Abstract
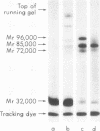
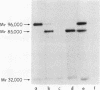
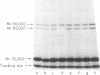
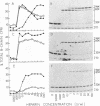
We have demonstrated that human plasma contains a heparin-dependent inhibitor of thrombin that is distinguishable from antithrombin III (AT III). When a 1:50 dilution of plasma was incubated with greater than or equal to 0.01 U/ml heparin and 1 U/ml 125I-thrombin, the labeled thrombin B-chains became incorporated into two complexes of Mr-96,000 and Mr-85,000 that were separated by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and beta-mercaptoethanol. Neither complex was detectable at heparin concentrations less than 0.01 U/ml. When a limiting amount of 125I-thrombin was present, the proportion of radioactivity incorporated into each of the two complexes varied with the heparin concentration. Thus, the Mr-85,000 complex predominated at 0.01-5 U/ml heparin, whereas the Mr-96,000 complex predominated at 5-100 U/ml heparin. The Mr-85,000 complex reacted with antibodies to human AT III and comigrated with the purified thrombin-AT III complex. The Mr-96,000 complex did not react with antibodies to AT III or to alpha 1-antitrypsin, and it was detected in normal quantities after incubating 125I-thrombin with plasma immunodepleted of AT III, alpha 2-antiplasmin, alpha 2-macroglobulin, C1 inactivator, alpha 1-antichymotrypsin, or inter-alpha-trypsin inhibitor. The protein that combines with thrombin to form the Mr-96,000 complex was estimated to be present at a minimum concentration of 90 +/- 26 micrograms/ml (mean +/- SD) in identical to any of the known plasma protease inhibitors and that at relatively high heparin concentrations in vitro it reacts with thrombin more rapidly than does AT III.
Full text
PDF


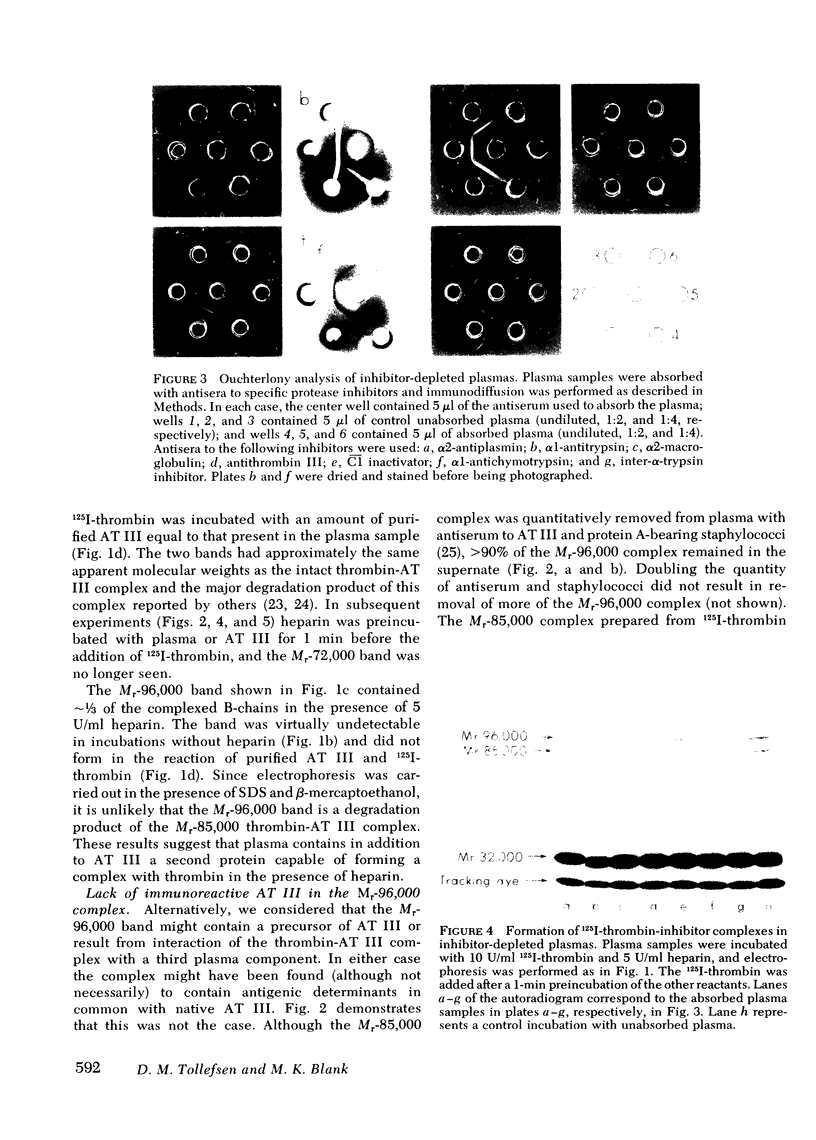
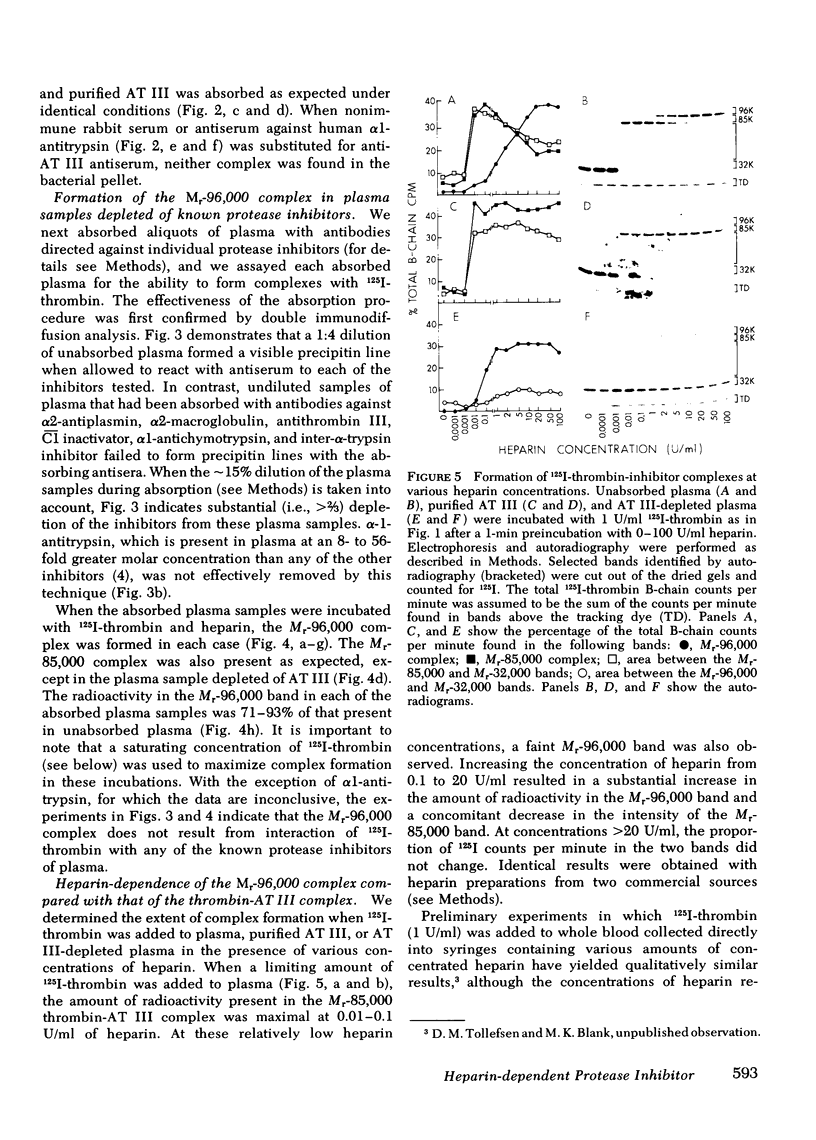



