Abstract
The genus Candida includes about 200 different species, but only a few species are human opportunistic pathogens and cause infections when the host becomes debilitated or immunocompromised. Candida infections can be superficial or invasive. Superficial infections often affect the skin or mucous membranes and can be treated successfully with topical antifungal drugs. However, invasive fungal infections are often life-threatening, probably due to inefficient diagnostic methods and inappropriate initial antifungal therapies. Here, we briefly review our current knowledge of pathogenic species of the genus Candida and yeast infection causes and then focus on current antifungal drugs and resistance mechanisms. An overview of new therapeutic alternatives for the treatment of Candida infections is also provided.
1. Introduction
Candida albicans is the most important fungal opportunistic pathogen. It usually resides as a commensal in the gastrointestinal and genitourinary tracts and in the oral and conjunctival flora [1–5]. However, it causes infection when the host becomes debilitated or immunocompromised. These infections can be superficial and affect the skin or mucous membrane [6] or can invade the bloodstream and disseminate to internal organs. Risk factors for invasive candidiasis include surgery (especially abdominal surgery), burns, long-term stay in an intensive care unit, and previous administration of broad-spectrum antibiotics and immunosuppressive agents [7–10]. Advances in medical management as antineoplasic chemotherapy, organ transplantation, hemodialysis, parenteral nutrition, and central venous catheters also contribute to fungal invasion and colonization [11]. Other Candida species found in healthy individuals include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei [12]. All five mentioned species cause more than 90% of invasive infections, although the relative prevalence of the species depends on the geographical location, patient population, and clinical settings [12–14]. Emergence of Candida guilliermondii, Candida kefyr, Candida rugosa, Candida dubliniensis, and Candida famata as pathogens has also been reported worldwide [6, 14]. In fact, the National Nosocomial Infections Surveillance System (NNISS) reports Candida species as the fourth most common nosocomial bloodstream pathogen [15]. Mortality rates are estimated to be as high as 45% [16], probably due to inefficient diagnostic methods and inappropriate initial antifungal therapies [17].
2. Antifungal Drugs in Clinical Treatments
Although the antifungal drugs used in clinical treatments appear to be diverse and numerous, only few classes of antifungal agents are currently available to treat mucosal or systemic infections with Candida spp. (Figure 1) [18–20].
Figure 1.
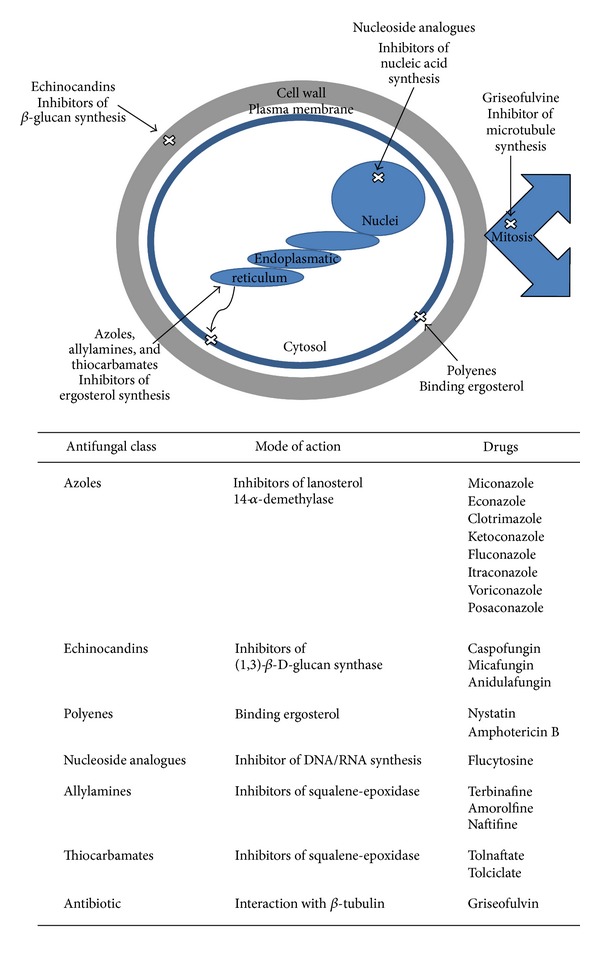
Primary targets and mode of action of several antifungal agents.
2.1. Azoles: Inhibitors of the Lanosterol 14-α-Demethylase
The largest family of antifungal drugs is the azole family. Azoles disrupt the cell membrane by inhibiting the activity of the lanosterol 14-α-demethylase [21], enzyme involved in the biosynthesis of ergosterol (Figure 1). Ergosterol, analogous to cholesterol in animal cells, is the largest sterol component of the fungal cell membrane. Since ergosterol and cholesterol have sufficient structural differences, most antifungal agents targeted to ergosterol binding or biosynthesis does not cross-react with host cells. The azole family includes imidazoles (miconazole, econazole, clotrimazole, and ketoconazole) and triazoles (fluconazole, itraconazole, and the latest agent voriconazole (second-generation, synthetic triazole derivative of fluconazole) and posaconazole (hydroxylated analogue of itraconazole)) [21, 22]. Many azoles are effective both for topical use and for the treatment and prophylaxis of invasive fungal infections [22]. In this regard, these agents have the approval of the US Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) [23].
2.2. Echinocandins: Inhibitors of the Glucan Synthesis
Echinocandins (caspofungin, micafungin, and anidulafungin) are lipopeptidic antifungal agents that inhibit the synthesis of fungal wall by noncompetitive blockage of the (1,3)-β-D-glucan synthase (Figure 1). This enzyme inhibition leads to the formation of fungal cell walls with impaired structural integrity, which finally results in cell vulnerability to osmotic lysis [24]. All three agents (caspofungin, micafungin, and anidulafungin) exhibit concentration-dependent fungicidal activity against most species of Candida [25, 26] and have been approved by the regulatory agency FDA for the treatment of esophageal and invasive candidiasis, including candidemia [27–29].
2.3. Polyenes: Binding Ergosterol
Polyenes such as nystatin and amphotericin B (both isolated from Streptomyces spp.) bind ergosterol and disrupt the major lipidic component of the fungal cell membrane resulting in the production of aqueous pores (Figure 1). Consequently, the cellular permeability is altered and leads to the leakage of cytosolic components and, therefore, fungal death [30].
2.4. Nucleoside Analogues: Inhibitors of DNA/RNA Synthesis
Flucytosine is a pyrimidine analogue. It is transported into fungal cells by cytosine permeases. Then, it is deaminated to 5-fluorouracil and phosphorylated to 5-fluorodeoxyuridine monophosphate. This fluorinated nucleotide inhibits thymidylate synthase and thus interferes with DNA synthesis (Figure 1, [31]). The 5-fluorodeoxyuridine monophosphate can be further phosphorylated and incorporated to RNA, thus affecting RNA and protein synthesis (Figure 1, [32]).
2.5. Other Antifungal Agents
Allylamines and thiocarbamates also disrupt the cell membrane by inhibiting the squalene-epoxidase [33], enzyme involved in the biosynthesis of ergosterol (Figure 1).
Griseofulvin (a tricyclic spirodiketone, first isolated from Penicillium griseofulvum) acts by disrupting spindle and cytoplasmic microtubule production, thereby inhibiting fungal mitosis (Figure 1, [34]).
2.6. Treatment of Systemic Infections
The antifungal therapy is driven by whether the agents are being used to treat mucosal or systemic infections. Superficial infections can be treated successfully with topical antifungal drugs. Systemic infections can be treated with oral or intravenous (IV) preparations. Table 1 shows the pharmacokinetic parameters of the main antifungal agents used for the treatment of systemic candidiasis. Pharmacokinetic parameters are not always directly comparable because data derive from multiple sources and trials [20]. However, the routes of administration and excretion are often important considerations in selecting an appropriate antifungal agent. Some drugs are available only as IV preparations (e.g., caspofungin, micafungin, anidulafungin, and amphotericin B), only as oral preparations (e.g., posaconazole and flucytosine) or can be administered by both IV and oral routes (e.g., fluconazole, itraconazole, and voriconazole) depending on the drug solubility [35]. Since fluconazole and caspofungin are primarily excreted into the urine as active forms (Table 1), they are agents of choice for the treatment of urinary tract fungal infections. Unfortunately, some of these antifungal drugs have been extensively used and led to an increased selective pressure and the development of antifungal resistance [36].
Table 1.
