Abstract
Background
The use of allogenic juvenile chondrocytes or autologous chondral fragments has shown promising laboratory results for the repair of chondral lesions.
Hypothesis/Purpose
The purpose of the study was to evaluate in vitro the extracellular matrix production of mixed adult/juvenile cultures of both chondrocytes (part 1) and minced cartilage fragments (part 2). The authors hypothesized that juvenile chondrocytes would not affect matrix production when mixed with adult chondrocytes or cartilage fragments.
Study design
Controlled laboratory study.
Methods
Cartilage sources consisted of three adult and three juvenile (human) donors. In part 1, per each donor, juvenile chondrocytes were mixed with adult chondrocytes in five different proportions: 100, 50, 25, 12.5 and 0 %. Three-dimensional cultures in low melt agarose were performed. At 6 weeks, biochemical and histological analyses were performed. In part 2, isolated adult, isolated juvenile, and mixed three-dimensional cultures (1:1) were performed with chondral fragments (<1mm), both with low melt agarose and a hyaluronic acid scaffold. At 2 and 6 weeks, cultures were evaluated with biochemical and histological analyses.
Results
Part 1: biochemical and histological analyses showed that isolated juvenile cultures performed significantly better than mixed and isolated adult cultures. No significant differences were noted between mixed cultures (1:1) and isolated adult cultures. Part 2: biochemical and histological results at 6 weeks showed that mixed cartilage fragment cultures performed better than isolated adult cultures in terms of PG/DNA ratio (p=0.014), percentage of safranin-O positive cells (p=0.012), Bern score (p=0.001), and Collagen type II. No statistical difference was noted between juvenile and mixed cultures.
Conclusion
Extracellular matrix production of juvenile chondrocytes is inhibited by adult chondrocytes. The addition of juvenile cartilage fragments to adult fragments improves matrix production, with a positive interaction between the two sources.
Clinical relevance
Even if the underlying mechanisms are still unknown, this study describes the behavior of juvenile/adult co-cultures using both chondrocytes and cartilage fragments, with potential for new research and clinical applications.
Keywords: juvenile, adult, chondrocytes, cartilage fragments, co-culture, cartilage repair
Introduction
Articular cartilage matrix undergoes substantial structural, molecular, and mechanical changes with age and arthritis. These include: surface fibrillation, alteration of proteoglycan structure and composition, increased collagen cross-linking, and decreased tensile strength and stiffness.5,6,10,21 Deterioration of chondrocyte function accompanies these changes in the matrix. The cells synthesize smaller aggrecan molecules and less functional link proteins, leading to the formation of smaller, more irregular proteoglycan aggregates.7-9,33 The mitotic and synthetic activity of human chondrocytes declines with age.20,24 Furthermore, human chondrocytes become less responsive to anabolic mechanical stimuli (i.e. insulin-like growth factor I).22,23 In part for these reasons, articular injuries in adults are more likely to promote osteoarthritis (OA) than in skeletally immature juveniles, suggesting an age- or maturation-related decline in the potential for cartilage repair.16,17,30,34 Juvenile chondrocytes have shown superior capabilities of producing cartilage extracellular matrix.1,2,12 For these reasons, an ex vivo pre-culture of autologous chondrocytes, combined with juvenile allogenic chondrocytes, could represent an interesting method to increase mature matrix production and cells expansion in classical “two stage” cartilage repair procedures, i.e. autologous chondrocyte implantation (ACI) and membrane-ACI (MACI).
Recently, cartilage fragments have been proposed as a valid source of cells in animal models (goat and horse), allowing to cover large cartilage defects, without requiring pre-cultivation of the cells.13,18,19 With this technique, manual mechanical fragmentation has shown to induce matrix breakdown and release of cells from cartilage fragments.19 Regenerating a functional repair tissue similar to hyaline cartilage, through implantation of chondral fragments held by scaffolds, would represent a fascinating one-stage cartilage repair alternative.27,32 Adding allogenic juvenile cartilage fragments to autologous fragments would theoretically increase the chondrogenic potential of the procedure and allow larger defects coverage.
In this in vitro study, adult/juvenile co-cultures were performed using both chondrocytes (part 1) and minced cartilage fragments (part 2). The co-cultures were compared to juvenile or adult monocultures.