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bagdy D., Barabas E., Gráf L., Petersen T. E., Magnusson S. Hirudin. Methods Enzymol. 1976;45:669–678. doi: 10.1016/s0076-6879(76)45057-7. [DOI] [PubMed] [Google Scholar]
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Beeler D., Rosenberg R., Jordan R. Fractionation of low molecular weight heparin species and their interaction with antithrombin. J Biol Chem. 1979 Apr 25;254(8):2902–2913. [PubMed] [Google Scholar]
- Briginshaw G. F., Shanberge J. N. Identification of two distinct heparin cofactors in human plasma. II. Inhibition of thrombin and activated factor X. Thromb Res. 1974 Mar;4(3):463–477. doi: 10.1016/0049-3848(74)90081-4. [DOI] [PubMed] [Google Scholar]
- Briginshaw G. F., Shanberge J. N. Identification of two distinct heparin cofactors in human plasma. Separation and partial purification. Arch Biochem Biophys. 1974 Apr 2;161(2):683–690. doi: 10.1016/0003-9861(74)90354-3. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Fish W. W., Orre K., Björk I. Routes of thrombin action in the production of proteolytically modified, secondary forms of antithrombin-thrombin complex. Eur J Biochem. 1979 Nov 1;101(1):39–44. doi: 10.1111/j.1432-1033.1979.tb04213.x. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Electrophoretic separation of two thrombin inhibitors in plasma and serum. Scand J Clin Lab Invest. 1969 Aug;24(1):11–14. doi: 10.3109/00365516909080125. [DOI] [PubMed] [Google Scholar]
- Holmer E., Söderström G., Andersson L. O. Properties of antithrombin III depleted plasma. I. Effect of heparin. Thromb Res. 1980 Jan 1;17(1-2):113–124. doi: 10.1016/0049-3848(80)90299-6. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn H., Cerskus A., Ofosu F., Blajchman M., Hirsh J. Preparation and partial characterization of human plasma depleted of antithrombin-III by heparin-sepharose affinity chromatography. Thromb Res. 1980 Oct 1;20(1):77–83. doi: 10.1016/0049-3848(80)90058-4. [DOI] [PubMed] [Google Scholar]