Administration routes and pharmacokinetic parameters of representative antifungal agents belonging to the major families of compounds.
| Drug family | Drug | Adm. routea | Pharmacokinetic parameters | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Oral bioavailability (%) |
C
max
b
μg/mL |
AUCc mg·h/L | Protein binding (%) | Half time (h) | Elimination | ||||
| Azoles | Fluconazole | Oral | >90 | 0.7 | 400.0 | 10–12 | 27–31 | Urine | [35, 37] |
| Itraconazole | Oral | >55 | 1.1 | 29.2 | 99.8 | 21–64 | Hepatic | [35, 37] | |
| Voriconazole | Oral | >90 | 4.6 | 20.3 | 60.0 | 6 | Renal | [35, 37, 38] | |
| Posaconazole | Oral | >98 | 7.8 | 17.0 | 99.0 | 15–35 | Feces | [35, 39] | |
|
| |||||||||
| Echinocandins | Caspofungin | IV | <5 | 9.5–12.1 | 93.5–100.5 | 96.0 | 10.6 | Urine | [20, 35, 40, 41] |
| Micafungin | IV | <5 | 7.1–10.9 | 59.9–111.3 | 99.8 | 11–17 | Feces | [20, 35, 40, 41] | |
| Anidulafungin | IV | <5 | 3.4–7.5 | 44.4–104.5 | 84.0 | 18.1–25.6 | Feces | [20, 35, 40, 41] | |
|
| |||||||||
| Polyenes | Amphotericin B | IV | <5 | 1.5–2.1 | 13–17 | >95 | 6.8–50 | Feces | [35, 42] |
|
| |||||||||
| Nucleoside analogues | Flucytosine | Oral | 76–89 | 80 | 62 | 4 | 3–6 | Renal | [31, 35] |
aAdm. route indicates administration route; fluconazole, itraconazole, and voriconazole can be administered by both intravenous and oral routes; IV: intravenous; b C max: maximal concentration; cAUC: area under the curve.
3. Mechanisms of Resistance against Antifungal Agents
Antifungal resistance is based on different mechanisms, namely, (i) reduced drug intracellular accumulation, (ii) decreased target affinity/processivity for the drug, and (iii) counteraction of the drug effect. Particularly, the mechanism of resistance will be different depending on the mode of action of antifungal compounds. Cellular and molecular mechanisms supporting resistance against antifungal classes mentioned above have been discussed in detail in previous reviews [43–46]. Below, we briefly summarize the main observations (Table 2).
Table 2.
Resistance mechanisms of major systemic antifungal drugs. Antifungal resistance is based on different mechanisms, namely, (i) reduced drug intracellular accumulation, (ii) decreased target affinity/processivity for the drug, and (iii) counteraction of the drug effect.
| Antifungal class | Genetic basis for resistance | Functional basis for resistance |
|---|---|---|
| Azoles | Upregulation of CDR1/CDR2 and MDR1 by point mutations in TAC1 and MRR1 transcription factors | (i) Upregulation of drug transporters |
| Point mutations in ERG11 | (ii) Decreased lanosterol 14-α-demethylase binding affinity for the drug | |
| Upregulation of ERG11 by gene duplication and transcription factor regulation | (iii) Increased concentration of lanosterol 14-α-demethylase | |
| Point mutations in ERG3 | (iii) Inactivation of C5 sterol desaturase leading to alterations in the ergosterol synthetic pathway | |
|
| ||
| Echinocandins | Point mutations in FKS1 and FKS2 | (ii) Decreased glucan synthase processivity for the drug |
|
| ||
| Polyenes | Point mutations in ERG3 and ERG6 | (iii) Decreased ergosterol content in cells |
|
| ||
| Nucleoside analogues | Point mutations in FCY2 | (i) Inactivation of cytosine permease affecting drug uptake |
| Point mutations in FCY1 | (iii) Inactivation of cytosine deaminase leading to alterations in the metabolism of 5-fluorocytosine | |
| Point mutations in FUR1 | (iii) Inactivation of uracil phosphoribosyl transferase leading to alterations in the metabolism of 5-fluorocytosine | |
3.1. Azole Resistance
Over the past 10 years, fluconazole and itraconazole have been used extensively for chemoprophylaxis and treatment of systemic fungal infections because of their favorable oral bioavailability and safety profiles [84–86]. Afterwards, fluconazole resistance has been described in a high percentage of patients [87]. In fact, azole-resistant C. albicans is frequent in HIV-infected patients with oropharyngeal candidiasis [88]. However, resistance is less important in patients with other diseases, such as vaginal candidiasis and candidemia [89]. An intrinsically reduced susceptibility to fluconazole has been also reported for non-albicans species of Candida like C. glabrata, C. krusei, and C. lusitaniae [90, 91]. It appears that variations in the structure of azoles are responsible for the cross-resistance patterns among Candida species [92–94]. Several major mechanisms leading to azole resistance have been elucidated (Table 2, [95]) and detailed below.
(i) Reduced Drug Intracellular Accumulation. A responsible mechanism for decreasing the intracellular concentration of azole relies on an upregulation of two principal families of efflux pumps (reviewed in [96]). These transporters differ in the source of energy used to pump out the drug and in the specificity of the azole molecule. The Cdr pumps belong to the superfamily of ATP-binding cassette (ABC) transporters and are able to extrude all azole antifungals. These pumps are encoded by Candida drug resistance 1 and 2 (CDR1 and CDR2) genes in C. albicans [96]. The other pump is a secondary transporter which utilizes proton gradient as a source of energy and is specific for fluconazole. This pump belongs to the major facilitator superfamily (MFS) transporters and is encoded by the MDR1 gene in C. albicans [96]. Upregulation of CDR1/CDR2 and MDR1 arises from mutations in TAC1 and MRR1 transcription factors, respectively [97, 98]. Gain-of-function mutations generate hyperactive alleles in C. albicans and subsequent loss of heterozigocyty (LOH) at the TAC1 and MRR1 loci [99]. Other transporter genes have been reported to be upregulated in azole-resistant C. glabrata (CgCDR1, CgCDR2 (formerly named PDH1) and CgSNQ2 (another ABC transporter)) [100–102], C. dubliniensis (CdCDR1 and CdCDR2) [103], C. krusei (ABC1 and 2) [104, 105], and C. tropicalis (CDR1-homologue) isolates [44]. In C. glabrata, CgCDR1, CgCDR2, and CgSNQ2 genes are regulated by the CgPDR1 transcription factor [106–108].
(ii) Decreased Target Affinity for the Drug. The target of azole antifungals is the lanosterol 14-α-demethylase encoded by the ERG11 gene. Several point mutations have been characterized and associated to azole minimum inhibitory concentration (MIC) increases (reviewed in [95]).
(iii) Counteraction of the Drug Effect. Two mechanisms contribute to counterbalancing the drug effects. The first system involves an upregulation of the ERG11 gene leading to an intracellular increase of the target protein. ERG11 overexpression occurs by transcription factor regulation and gene duplication (reviewed in [95]). The second mechanism, although very uncommon, has been identified in several clinical isolates of C. albicans [109]. Alteration of the late steps of the biosynthesis of ergosterol through ERG3 inactivation leads to the total inactivation of the C5 sterol desaturase [110]. Thus, toxic 14α-methylated sterols are no longer accumulated, and yeast strains produce cell membranes devoid of ergosterol but containing other sterols [110].
3.2. Echinocandin Resistance
Echinocandin drugs are recommended as the first line for invasive candidiasis. However, reports of echinocandin resistance in patients with infections due to C. albicans, C. glabrata, C. tropicalis, and C. krusei are rising [111–116]. In fact, resistance in C. glabrata increased from 4.9% to 12.3% between 2001 and 2010 [115]. Even more, emergence of coresistance to both echinocandins and azoles in clinical isolates of C. glabrata has been reported [115]. In addition, intrinsic echinocandin resistance of C. parapsilosis, C. orthopsilosis, C. metapsilosis, and C. guilliermondii has been described [117, 118].
Secondary resistance to echinocandins is associated with the following mechanism.
(ii) Decreased Target Processivity for the Drug. Resistance is attributed to point mutations in the FKS1 and/or FKS2 genes (Table 2, [119–121]) which encode the (1,3)-β-D-glucan synthase complex [121]. Mutations in FKS1 did not alter substrate binding but lowered V max values [122].
