolvin and protectin production in this regard. In contrast, expression of the ALOX5-activating protein Alox5ap did not change with n-3PUFA consumption, which may indicate basal levels of expression are sufficient to facilitate ALOX5 activity.
Our data also show that placental RvD2 levels increased with Hn3 diet at both gestational days, whereas RvD1 and 17R-RvD1 were increased only at day 22. Furthermore, levels of RvD2 were 3- to 4-fold higher than RvD1 in these tissues, and significantly higher than levels found in human adipose tissue (approximately 170- to 300-fold) (31). Specific cell surface-binding sites for RvD1 (G protein-coupled receptor 32 and lipoxin A4 receptor) (36) have been identified in human leukocytes; however, whether these receptors are present in the placenta is currently unknown. We have confirmed that the E-series resolvin receptors leukotriene B4 receptor 1 and chemokine-like receptor 1 (37) are expressed in the human placenta (J. A. Keelan, unpublished observations), although the cellular location remains unknown. Further studies are required to delineate the effects of resolvin/protectin receptor activation in the placenta. Collectively, these data suggest that maternal dietary supplementation with n-3PUFAs effectively enhances a number of resolvins and protectins in the placental LZ. Given the primary function of the LZ in materno-fetal exchange (38), a greater capacity to resolve inflammation in this zone may protect the tissue from damaging effects of heightened inflammation associated with pregnancy (32), contributing to enhanced placental LZ and fetal growth observed in this animal model (8).
We observed that the majority of pro-inflammatory mediators increased in abundance toward term, consistent with the conventional view that pregnancy is a state of heightened inflammation. This may be in preparation for the parturition process (39). Clear gestational increases in maternal systemic levels of TNFα and placental gene expression of Tnfα and Il1β mRNA were evident in this study. Furthermore, Ptgs1 and Ptgs2 expression in the LZ increased between days 17 and 22 of pregnancy, consistent with a role for these enzymes in the regulation of parturition (40).
Maternal systemic levels of TNFα were reduced at day 22 by dietary n-3PUFA supplementation, consistent with the well-documented anti-inflammatory effects of these fatty acids. In contrast, other measures of systemic and placental pro-inflammatory mediators were not suppressed by the Hn3 diet. Indeed, maternal systemic levels of IL1β and LZ gene expression of Il1β, Il6, and Ptgs1 all increased with the Hn3 diet (although, notably, LZ expression of Ptgs2 was unaffected). These data suggest that unlike the well-documented anti-inflammatory effects of n-3PUFAs in other settings (11), the inflammatory status of the placenta was not reduced by n-3PUFA supplementation. Interestingly, we recently demonstrated that glucocorticoids, also well recognized for their potent anti-inflammatory effects (41), were similarly unable to suppress placental expression of pro-inflammatory mediators in late gestation (42). Collectively, these and other data suggest that regulation of inflammation in the late gestation placenta may be unconventional, possibly driven by additional signaling pathways that promote parturition. While further studies are required to explore this possibility, the observed increases in placental resolvin and protectin levels in response to the Hn3 diet are still likely to provide the placenta with a greater capacity to resolve inflammation in the event of an inflammatory challenge (e.g., infection or ischemia-reperfusion injury). This hypothesis would need to be tested in a suitable animal model. Furthermore, despite minimal anti-inflammatory effects observed here, n-3PUFA supplementation remains beneficial to placental oxidative status, as evidenced by reduced placental LZ levels of F2-isoprostanes (a highly reliable marker of oxidative damage) following n-3PUFA supplementation [31% and 11% decrease at day 17 and day 22, respectively (8)].
In conclusion, this study demonstrates that the resolvins 17R-RvD1, RvD1, and RvD2, the protectins PD1 and 10S,17S-DiHDHA, and their respective precursors 18R/S-HEPE and 17R/S-HDHA are present in the rat placental LZ and that levels of the protectins increase toward term. We have also shown that maternal dietary supplementation with n-3PUFAs effectively increases placental levels of these fatty acid-derived mediators, potentially enhancing the placental capacity to resolve inflammation. This effect may contribute to increased fetal and placental growth as demonstrated previously for this same cohort of animals (8), and it highlights the therapeutic potential of n-3PUFAs to limit placental inflammation associated with pregnancy disorders.
Acknowledgments
The authors acknowledge the technical assistance of Ms. Tracey Lee-Pullen (Centre for Microscopy, Characterisation and Analysis, University of Western Australia, Australia) for the Milliplex cytokine assay.