- Jesty J. Dissociation of complexes and their derivatives formed during inhibition of bovine thrombin and activated factor X by antithrombin III. J Biol Chem. 1979 Feb 25;254(4):1044–1049. [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kurachi K., Schmer G., Hermodson M. A., Teller D. C., Davie E. W. Characterization of human, bovine, and horse antithrombin III. Biochemistry. 1976 Jan 27;15(2):368–373. doi: 10.1021/bi00647a020. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lau H. K., Rosenberg R. D. The isolation and characterization of a specific antibody population directed against the thrombin antithrombin complex. J Biol Chem. 1980 Jun 25;255(12):5885–5893. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Marlar R. A., Griffin J. H. Deficiency of protein C inhibitor in combined factor V/VIII deficiency disease. J Clin Invest. 1980 Nov;66(5):1186–1189. doi: 10.1172/JCI109952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Owen W. G. Evidence for the formation of an ester between thrombin and heparin cofactor. Biochim Biophys Acta. 1975 Oct 20;405(2):380–387. doi: 10.1016/0005-2795(75)90103-8. [DOI] [PubMed] [Google Scholar]
- PENSKY J., LEVY L. R., LEPOW I. H. Partial purification of a serum inhibitor of C'1-esterase. J Biol Chem. 1961 Jun;236:1674–1679. [PubMed] [Google Scholar]
- Rosenberg R. D. Biologic actions of heparin. Semin Hematol. 1977 Oct;14(4):427–440. [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Thaler E., Schmer G. A simple two-step isolation procedure for human and bovine antithrombin II/III (heparin cofactor): a comparison of two methods. Br J Haematol. 1975 Oct;31(2):233–243. doi: 10.1111/j.1365-2141.1975.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]