3.3. Polyene Resistance
Despite more than 30 years of clinical use, minimal resistance to amphotericin B has been developed. However, the main problem associated with the prophylactic use of conventional amphotericin B has always been due to its well-known side effects and toxicity [123, 124]. Resistance tends to be species dependent. C. glabrata and C. krusei are usually considered to be susceptible to amphotericin B, although they show higher MICs to polyenes than C. albicans. In this regard, higher than usual doses of amphotericin B have been recommended by the Infectious Diseases Society of America for treating candidemia caused by C. glabrata and C. krusei [125]. In fact, a significant proportion of isolates of C. glabrata and C. krusei species resistant to amphotericin B has been reported [126]. Additionally, some Candida spp. including C. lusitaniae and C. guilliermondii, besides C. glabrata, are capable of expressing resistance to amphotericin B [127]. It is noteworthy that even the antifungal lipopeptide caspofungin led to drug resistance in transplanted patients [112]. When resistance to polyenes occurs, it may result from the following mechanism.
(iii) Counteraction of the Drug Effect. Acquired resistance is probably due to a decrease or lack of ergosterol content in cell membranes. In fact, membranes of polyene-resistant Candida isolates have relatively low ergosterol content, compared to those of polyene-susceptible isolates. These deficiencies are probably consequences of loss of function mutations in the ERG3 or ERG6 genes which encode some of the enzymes involved in ergosterol biosynthesis (Table 2, [128–130]).
3.4. Flucytosine Resistance
Primary resistance to flucytosine remains low (<2%). Secondary resistance relies on inactivation of different enzymes of the pyrimidine pathway (Table 2) as described below.
(i) Reduced Drug Intracellular Accumulation. Uptake of the drug is affected by point mutations in the FCY2 gene which encodes the cytosine permease [46, 128].
(iii) Counteraction of the Drug Effect. Acquired resistance to flucytosine also results from point mutations in the FCY1 gene which encodes for the cytosine deaminase or FUR1 gene which encodes for the uracil phosphoribosyl transferase. These enzymes catalyze the conversion of 5-fluorocytosine to 5-fluorouracil and 5-fluorouracil to 5-fluorouridine monophosphate, respectively. The most frequently acquired resistance to flucytosine is based on point mutations in the FUR1 gene. Several point mutations have been described in C. albicans, C. glabrata, and C. lusitaniae [46, 128, 131, 132].
The rapid development of antifungal resistance, the toxicity and the variability in available formulations of some agents, and the increase in the frequency of non-albicans Candida spp. infections support the need for more effective and less toxic treatment strategies.
4. Need of New Antifungal Agents
Potential pharmacological strategies include the use of (i) new formulations of antifungals, such as liposomal amphotericin B, amphotericin B lipid complex, amphotericin B colloidal dispersion, amphotericin B into a lipid nanosphere formulation, itraconazole, and β-cyclodextrin itraconazole or (ii) combination therapies of one or more antifungal compounds, for example, amphotericin B + flucytosine, fluconazole + flucytosine, amphotericin B + fluconazole, caspofungin + liposomal amphotericin B, and caspofungin + fluconazole.
Potential alternative therapies include the use of new active principles obtained from different general sources, as natural products, synthetic agents or polymeric materials that have been shown to be active in vitro (Table 3). Among the natural products, plants contain diverse components that are important sources of biologically active molecules [50, 133, 134]. In fact, the activity of plant crude extracts against different microorganisms has been reported, that is, strong antifungal activity of some major components of essential oils [135, 136]. In this regard, the antibiofilm activity of terpenes and the exceptional efficiency of carvacrol, geraniol, and thymol, in the treatment of candidiasis associated with medical devices, have been demonstrated [137]. In another work, terpenoids exhibited excellent activity against C. albicans yeast and hyphal form growth at concentrations that were nontoxic to HeLa cells [138]. Thus, terpenoids may be useful in the near future not only as an antifungal chemotherapeutic agent but also to synergize effects of conventional drugs like fluconazole [138]. Other compounds with antimycological activity obtained from plants are saponins, alkaloids, peptides, and proteins [47, 48]. Marine organisms, endophytic fungi and microorganisms of terrestrial environment are also specific sources of antifungal compounds, although to a lesser extent [50, 139]. Among them, good antimicrobial activities of anthracycline-related compounds, peptides, pyrones, lipopeptides, and terpenoids isolated from these specific sources have been reported [49–51].
Table 3.
Some natural products, synthetic agents, and polymeric materials with reported antifungal activities.
| General source | Specific source | Biological active molecules | Examples | References |
|---|---|---|---|---|
| Natural products | Plants | Essential oils; terpenoids; saponins; phenolic compounds; alkaloids; peptides; proteins | Steroidal saponins, sesquiterpenoids | [47, 48] |
| Marine organisms | Anthracycline-related compounds; lipopeptides; pentacyclic compounds | Xestodecalactone B, seragikinone A | [49] | |
| Endophytic fungi | Secondary metabolites; peptides; pyrones | cryptocandin, pestalopyrone | [50] | |
| Microorganisms of terrestrial environment | Lipopeptides; terpenoids | Echinocandins, enfumafungin | [50, 51] | |
|
| ||||
| Synthetic agents | Organically synthesized or derived compounds (not polymeric materials) |
Compounds based on N,N-dimethylbiguanide complexes | Me (N,N-dimethylbiguanide)2(CH3COO)2·nH2O where Me: Mn, Ni, Cu, and Zn | [52, 53] |
| Derived compounds from traditional antifungal structures | Imidazole derivatives, amine-derived bis-azoles | [54, 55] | ||
| Synthetic derived peptides | Lactoferrin-derived peptides | [56] | ||
| Derived compounds from natural products | Micafungin sodium, anidulafungin, caspofungin acetate, pneumocandin, and enfumafungin derivatives | [57, 58] | ||
|
| ||||
| Polymeric materials | Polymeric materials | Polymers with quaternary nitrogen atoms | Polymers containing aromatic or heterocyclic structures | [59] |
| Cationic conjugated polyelectrolytes | [58] | |||
| Polymers with quaternary nitrogen atoms within the main chain. | [60] | |||
| Block copolymers containing quaternary ammonium salt | [61] | |||
| Synthetic peptides, synthetic dendrimeric peptides | [62, 63] | |||
| Antifungal peptides mimics | Arylamide and phenylene ethynylene backbone polymers | [64] | ||
| Polynorbornene derivatives | [65] | |||
| Polymethacrylate and polymethacrylamide platforms containing hydrophobic and cationic side chains | [66, 67] | |||
| Polymers with superficial activity | Fluorine-containing polymers | [68] | ||
| Polymers containing different contents of halogens | Chlorine-containing phenyl methacrylate polymers | [69, 70] | ||
| Polymeric N-halamines | [71] | |||
| Chelates | Polymer-copper(II)-bipyridyl complex | [72] | ||
| N-vinylimidazole copolymerized with phenacyl methacrylate | [73] | |||
| Imidazole derivative polymers | 2-[(5-methylisoxazol-3-yl)amino]-2-oxo-ethyl methacrylate and ethyl methacrylate | [71] | ||
| Polymers loaded with antifungal compounds | Organic compounds | [74–76] | ||
| Inorganic compounds | [77–79] | |||
A second general source of antifungal agents comprises nonpolymeric synthetic agents, which can be classified into four groups (Table 3). The first group includes chemicals based on N,N-dimethylbiguanide complexes [52]. These compounds displayed low cytotoxicity and could be considered as potential broad-spectrum agents [53]. The second group involves derived compounds of traditional antifungal structures [54, 55] where some of them present better antimicrobial action than the original structures [55, 140]. The third group is formed by synthetic derived peptides, that is, the “human lactoferrin derived peptide” which was well tolerated in preclinical tests and clinical trials [56]. Finally, the last group includes compounds which are derived from semisynthetic natural products, such as compounds derived from echinocandins: micafungin sodium, anidulafungin, caspofungin acetate, and pneumocandin. These agents showed improved properties over the parental compounds [50, 141]. Unfortunately, echinocandins derivatives are poorly absorbed when administered orally and, therefore, are used only for IV administration. A natural antifungal with comparable activity to that of caspofungin acetate against Candida pathogenic fungal strains was isolated [51]. The compound, named enfumafungin, is a new triterpene glycoside that inhibits the (1,3)-β-D-glucan synthase. Several synthetic products derived from enfumafungin are currently under development in order to optimize in vivo antifungal activity and oral efficacy [57].