The starting hypothesis was that chondrocytes of juvenile origin would not affect matrix production when mixed with adult chondrocytes or minced cartilage. The aim of part 1 of this study was to validate in vitro the combined use of both autologous and juvenile allogenic chondrocytes, when performing most common two-stage cartilage repair procedures. The aim of part 2 was to prove in vitro the concept of a new “one-stage” surgical procedure for cartilage repair, directly combining “in situ” autologous and allogenic juvenile cartilage fragments, as a viable source of cells.
Material and methods
Cartilage sources
Adult cartilage was harvested from intra-operative pieces of three different patients, following informed consent for use in medical research: a) 62 year old female (WE), affected by severe knee arthritis undergoing total knee replacement; b) 69 year old female (DD), affected by initial knee arthritis and undergoing unicompartmental knee replacement; c) 18 year old male (GC), affected by hip osteochondral defect undergoing hip arthroscopy and debridement. The intra-operative pieces were shipped en bloc on wet ice in physiologically buffered solution and stored for no more than 6 hours at 4°C before processing. The cartilage was harvested in a sterile fashion with a scalpel (n°13 blade), and manually minced in order to obtain fragments <1 mm3. Both macroscopically healthy and degenerated articular cartilage were harvested from WE and DD. On the other hand, the large chondral flap obtained from GC did not show any areas of degeneration.
The juvenile cartilage, provided by ISTO technologies company (St. Louis, MO), was harvested from the distal femur and proximal tibia of three different donors (<6 years old) and minced in <1 mm3 fragments. The cartilage from the three different juvenile sources was mixed together in equal proportions, in order to limit the variability related to the single donors and obtain only one pool of juvenile cartilage.
Part 1
Culture preparation
Chondrocytes from both adult and juvenile cartilage were isolated by enzymatic digestion according to the methods previously described.1 Briefly, the cartilage was washed, and transferred to 50 ml sterile conical tubes (4 g tissue per tube) for 30-minute digestion in protease from Streptomyces griseus (2 mg/ml, Sigma, St Louis, MO), followed by overnight incubation in HL-1™ containing 2000 units CLS2 collagenase (Worthington, Lakewood, NJ) and 5 mg Type VIII hyaluronidase (Sigma, St Louis, MO) at 37°C with mechanical agitation. The next morning, cell suspensions were diluted with 10 ml of fresh media, vortexed gently, and tissue debris was removed by gravity filtration through 70-μm Falcon cell strainer units (Beckton Dickenson, Franklin Lakes, NJ). Cells were pelleted at 2000 rpm for 8 minutes and counted to determine viability (>98%). Cells were then suspended in low melt agarose at 1 × 107 cells/ml concentration, in order to obtain three-dimensional cultures. Per each donor, juvenile cells were mixed with adult ones in five different proportions: 100, 50, 25, 12.5 and 0 % of adult chondrocytes combined with, respectively, 0, 50, 75, 87.5, 100% of juvenile chondrocytes. The cultures were maintained in chondrogenic medium and humidified incubator (37°C, 5% CO2) for 6 weeks. Chondrogenic medium was composed by basic medium supplemented with 10% fetal bovine serum, with further addition of growth factors: 1 ng/ml of transforming growth factor β1 (TGF β1), 5 ng/ml of fibroblast growth factor 2 (FGF-2), and 10 ng/ml of platelet-derived growth factor type BB (PDGF-BB). Medium exchange was performed twice a week.
Biochemical analysis
At 6 weeks, half of each sample underwent biochemical analysis and the other half was cryo-embedded and cut at different depths with the microtome for histological evaluation. Biochemically, a s-GAG assay (DMMB) for proteoglycans content (PGcon) in the neo-tissue was conducted, according to the methods described by Hoemann et al.15 A VMax® microplate reader (Molecular Devices, Sunnyvale, CA) spectrophotometer was used. An assay for the lactate released in the culture medium was used as a marker of metabolic activity in juvenile, adult and 1:1 mixed cultures.26 The assay was performed using Sigma (St. Louis, MO) Lactate Reagent. In addition, an assay for the PG released (PGrel) in the culture medium was performed in juvenile, adult and 1:1 mixed cultures. The PGrel/PGcon ratio was calculated in juvenile, adult and 1:1 mixed cultures, as an indicator of the amount of PG lost in the culture medium.
Histological analysis
Histological evaluation consisted of safranin-O fast green and immunofluorescence (Link protein and Collagen type II) staining. Titration of safranin-O positive cell (% of the total) was performed for each sample at 10× microscope magnification, at different depth cuts and in multiple fields per slide. The Bern scoring system (minimum score 0, maximum 9) for safranin-O stained in vitro-generated neo-cartilage was used.14 Histological evaluation was performed in a blinded fashion by one investigator expert in cartilage histology.