Footnotes
Abbreviations:
- 10S,17SDiHDHA
- 10S,17S-dihydroxy-4Z,7Z,11E,13Z,15E,19Z-docosahexaenoicacid
- 17R-RvD1, 7S,8R
- 17R-trihydroxy-4Z,9E,11E,13Z,15E19Z-docosahexaenoicacid
- 17R/S-HDHA
- 17S-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid
- 18R/S-HEPE
- 18R/S-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid
- ALOX
- lipoxygenase
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- Hn3
- high n-3PUFA semipure diet
- Il
- interleukin
- LZ
- labyrinth zone
- n-3PUFA
- omega-3 polyunsaturated fatty acid
- PD1
- 10R,17S-dihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid
- Ppia
- peptidylprolyl isomerase A
- Ptgs
- prostaglandin-endoperoxide synthase
- Std
- standard semipure diet
- RvD1
- 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid
- RvD2
- 7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid
- Sdha
- succinate dehydrogenase subunit A
- Tnfα
- tumor necrosis factor-α
- Ywhaz
- tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein
This study was supported by Project Grant 572621 from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Calder P. C. 2012. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 71: 284–289 [DOI] [PubMed] [Google Scholar]
- 2.Haggarty P. 2010. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 30: 237–255 [DOI] [PubMed] [Google Scholar]
- 3.Szajewska H., Horvath A., Koletzko B. 2006. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 83: 1337–1344 [DOI] [PubMed] [Google Scholar]
- 4.Oken E., Ning Y., Rifas-Shiman S. L., Rich-Edwards J. W., Olsen S. F., Gillman M. W. 2007. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann. Epidemiol. 17: 663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen S. F., Secher N. J., Tabor A., Weber T., Walker J. J., Gluud C. 2000. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish oil trials in pregnancy (FOTIP) team. BJOG. 107: 382–395 [DOI] [PubMed] [Google Scholar]
- 6.Zhou S. J., Yelland L., McPhee A. J., Quinlivan J., Gibson R. A., Makrides M. 2012. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. Am. J. Clin. Nutr. 95: 1378–1384 [DOI] [PubMed] [Google Scholar]
- 7.Olsen S. F., Olsen J. R., Frische G. 1990. Does fish consumption during pregnancy increase fetal growth? A study of the size of the newborn, placental weight and gestational age in relation to fish consumption during pregnancy. Int. J. Epidemiol. 19: 971–977 [DOI] [PubMed] [Google Scholar]
- 8.Jones M. L., Mark P. J., Mori T. A., Keelan J. A., Waddell B. J. 2013. Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol. Reprod. 88 In press. [DOI] [PubMed] [Google Scholar]
- 9.Jawerbaum A., Capobianco E. 2011. Review: Effects of PPAR activation in the placenta and the fetus: implications in maternal diabetes. Placenta. 32(Suppl. 2): S212–S217 [DOI] [PubMed] [Google Scholar]
- 10.Calder P. C. 2003. n−3 Polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 38: 343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calder P. C. 2011. Fatty acids and inflammation: the cutting edge between food and pharma. Eur. J. Pharmacol. 668(Suppl. 1): S50–S58 [DOI] [PubMed] [Google Scholar]
- 12.Serhan C. N., Petasis N. A. 2011. Resolvins and protectins in inflammation resolution. Chem. Rev. 111: 5922–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan C. N. 2007. Resolution phase of inflammation: novel endogenous anti-Inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25: 101–137 [DOI] [PubMed] [Google Scholar]
- 14.Seki H., Tani Y., Arita M. 2009. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 89: 126–130 [DOI] [PubMed] [Google Scholar]
- 15.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192: 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R-L. 2002. A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196: 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weylandt K. H., Chiu C-Y., Gomolka B., Waechter S. F., Wiedenmann B. 2012. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 97: 73–82 [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Periz A., Planaguma A., Gronert K., Miquel R., Lopez-Parra M., Titos E., Horrillo R., Ferre N., Deulofeu R., Arroyo V., et al. 2006. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 20: 2537–2539 [DOI] [PubMed] [Google Scholar]
- 19.Poulsen R. C., Gotlinger K. H., Serhan C. N., Kruger M. C. 2008. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and omega-3 intake. Am. J. Hematol. 83: 437–445 [DOI] [PubMed] [Google Scholar]