The third general source of antifungal compounds, namely, polymeric materials could be classified into seven groups (Table 3). (1) Polymers with quaternary nitrogen atoms [60] that can exist in different structures, that is, aromatic or heterocyclic structures [59], cationic conjugated polyelectrolytes [58], quaternary nitrogen atoms within the main chain [60], block copolymers [61], and synthetic and dendrimeric peptides [62, 63]. All of them were shown to be effective against a variety of microorganisms based on the exposure of its quaternary ammonium group. (2) Mimic antimicrobial peptides; among them are arylamide and phenylene ethynylene backbone polymers [64]; polynorbornene derivatives, which depending on their structure may exhibit substantial antimicrobial and low hemolytic activity [65], and polymethacrylate and polymethacrylamide with hydrophobic and cationic side chains [66, 67]. (3) Polymers with antimicrobial activity derived from their superficial activity (surfactants) based on fluorine-containing compounds [68]. (4) Polymers containing different contents of halogens, where the halogen group is the commander of the inhibition process, such as phenyl methacrylate polymers with different contents of chlorine [69, 70]. The halogen may form a covalent bond to nitrogen yielding polymeric N-halamines with a broad-spectrum antimicrobial activity without causing environmental concerns [71]. (5) Chelates; the antimicrobial activity of different chelates, such as N-vinylimidazole copolymerized with phenacyl methacrylate or poly (1,3-thiazol-2-yl-carbamoyl) methyl methacrylate with Cd(II), Cu(II), or Ni (II), has been analyzed in 2011 by Soykan et al. [73]. The Ni(II) complexes showed higher activity than those of Cu(II) and Co(II) ions. However, all of them exhibited lower activity than fluconazole. Another complex containing Cu(II) was found to have good antifungal activity due to electrostatic binding to fungal DNA [72]. (6) Imidazole derivatives, polymers and copolymers, with antimicrobial effectiveness depending on the polymeric structures [71, 142]. (7) Polymers loaded with antimicrobial organic or inorganic compounds. Antimicrobial organic agents are based on organic drugs; that is, chlorhexidine has been incorporated into polymeric microparticles and into polymeric hydrogels to modulate the release of the drug [74, 75]. Another research group loaded triclosan into polymeric nanoparticles [76]. Antimicrobial inorganic agents frequently incorporate metals into polymers, such as silver. This metal exhibits much higher toxicity to microorganisms than to mammalian cells. Polymeric nanotubes [77] and nanofibers [78] with silver nanoparticles have been prepared by chemical oxidation polymerization of rhodanine. Other silver nanocomposites have been reported in the literature based on different silver-loaded nanoparticles such as silver-zirconium phosphate nanoparticles [79] or silver zeolites [142]. Another example of inorganic compound loaded into polymers is copper. Copper particles are also known for their antimicrobial activity, although they are relatively less studied than silver [143].
The mentioned agents have been tried in vitro against Candida; however, many of them are not used in clinical treatments; in this regard, there are three agents with actual promise: E1210, albaconazole, and isavuconazole (Figure 2).
Figure 2.
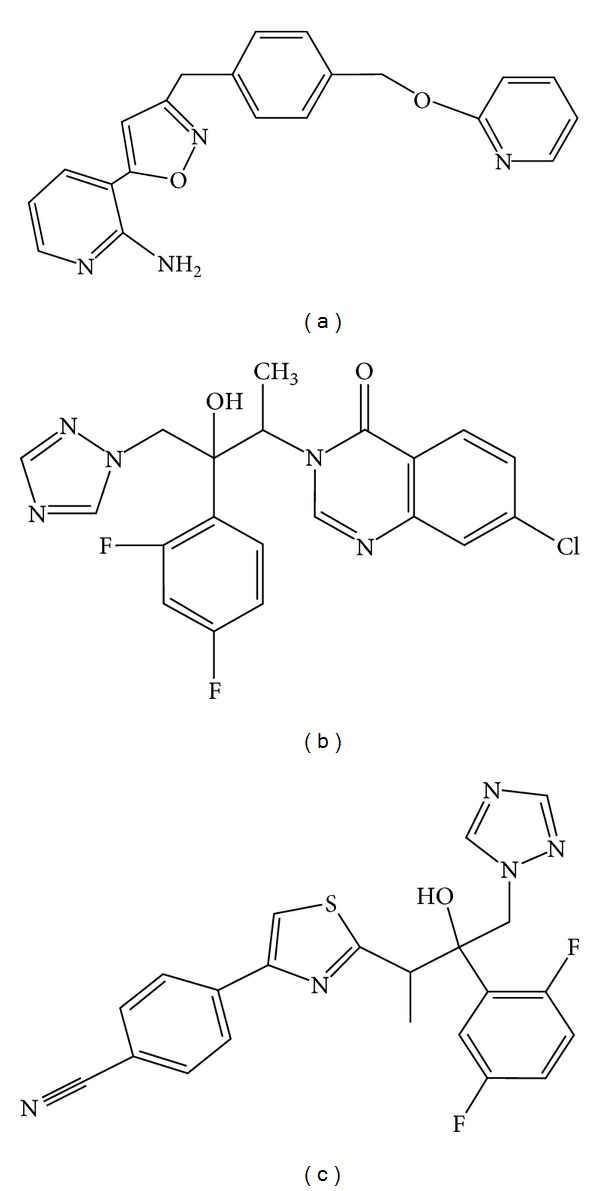
Chemical structures of three agents with actual promise: E1210 (a), albaconazole (b), and isavuconazole (c).
E1210 is a broad-spectrum antifungal agent with a novel mechanism of action based on the inhibition of fungal glycosylphosphatidylinositol biosynthesis [144, 145]. The efficacy of oral E1210 was evaluated in murine models of oropharyngeal and disseminated candidiasis [80].
Results indicate that E1210 significantly reduced the number of viable Candida in the oral cavity in comparison to that of the control treatment and prolonged survival of mice infected with Candida spp. Therapeutic responses were dose dependent [80]. Table 4 shows the major pharmacokinetic parameters after administration of E1210 in mice. E1210 was also highly effective in the treatment of disseminated candidiasis caused by azole-resistant C. albicans or C. tropicalis [80]. Currently, E1210 is in Phase II.
Table 4.
Pharmacokinetic parameters of some lead drugs.
| Drug | Available forms | Experimental organisms | Pharmacokinetic parameters | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Oral bioavailability (%) | C max b (μg/mL) | t max c (h) | Protein binding (%) | Half time (h) | Elimination | ||||
| E1210 | Oral/IVa | Mice | 57.5 | 0.11 | 0.5 | High | 2.2 | nrd | [80] |
| Albaconazole | Oral | Healthy human volunteers | nrd | 5–80 (proportional to dose) |
2–4 | 98 | 30–56 | Feces | [81, 82] |
| Isavuconazonium | Oral | Healthy human volunteers | Very high | 1.03 (100 mg dose) |
0.75–1 | 98 | 56–77 | Feces | [81–83] |
| Isavuconazole | IVa | Healthy human volunteers | nrd | 1.45 (100 mg dose) |
1.3–5 | 98 | 76–104 | Feces | [81–83] |
aIV: intravenous; b C max: maximal concentration; c t max: time to reach maximal plasma concentrations after oral administration; dnr: not reported.
Albaconazole is a new oral triazole with broad-spectrum antifungal activity, unique pharmacokinetics, and excellent tolerability [146]. It has been demonstrated that this compound was highly effective in vitro against pathogenic yeasts and also in animal models of systemic candidiasis [146]. Oral bioavailability was calculated to be 80% in rats and 100% in dogs [81]. Assays in healthy human volunteers showed that albaconazole was rapidly absorbed and presented good pharmacokinetic parameters (Table 4). In fact, the therapeutic efficacy of a single dose of albaconazole at ≥40 mg was more effective than 150 mg of fluconazole for the treatment of acute vulvovaginal candidiasis [81]. Currently, albaconazole is in Phase II. In addition, low toxicity was observed when albaconazole was administered to animals and human volunteers [82].
Finally, isavuconazole (the active metabolite of the water-soluble prodrug isavuconazonium) is a novel second-generation water-soluble triazole with broad-spectrum antifungal activity, also against azole-resistant strains. Studies carried out with neutropenic mice of disseminated C. tropicalis or C. krusei infections showed that the treatment significantly reduced kidney burden in mice infected with C. tropicalis and both kidney and brain burden in mice infected with C. krusei [147]. This azole is currently under Phase III trials in patients with systemic candidiasis. Both oral and intravenous formulations showed favorable pharmacokinetic (Table 4) and pharmacodynamic profiles [82]. This drug has the potential to become an important agent for the treatment of invasive fungal infections, principally because of its relatively broad and potent in vitro antifungal activity, its favorable pharmacokinetic profile, and the absence of severe adverse effects [82, 148, 149].
5. Conclusions
Although the antifungal drugs used in clinical treatments appear to be diverse and numerous, only few classes of antifungal agents are currently available in oral and intravenous forms. Additionally antifungal resistance based on different mechanisms continues to grow and evolve and exacerbate the need of new treatments against Candida infections. In this regard, new formulations of antifungals, combination therapies and development of new bioactive compounds might be useful for a better therapeutic outcome. Particularly, there are three compounds in Phase II or III studies with actual promise for the treatment of invasive candidiasis.
Acknowledgments
This paper was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) to Claudia Spampinato (PICT 0458) and Darío Leonardi (PICT 2643), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) to Claudia Spampinato (PIP 0018), and Universidad Nacional de Rosario (UNR) to Claudia Spampinato (BIO 221) and Darío Leonardi (BIO 328). Both authors are members of the Researcher Career of CONICET.
References
- 1.Jackson BE, Wilhelmus KR, Mitchell BM. Genetically regulated filamentation contributes to Candida albicans virulence during corneal infection. Microbial Pathogenesis. 2007;42(2-3):88–93. doi: 10.1016/j.micpath.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu TG, Mitchell BM, Carothers TS, et al. Molecular analysis of the pediatric ocular surface for fungi. Current Eye Research. 2003;26(1):33–36. doi: 10.1076/ceyr.26.1.33.14253. [DOI] [PubMed] [Google Scholar]
- 3.Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clinical Microbiology Reviews. 2010;23(2):253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbach A, Dignard D, Pierce JV, Whiteway M, Kumamoto CA. Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryotic Cell. 2010;9(7):1075–1086. doi: 10.1128/EC.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naglik JR, Moyes DL, Wächtler B, Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes and Infection. 2011;13(12-13):963–976. doi: 10.1016/j.micinf.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Martínez R. Candidosis, a new challenge. Clinics in Dermatology. 2010;28:178–184. doi: 10.1016/j.clindermatol.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Kontoyiannis DP, Mantadakis E, Samonis G. Systemic mycoses in the immunocompromised host: an update in antifungal therapy. Journal of Hospital Infection. 2003;53(4):243–258. doi: 10.1053/jhin.2002.1278. [DOI] [PubMed] [Google Scholar]
- 8.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clinical Infectious Diseases. 2005;41(9):1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 9.Sydnor ERM, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clinical Microbiology Reviews. 2011;24(1):141–173. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus . Journal of Clinical Microbiology. 2004;42(10):4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikulska M, del Bono V, Ratto S, Viscoli C. Occurrence, presentation and treatment of candidemia. Expert Review of Clinical Immunology. 2012;8:755–765. doi: 10.1586/eci.12.52. [DOI] [PubMed] [Google Scholar]
- 12.MacCallum DM. Hosting infection: experimental models to assay Candida virulence. International Journal of Microbiology. 2012;2012:12 pages. doi: 10.1155/2012/363764.363764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clinical Microbiology Reviews. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miceli MH, Díaz JA, Lee SA. Emerging opportunistic yeast infections. The Lancet Infectious Diseases. 2011;11(2):142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 15.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical Infectious Diseases. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 16.Cheng M-F, Yang Y-L, Yao T-J, et al. Risk factors for fatal candidemia caused by Candida albicans and non-albicans Candida species . BMC Infectious Diseases. 2005;5, article 22 doi: 10.1186/1471-2334-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrobial Agents and Chemotherapy. 2005;49(9):3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew BP, Nath M. Recent approaches to antifungal therapy for invasive mycoses. ChemMedChem. 2009;4(3):310–323. doi: 10.1002/cmdc.200800353. [DOI] [PubMed] [Google Scholar]
- 19.Kathiravan MK, Salake AB, Chothe AS, et al. The biology and chemistry of antifungal agents: a review. Bioorganic & Medicinal Chemistry. 2012;20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 20.Denning DW, Hope WW. Therapy for fungal diseases: opportunities and priorities. Trends in Microbiology. 2010;18(5):195–204. doi: 10.1016/j.tim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Hof H. A new, broad-spectrum azole antifungal: posaconazole—mechanisms of action and resistance, spectrum of activity. Mycoses. 2006;49(1):2–6. doi: 10.1111/j.1439-0507.2006.01295.x. [DOI] [PubMed] [Google Scholar]
- 22.Hay R. Antifungal drugs. In: Katsambas A, Lotti T, editors. European Handbook of Dermatological Treatments. Berlin, Germany: Springer; 2003. pp. 700–710. [Google Scholar]
- 23.Aparicio JF, Mendes MV, Antón N, Recio E, Martín JF. Polyene macrolide antiobiotic biosynthesis. Current Medicinal Chemistry. 2004;11(12):1645–1656. doi: 10.2174/0929867043365044. [DOI] [PubMed] [Google Scholar]
- 24.Grover N. Echinocandins: a ray of hope in antifungal drug therapy. Indian Journal of Pharmacology. 2010;42(1):9–11. doi: 10.4103/0253-7613.62396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappelletty D, Eiselstein-McKitrick K. The echinocandins. Pharmacotherapy. 2007;27(3):369–388. doi: 10.1592/phco.27.3.369. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez JA. Anidulafungin: a new echinocandin with a novel profile. Clinical Therapeutics. 2005;27(6):657–673. doi: 10.1016/j.clinthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Ostrosky-Zeichner L, Kontoyiannis D, Raffalli J, et al. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. European Journal of Clinical Microbiology and Infectious Diseases. 2005;24(10):654–661. doi: 10.1007/s10096-005-0024-8. [DOI] [PubMed] [Google Scholar]
- 28.de Wet N, Llanos-Cuentas A, Suleiman J, et al. A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clinical Infectious Diseases. 2004;39(6):842–849. doi: 10.1086/423377. [DOI] [PubMed] [Google Scholar]
- 29.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. The New England Journal of Medicine. 2002;347(25):2020–2029. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 30.Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. The Lancet Infectious Diseases. 2002;2(2):73–85. doi: 10.1016/s1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 31.Vermes A, Guchelaar H-J, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. Journal of Antimicrobial Chemotherapy. 2000;46(2):171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 32.Onishi J, Meinz M, Thompson J, et al. Discovery of novel antifungal (1,3)-β-D-glucan synthase inhibitors. Antimicrobial Agents and Chemotherapy. 2000;44(2):368–377. doi: 10.1128/aac.44.2.368-377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanglard D, Coste A, Ferrari S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Research. 2009;9(7):1029–1050. doi: 10.1111/j.1567-1364.2009.00578.x. [DOI] [PubMed] [Google Scholar]
- 34.François IEJA, Aerts AM, Cammue BPA, Thevissen K. Currently used antimycotics: spectrum, mode of action and resistance occurrence. Current Drug Targets. 2005;6(8):895–907. doi: 10.2174/138945005774912744. [DOI] [PubMed] [Google Scholar]
- 35.Ashley ESD, Lewis R, Lewis JS, Martin C, Andes D. Pharmacology of systemic antifungal agents. Clinical Infectious Diseases. 2006;43(1):S28–S39. [Google Scholar]
- 36.Espinel-Ingroff A. Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005–2009) Revista Iberoamericana de Micologia. 2009;26(1):15–22. doi: 10.1016/S1130-1406(09)70004-X. [DOI] [PubMed] [Google Scholar]
- 37.Lipp H-P. Clinical pharmacodynamics and pharmacokinetics of the antifungal extended-spectrum triazole posaconazole: an overview. British Journal of Clinical Pharmacology. 2010;70(4):471–480. doi: 10.1111/j.1365-2125.2010.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clinical Pharmacokinetics. 2006;45(7):649–663. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Theuretzbacher U, Clancy CJ, Nguyen MH, Derendorf H. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clinical Pharmacokinetics. 2010;49(6):379–396. doi: 10.2165/11319340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Theuretzbacher U. Pharmacokinetics/pharmacodynamics of echinocandins. European Journal of Clinical Microbiology and Infectious Diseases. 2004;23(11):805–812. doi: 10.1007/s10096-004-1228-z. [DOI] [PubMed] [Google Scholar]