- 20.Oh S. F., Pillai P. S., Recchiuti A., Yang R., Serhan C. N. 2011. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 121: 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N. A., Serhan C. N. 2005. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201: 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mark P. J., Augustus S., Lewis J. L., Hewitt D. P., Waddell B. J. 2009. Changes in the placental glucocorticoid barrier during rat pregnancy: impact on placental corticosterone levels and regulation by progesterone. Biol. Reprod. 80: 1209–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imperato-McGinley J., Binienda Z., Gedney J., Vaughan E. D. J. 1986. Nipple differentiation in fetal male rats treated with an inhibitor of the enzyme 5{alpha}-reductase: definition of a selective role for dihydrotestosterone. Endocrinology. 118: 132–137 [DOI] [PubMed] [Google Scholar]
- 24.Mas E., Croft K. D., Zahra P., Barden A., Mori T. A. 2012. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 58: 1476–1484 [DOI] [PubMed] [Google Scholar]
- 25.Sun Y-P., Oh S. F., Uddin J., Yang R., Gotlinger K., Campbell E., Colgan S. P., Petasis N. A., Serhan C. N. 2007. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 282: 9323–9334 [DOI] [PubMed] [Google Scholar]
- 26.Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., Flower R. J., Perretti M., Serhan C. N. 2009. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 461: 1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serhan C. N., Gotlinger K., Hong S., Lu Y., Siegelman J., Baer T., Yang R., Colgan S. P., Petasis N. A. 2006. Anti-inflammatory actions of neuroprotectin d1/protectin d1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 176: 1848–1859 [DOI] [PubMed] [Google Scholar]
- 28.Rozen S., Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132: 365–386 [DOI] [PubMed] [Google Scholar]
- 29.Vandesompele J., Preter K. D., Pattyn F., Poppe B., Roy N. V., Paepe A. D., Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snedecor G., Cochran W. 1989. Statistical Methods. 8th edition. Iowa State University Press, Ames, IA. [Google Scholar]
- 31.Claria J., Nguyen B. T., Madenci A., Ozaki C. K., Serhan C. N. Diversity of lipid mediators in human adipose tissue depots. Am. J. Physiol. Cell Physiol.Epub ahead of print. January 30, 2013; 10.1152/ajpcell.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Challis J. R., Lockwood C. J., Myatt L., Norman J. E., Strauss J. F., Petraglia F. 2009. Inflammation and pregnancy. Reprod. Sci. 16: 206–215 [DOI] [PubMed] [Google Scholar]
- 33.Chiu C-Y., Gomolka B., Dierkes C., Huang N. R., Schroeder M., Purschke M., Manstein D., Dangi B., Weylandt K. H. 2012. Omega-6 docosapentaenoic acid-derived resolvins and 17-hydroxydocosahexaenoic acid modulate macrophage function and alleviate experimental colitis. Inflamm. Res. 61: 967–976 [DOI] [PubMed] [Google Scholar]
- 34.Krishnamurthy V. R., Dougherty A., Haller C. A., Chaikof E. L. 2011. Total synthesis and bioactivity of 18(R)-hydroxyeicosapentaenoic acid. J. Org. Chem. 76: 5433–5437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weylandt K. H., Krause L. F., Gomolka B., Chiu C-Y., Bilal S. l., Nadolny A., Waechter S. F., Fischer A., Rothe M., Kang J. X. 2011. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-a. Carcinogenesis. 32: 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S. T., Lee C-H., Yang R., Petasis N. A., Serhan C. N. 2010. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA. 107: 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arita M., Ohira T., Sun Y-P., Elangovan S., Chiang N., Serhan C. N. 2007. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178: 3912–3917 [DOI] [PubMed] [Google Scholar]
- 38.Takata K., Fujikura K., Shin B. C. 1997. Ultrastructure of the rodent placental labyrinth: a site of barrier and transport. J. Reprod. Dev. 43: 13–24 [Google Scholar]
- 39.Paulesu L., Bhattacharjee J., Bechi N., Romagnoli R., Jantra S., Ietta F. 2010. Pro-inflammatory cytokines in animal and human gestation. Curr. Pharm. Des. 16: 3601–3615 [DOI] [PubMed] [Google Scholar]
- 40.Keelan J. A., Blumenstein M., Helliwell R. J. A., Sato T. A., Marvin K. W., Mitchell M. D. 2003. Cytokines, prostaglandins and parturition--a review. Placenta. 24: S33–S46 [DOI] [PubMed] [Google Scholar]
- 41.Vandevyver S., Dejager L., Tuckermann J., Libert C. 2013. New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology. 154: 993–1007 [DOI] [PubMed] [Google Scholar]
- 42.Mark P. J., Lewis J. L., Jones M. L., Keelan J. A., Waddell B. J. 2013. The inflammatory state of the rat placenta increases in late gestation and is further enhanced by glucocorticoids in the labyrinth zone. Placenta. 34: 559–566. [DOI] [PubMed] [Google Scholar]