- 41.Wagner C, Graninger W, Presterl E, Joukhadar C. The echinocandins: comparison of their pharmacokinetics, pharmacodynamics and clinical applications. Pharmacology. 2006;78(4):161–177. doi: 10.1159/000096348. [DOI] [PubMed] [Google Scholar]
- 42.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrobial Agents and Chemotherapy. 2002;46(3):828–833. doi: 10.1128/AAC.46.3.828-833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanafani ZA, Perfect JR. Resistance to antifungal agents: mechanisms and clinical impact. Clinical Infectious Diseases. 2008;46(1):120–128. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 44.Vandeputte P, Ferrari S, Coste AT. Antifungal resistance and new strategies to control fungal infections. International Journal of Microbiology. 2012;2012:26 pages. doi: 10.1155/2012/713687.713687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perlin DS. Antifungal drug resistance: do molecular methods provide a way forward? Current Opinion in Infectious Diseases. 2009;22(6):568–573. doi: 10.1097/QCO.0b013e3283321ce5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pemán J, Cantón E, Espinel-Ingroff A. Antifungal drug resistance mechanisms. Expert Review of Anti-Infective Therapy. 2009;7(4):453–460. doi: 10.1586/eri.09.18. [DOI] [PubMed] [Google Scholar]
- 47.Abad MJ, Ansuategui M, Bermejo P. Active antifungal substances from natural sources. Arkivoc. 2007;2007(7):116–145. [Google Scholar]
- 48.Li Y-Y, Hu Z-Y, Lu C-H, Shen Y-M. Four new terpenoids from Xylaria sp. 101. Helvetica Chimica Acta. 2010;93(4):796–802. [Google Scholar]
- 49.Bhadury P, Mohammad BT, Wright PC. The current status of natural products from marine fungi and their potential as anti-infective agents. Journal of Industrial Microbiology and Biotechnology. 2006;33(5):325–337. doi: 10.1007/s10295-005-0070-3. [DOI] [PubMed] [Google Scholar]
- 50.Sortino M, Derita M, Svetaz L, et al. 6. The role of natural products in discovery of new anti-infective agents with emphasis on antifungal compounds. In: Filho VC, editor. Plant Bioactives and Drug Discovery: Principles, Practice, and Perspectives. 2012. pp. 205–239. [Google Scholar]
- 51.Peláez F, Cabello A, Platas G, et al. The discovery of enfumafungin, a novel antifungal compound produced by an endophytic Hormonema species biological activity and taxonomy of the producing organisms. Systematic and Applied Microbiology. 2000;23(3):333–343. doi: 10.1016/s0723-2020(00)80062-4. [DOI] [PubMed] [Google Scholar]
- 52.Olar R, Badea M, Marinescu D, et al. Prospects for new antimicrobials based on N,N-dimethylbiguanide complexes as effective agents on both planktonic and adhered microbial strains. European Journal of Medicinal Chemistry. 2010;45(7):2868–2875. doi: 10.1016/j.ejmech.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Olar R, Badea M, Marinescu D, et al. N, N-dimethylbiguanide complexes displaying low cytotoxicity as potential large spectrum antimicrobial agents. European Journal of Medicinal Chemistry. 2010;45(7):3027–3034. doi: 10.1016/j.ejmech.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 54.Anderson EB, Long TE. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer. 2010;51(12):2447–2454. [Google Scholar]
- 55.Fang B, Zhou C-H, Rao X-C. Synthesis and biological activities of novel amine-derived bis-azoles as potential antibacterial and antifungal agents. European Journal of Medicinal Chemistry. 2010;45(9):4388–4398. doi: 10.1016/j.ejmech.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Brouwer CPJM, Rahman M, Welling MM. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides. 2011;32(9):1953–1963. doi: 10.1016/j.peptides.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Heasley BH, Pacofsky GJ, Mamai A, et al. Synthesis and biological evaluation of antifungal derivatives of enfumafungin as orally bioavailable inhibitors of β-1, 3-glucan synthase. Bioorganic & Medicinal Chemistry Letters. 2012;22:6811–6816. doi: 10.1016/j.bmcl.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 58.Melo LD, Mamizuka EM, Carmona-Ribeiro AM. Antimicrobial particles from cationic lipid and polyelectrolytes. Langmuir. 2010;26(14):12300–12306. doi: 10.1021/la101500s. [DOI] [PubMed] [Google Scholar]
- 59.Timofeeva LM, Kleshcheva NA, Moroz AF, Didenko LV. Secondary and tertiary polydiallylammonium salts: novel polymers with high antimicrobial activity. Biomacromolecules. 2009;10(11):2976–2986. doi: 10.1021/bm900435v. [DOI] [PubMed] [Google Scholar]
- 60.Cakmak I, Ulukanli Z, Tuzcu M, Karabuga S, Genctav K. Synthesis and characterization of novel antimicrobial cationic polyelectrolytes. European Polymer Journal. 2004;40(10):2373–2379. [Google Scholar]
- 61.Sauvet G, Fortuniak W, Kazmierski K, Chojnowski J. Amphiphilic block and statistical siloxane copolymers with antimicrobial activity. Journal of Polymer Science A. 2003;41(19):2939–2948. [Google Scholar]
- 62.Zhu J, Luther PW, Leng Q, Mixson AJ. Synthetic histidine-rich peptides inhibit Candida species and other fungi in vitro: role of endocytosis and treatment implications. Antimicrobial Agents and Chemotherapy. 2006;50(8):2797–2805. doi: 10.1128/AAC.00411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tam JP, Lu Y-A, Yang J-L. Antimicrobial dendrimeric peptides. European Journal of Biochemistry. 2002;269(3):923–932. doi: 10.1046/j.0014-2956.2001.02728.x. [DOI] [PubMed] [Google Scholar]
- 64.Tew GN, Clements D, Tang H, Arnt L, Scott RW. Antimicrobial activity of an abiotic host defense peptide mimic. Biochimica et Biophysica Acta. 2006;1758(9):1387–1392. doi: 10.1016/j.bbamem.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Som A, Choi Y, Tew GN. Monovalent salt effects on the membrane activity of antimicrobial polymers. Macromolecular Symposia. 2009;283-284(1):319–325. [Google Scholar]
- 66.Palermo EF, Kuroda K. Chemical structure of cationic groups in amphiphilic polymethacrylates modulates the antimicrobial and hemolytic activities. Biomacromolecules. 2009;10(6):1416–1428. doi: 10.1021/bm900044x. [DOI] [PubMed] [Google Scholar]
- 67.Palermo EF, Sovadinova I, Kuroda K. Structural determinants of antimicrobial activity and biocompatibility in membrane-disrupting methacrylamide random copolymers. Biomacromolecules. 2009;10(11):3098–3107. doi: 10.1021/bm900784x. [DOI] [PubMed] [Google Scholar]
- 68.Caillier L, Taffin de Givenchy E, Levy R, Vandenberghe Y, Geribaldi S, Guittard F. Polymerizable semi-fluorinated gemini surfactants designed for antimicrobial materials. Journal of Colloid and Interface Science. 2009;332(1):201–207. doi: 10.1016/j.jcis.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 69.Patel MB, Patel SA, Ray A, Patel RM. Synthesis, characterization, and antimicrobial activity of acrylic copolymers. Journal of Applied Polymer Science. 2003;89(4):895–900. [Google Scholar]
- 70.Patel JN, Dolia MB, Patel KH, Patel RM. Homopolymer of 4-chloro-3-methyl phenyl methacrylate and its copolymers with butyl methacrylate: synthesis, characterization, reactivity ratios and antimicrobial activity. Journal of Polymer Research. 2006;13(3):219–228. [Google Scholar]
- 71.Muñoz-Bonilla A, Fernández-García M. Polymeric materials with antimicrobial activity. Progress in Polymer Science. 2012;37:281–339. [Google Scholar]
- 72.Senthil Kumar R, Sasikala K, Arunachalam S. DNA interaction of some polymer-copper(II) complexes containing 2,2′-bipyridyl ligand and their antimicrobial activities. Journal of Inorganic Biochemistry. 2008;102(2):234–241. doi: 10.1016/j.jinorgbio.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Soykan C, Coskun R, Kirbag S. Poly(crotonic acid-co-2-acrylamido-2-methyl-1-propanesulfonic acid)-metal complexes with copper(II), cobalt(II), and nickel(II): synthesis, characterization and antimicrobial activity. European Polymer Journal. 2007;43(9):4028–4036. [Google Scholar]
- 74.Yue IC, Poff J, Cortés ME, et al. A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease. Biomaterials. 2004;25(17):3743–3750. doi: 10.1016/j.biomaterials.2003.09.113. [DOI] [PubMed] [Google Scholar]
- 75.Kiremitçi AS, Çiftçi A, Özalp M, Gümüşderelioğlu M. Novel chlorhexidine releasing system developed from thermosensitive vinyl ether-based hydrogels. Journal of Biomedical Materials Research B. 2007;83:609–614. doi: 10.1002/jbm.b.30834. [DOI] [PubMed] [Google Scholar]
- 76.Zhang H, Wang D, Butler R, et al. Formation and enhanced biocidal activity of water-dispersable organic nanoparticles. Nature Nanotechnology. 2008;3(8):506–511. doi: 10.1038/nnano.2008.188. [DOI] [PubMed] [Google Scholar]
- 77.Kong H, Song J, Jang J. One-step preparation of antimicrobial polyrhodanine nanotubes with silver nanoparticles. Macromolecular Rapid Communications. 2009;30(15):1350–1355. doi: 10.1002/marc.200900106. [DOI] [PubMed] [Google Scholar]
- 78.Kong H, Jang J. Synthesis and antimicrobial properties of novel silver/polyrhodanine nanofibers. Biomacromolecules. 2008;9(10):2677–2681. doi: 10.1021/bm800574x. [DOI] [PubMed] [Google Scholar]
- 79.Duan Y-Y, Jia J, Wang S-H, Yan W, Jin L, Wang Z-Y. Preparation of antimicrobial poly(e-caprolactone) electrospun nanofibers containing silver-loaded zirconium phosphate nanoparticles. Journal of Applied Polymer Science. 2007;106(2):1208–1214. [Google Scholar]
- 80.Hata K, Horii T, Miyazaki M, et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrobial Agents and Chemotherapy. 2011;55(10):4543–4551. doi: 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasqualotto AC, Denning DW. New and emerging treatments for fungal infections. The Journal of Antimicrobial Chemotherapy. 2008;61:i19–i30. doi: 10.1093/jac/dkm428. [DOI] [PubMed] [Google Scholar]
- 82.Girmenia C. New generation azole antifungals in clinical investigation. Expert Opinion on Investigational Drugs. 2009;18(9):1279–1295. doi: 10.1517/13543780903176407. [DOI] [PubMed] [Google Scholar]
- 83.Schmitt-Hoffmann A, Roos B, Heep M, et al. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrobial Agents and Chemotherapy. 2006;50(1):279–285. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meis JFGM, Verweij PE. Current management of fungal infections. Drugs. 2001;61(1):13–25. doi: 10.2165/00003495-200161001-00002. [DOI] [PubMed] [Google Scholar]
- 85.Hoffman HL, Ernst EJ, Klepser ME. Novel triazole antifungal agents. Expert Opinion on Investigational Drugs. 2000;9(3):593–605. doi: 10.1517/13543784.9.3.593. [DOI] [PubMed] [Google Scholar]
- 86.Livermore DM. The need for new antibiotics. Clinical Microbiology and Infection. 2004;10(4):1–9. doi: 10.1111/j.1465-0691.2004.1004.x. [DOI] [PubMed] [Google Scholar]
- 87.Redding SW, Kirkpatrick WR, Saville S, et al. Multiple patterns of resistance to fluconazole in Candida glabrata isolates from a patient with oropharyngeal candidiasis receiving head and neck radiation. Journal of Clinical Microbiology. 2003;41(2):619–622. doi: 10.1128/JCM.41.2.619-622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skiest DJ, Vazquez JA, Anstead GM, et al. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clinical Infectious Diseases. 2007;44(4):607–614. doi: 10.1086/511039. [DOI] [PubMed] [Google Scholar]
- 89.Ribeiro M, Paula CR, Perfect JR, Cox GM. Phenotypic and genotypic evaluation of fluconazole resistance in vaginal Candida strains isolated from HIV-infected women from Brazil. Medical Mycology. 2005;43(7):647–650. doi: 10.1080/13693780500093838. [DOI] [PubMed] [Google Scholar]
- 90.Vazquez JA, Peng G, Sabel JO, et al. Evolution of antifungal susceptibility among Candida species isolates recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis. Clinical Infectious Diseases. 2001;33(7):1069–1075. doi: 10.1086/322641. [DOI] [PubMed] [Google Scholar]
- 91.Safdar A, van Rhee F, Henslee-Downey JP, Singhal S, Mehta J. Candida glabrata and Candida krusei fungemia after high-risk allogeneic marrow transplantation: no adverse effect of low-dose fluconazole prophylaxis on incidence and outcome. Bone Marrow Transplantation. 2001;28(9):873–878. doi: 10.1038/sj.bmt.1703252. [DOI] [PubMed] [Google Scholar]
- 92.Cuenca-Estrella M, Gomez-Lopez A, Mellado E, Buitrago MJ, Monzon A, Rodriguez-Tudela JL. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrobial Agents and Chemotherapy. 2006;50(3):917–921. doi: 10.1128/AAC.50.3.917-921.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pfaller MA, Messer SA, Boyken L, et al. Use of fluconazole as a surrogate marker to predict susceptibility and resistance to voriconazole among 13,338 clinical isolates of Candida spp. tested by clinical and laboratory standards institute-recommended broth microdilution methods. Journal of Clinical Microbiology. 2007;45(1):70–75. doi: 10.1128/JCM.01551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pfaller MA, Messer SA, Boyken L, et al. Cross-resistance between fluconazole and ravuconazole and the use of fluconazole as a surrogate marker to predict susceptibility and resistance to ravuconazole among 12,796 clinical isolates of Candida spp. Journal of Clinical Microbiology. 2004;42(7):3137–3141. doi: 10.1128/JCM.42.7.3137-3141.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noël T. The cellular and molecular defense mechanisms of the Candida yeasts against azole antifungal drugs. Journal de Mycologie Médicale. 2012;22:173–178. doi: 10.1016/j.mycmed.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Cannon RD, Lamping E, Holmes AR, et al. Efflux-mediated antifungal drug resistance. Clinical Microbiology Reviews. 2009;22(2):291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coste AT, Karababa M, Ischer F, Bille J, Sanglard D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2 . Eukaryotic Cell. 2004;3(6):1639–1652. doi: 10.1128/EC.3.6.1639-1652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coste A, Turner V, Ischer F, et al. A mutation in TAC1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans . Genetics. 2006;172(4):2139–2156. doi: 10.1534/genetics.105.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coste AT, Crittin J, Bauser C, Rohde B, Sanglard D. Functional analysis of cis-and trans-acting elements of the Candida albicans CDR2promoter with a novel promoter reporter system. Eukaryotic Cell. 2009;8(8):1250–1267. doi: 10.1128/EC.00069-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Torelli R, Posteraro B, Ferrari S, et al. The ATP-binding cassette transporter-encoding gene CgSNQ2 is contributing to the CgPDR1-dependent azole resistance of Candida glabrata . Molecular Microbiology. 2008;68(1):186–201. doi: 10.1111/j.1365-2958.2008.06143.x. [DOI] [PubMed] [Google Scholar]
- 101.Sanglard D, Ischer F, Calabrese D, Majcherczyk PA, Bille J. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrobial Agents and Chemotherapy. 1999;43(11):2753–2765. doi: 10.1128/aac.43.11.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bennett JE, Izumikawa K, Marr KA. Mechanism of Increased Fluconazole Resistance in Candida glabrata during Prophylaxis. Antimicrobial Agents and Chemotherapy. 2004;48(5):1773–1777. doi: 10.1128/AAC.48.5.1773-1777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moran GP, Sanglard D, Donnelly SM, Shanley DB, Sullivan DJ, Coleman DC. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis . Antimicrobial Agents and Chemotherapy. 1998;42(7):1819–1830. doi: 10.1128/aac.42.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lamping E, Ranchod A, Nakamura K, et al. Abc1p is a multidrug efflux transporter that tips the balance in favor of innate azole resistance in Candida krusei . Antimicrobial Agents and Chemotherapy. 2009;53(2):354–369. doi: 10.1128/AAC.01095-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Katiyar SK, Edlind TD. Identification and expression of multidrug resistance-related ABC transporter genes in Candida krusei . Medical Mycology. 2001;39(1):109–116. doi: 10.1080/mmy.39.1.109.116. [DOI] [PubMed] [Google Scholar]
- 106.Vermitsky J-P, Edlind TD. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrobial Agents and Chemotherapy. 2004;48(10):3773–3781. doi: 10.1128/AAC.48.10.3773-3781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vermitsky J-P, Earhart KD, Smith WL, Homayouni R, Edlind TD, Rogers PD. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Molecular Microbiology. 2006;61(3):704–722. doi: 10.1111/j.1365-2958.2006.05235.x. [DOI] [PubMed] [Google Scholar]
- 108.Tsai H-F, Krol AA, Sarti KE, Bennett JE. Candida glabrata PDR1 , a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrobial Agents and Chemotherapy. 2006;50(4):1384–1392. doi: 10.1128/AAC.50.4.1384-1392.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martel CM, Parker JE, Bader O, et al. Identification and characterization of four azole-resistant erg3 mutants of Candida albicans . Antimicrobial Agents and Chemotherapy. 2010;54(11):4527–4533. doi: 10.1128/AAC.00348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miyazaki Y, Geber A, Miyazaki H, et al. Cloning, sequencing, expression and allelic sequence diversity of ERG3 (C-5 sterol desaturase gene) in Candida albicans . Gene. 1999;236(1):43–51. doi: 10.1016/s0378-1119(99)00263-2. [DOI] [PubMed] [Google Scholar]
- 111.Hernandez S, López-Ribot JL, Najvar LK, McCarthy DI, Bocanegra R, Graybill JR. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive candida esophagitis. Antimicrobial Agents and Chemotherapy. 2004;48(4):1382–1383. doi: 10.1128/AAC.48.4.1382-1383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clinical Infectious Diseases. 2006;42(7):938–944. doi: 10.1086/500939. [DOI] [PubMed] [Google Scholar]
- 113.Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrobial Agents and Chemotherapy. 2006;50(7):2522–2524. doi: 10.1128/AAC.00148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pasquale T, Tomada JR, Ghannoun M, Dipersio J, Bonilla H. Emergence of Candida tropicalis resistant to caspofungin. Journal of Antimicrobial Chemotherapy. 2008;61(1):p. 219. doi: 10.1093/jac/dkm453. [DOI] [PubMed] [Google Scholar]
- 115.Alexander B, Johnson M, Pfeiffer C, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clinical Infectious Diseases. 2013;56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata . Journal of Clinical Microbiology. 2012;50(4):1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrobial Agents and Chemotherapy. 2008;52(7):2305–2312. doi: 10.1128/AAC.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cantón E, Pemán J, Sastre M, Romero M, Espinel-Ingroff A. Killing kinetics of caspofungin, micafungin, and amphotericin B against Candida guilliermondii . Antimicrobial Agents and Chemotherapy. 2006;50(8):2829–2832. doi: 10.1128/AAC.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kahn JN, Garcia-Effron G, Hsu M-J, Park S, Marr KA, Perlin DS. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrobial Agents and Chemotherapy. 2007;51(5):1876–1878. doi: 10.1128/AAC.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrobial Agents and Chemotherapy. 2005;49(8):3264–3273. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balashov SV, Park S, Perlin DS. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1 . Antimicrobial Agents and Chemotherapy. 2006;50(6):2058–2063. doi: 10.1128/AAC.01653-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrobial Agents and Chemotherapy. 2009;53(1):112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Revista Iberoamericana de Micologia. 2009;26(4):223–227. doi: 10.1016/j.riam.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 124.Ellis D. Amphotericin B: spectrum and resistance. Journal of Antimicrobial Chemotherapy. 2002;49(supplement 1):7–10. doi: 10.1093/jac/49.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 125.Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Clinical Infectious Diseases. 2000;30(4):662–678. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 126.Kontoyiannis DP, Lewis RE. Antifungal drug resistance of pathogenic fungi. The Lancet. 2002;359(9312):1135–1144. doi: 10.1016/S0140-6736(02)08162-X. [DOI] [PubMed] [Google Scholar]
- 127.Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of Candidiasis. Clinical Infectious Diseases. 2004;38(2):161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 128.Espinel-Ingroff A. Mechanisms of resistance to antifungal agents: yeasts and filamentous fungi. Revista Iberoamericana de Micologia. 2008;25(2):101–106. doi: 10.1016/s1130-1406(08)70027-5. [DOI] [PubMed] [Google Scholar]
- 129.Vandeputte P, Tronchin G, Bergès T, Hennequin C, Chabasse D, Bouchara J-P. Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrobial Agents and Chemotherapy. 2007;51(3):982–990. doi: 10.1128/AAC.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kelly SL, Lamb DC, Kelly DE, et al. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6-desaturation. FEBS Letters. 1997;400(1):80–82. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 131.Chapeland-Leclerc F, Bouchoux J, Goumar A, Chastin C, Villard J, Noël T. Inactivation of the FCY2 gene encoding purine-cytosine permease promotes cross-resistance to flucytosine and fluconazole in Candida lusitaniae . Antimicrobial Agents and Chemotherapy. 2005;49(8):3101–3108. doi: 10.1128/AAC.49.8.3101-3108.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vandeputte P, Pineau L, Larcher G, et al. Molecular mechanisms of resistance to 5-fluorocytosine in laboratory mutants of Candida glabrata . Mycopathologia. 2011;171(1):11–21. doi: 10.1007/s11046-010-9342-1. [DOI] [PubMed] [Google Scholar]
- 133.Duarte MCT, Figueira GM, Sartoratto A, Rehder VLG, Delarmelina C. Anti-Candida activity of Brazilian medicinal plants. Journal of Ethnopharmacology. 2005;97(2):305–311. doi: 10.1016/j.jep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 134.Butler MS. The role of natural product chemistry in drug discovery. Journal of Natural Products. 2004;67(12):2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 135.Mondello F, de Bernardis F, Girolamo A, Cassone A, Salvatore G. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species . BMC Infectious Diseases. 2006;6, article 158 doi: 10.1186/1471-2334-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Manohar V, Ingram C, Gray J, et al. Antifungal activities of origanum oil against Candida albicans . Molecular and Cellular Biochemistry. 2001;228(1-2):111–117. doi: 10.1023/a:1013311632207. [DOI] [PubMed] [Google Scholar]
- 137.Dalleau S, Cateau E, Bergès T, Berjeaud J-M, Imbert C. In vitro activity of terpenes against Candida biofilms. International Journal of Antimicrobial Agents. 2008;31(6):572–576. doi: 10.1016/j.ijantimicag.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 138.Zore GB, Thakre AD, Jadhav S, Karuppayil SM. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine. 2011;18(13):1181–1190. doi: 10.1016/j.phymed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 139.Gunatilaka AAL. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. Journal of Natural Products. 2006;69(3):509–526. doi: 10.1021/np058128n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aher NG, Pore VS, Mishra NN, et al. Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorganic and Medicinal Chemistry Letters. 2009;19(3):759–763. doi: 10.1016/j.bmcl.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 141.Kofla G, Ruhnke M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis—review of the literature. European Journal of Medical Research. 2011;16(4):159–166. doi: 10.1186/2047-783X-16-4-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fernández A, Soriano E, Hernández-Muñoz P, Gavara R. Migration of antimicrobial silver from composites of polylactide with silver zeolites. Journal of Food Science. 2010;75(3):E186–E193. doi: 10.1111/j.1750-3841.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 143.Ozay O, Akcali A, Otkun MT, Silan C, Aktas N, Sahiner N. P(4-VP) based nanoparticles and composites with dual action as antimicrobial materials. Colloids and Surfaces B. 2010;79(2):460–466. doi: 10.1016/j.colsurfb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 144.Watanabe N-A, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrobial Agents and Chemotherapy. 2012;56(2):960–971. doi: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miyazaki M, Horii T, Hata K, et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrobial Agents and Chemotherapy. 2011;55(10):4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bartroli J, Merlos M. Overview of albaconazole. European Infectious Disease. 2011;5(2):88–91. [Google Scholar]
- 147.Majithiya J, Sharp A, Parmar A, Denning DW, Warn PA. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei . Journal of Antimicrobial Chemotherapy. 2009;63(1):161–166. doi: 10.1093/jac/dkn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Odds FC. Drug evaluation: BAL-8557—a novel broad-spectrum triazole antifungal. Current Opinion in Investigational Drugs. 2006;7(8):766–772. [PubMed] [Google Scholar]
- 149.Livermore J, Hope W. Evaluation of the pharmacokinetics and clinical utility of isavuconazole for treatment of invasive fungal infections. Expert Opinion on Drug Metabolism & Toxicology. 2012;8:759–765. doi: 10.1517/17425255.2012.683859. [DOI] [PubMed] [Google Scholar]