Part 2
Culture preparation
All cartilage fragments were manually minced in order to obtain pieces smaller than 1 mm. The cartilage of every adult donor was cultured alone and with juvenile fragments. Juvenile cartilage as well was cultured alone. The use of fragments instead of cells did not allow a precise cells titration and every co-culture was composed by 0.5 mg of adult cartilage fragments and 0.5 mg of juvenile cartilage fragments. Each culture was made both with agarose and with a Hyaluronic-Acid Scaffold (Hyaff 11, Fidia Advanced Biopolymers, Italy). In the groups with the agarose, the fragments were mixed in the wells and then the agarose was poured on top. In the groups with the scaffold, this was put on the bottom of the wells and the fragments were laid on top of it. The cultures were maintained in chondrogenic medium and humidified incubator (37°C, 5% CO2) for 6 weeks. Chondrogenic medium was the same described in part 1. Medium exchange occurred twice a week.
Confocal microscopic evaluation
At 4 weeks a Confocal (BioRad MRC 1024 confocal system, Hercules, CA, with Nikon Eclipse E600 upright microscope, Melville, NY) microscopic examination was conducted without impairing the cultures, to confirm the matrix breakdown and release of cells from the cartilage fragments.
Biochemical analysis
At 2 and 6 weeks, half of each sample underwent biochemical analysis and the other half was cryo-embedded and cut at different depths with the microtome for histological evaluation. Biochemically, a s-GAG assay (DMMB) for proteoglycans (PG) was conducted according to the methods described by Hoemann et al.15 The value obtained was normalized dividing it for the DNA amount (DNA assay). 15 A VMax® microplate reader (Molecular Devices, Sunnyvale, CA) spectrophotometer was used. The PG/DNA ratio was considered the best approximation of the real amount of matrix production per cell. The increment (n times) of the PG/DNA ratio from week 2 to week 6, was calculated by dividing the value at 6 weeks by the value obtained at 2 weeks.
Histological analysis
At 6 weeks, histological evaluation consisted of safranin-O fast green and immunofluorescence (Collagen type II) staining. Titration of safranin-O positive cells was performed for each sample, at 10× microscope magnification, at different depth cuts and in multiple fields per slide. The Bern score was calculated for each sample (minimum score 0, maximum 9). 14 Histological evaluation was performed in a blinded fashion by one investigator expert in cartilage histology.
Statistical analysis
One way analysis of variance (ANOVA) with post hoc Bonferroni's correction was used to determine significant differences between the groups. Differences were considered significant at p < 0.05; a 95% confidence interval was used. The analysis was conducted with a SPSS for Windows 13 (SPSS Inc., Chicago, IL).
Results
Part 1
Biochemical analysis
The lactate and the PG released (PGrel) into the culture medium were significantly higher in isolated juvenile cultures compared to isolated adult and 1:1 mixed cultures (p<0.001) (Figure 1). Sulfated-GAG assays showed that PG content (PGcon) was more than 7-fold greater in juvenile than in adult isolated cell cultures (Figure 2). In addition, PGcon was significantly higher (p<0.001) in isolated juvenile cultures than in all the mixed cultures (Table 1). When increasing the percentage of adult cells, the PGcon declined logarithmically, rather than linearly, as first hypothesized (Figure 2).
Figure 1.
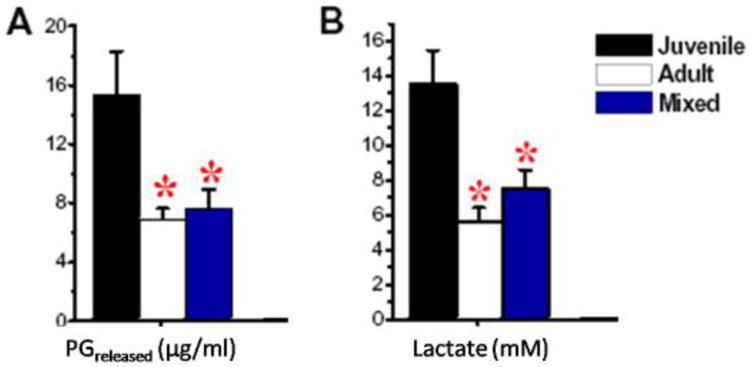
Lactate and PG released in the culture medium at 6 weeks. Results are shown for pure cultures of juvenile (black) or adult cells (white), and for 1:1 mixed cultures (blue). A) Significantly less PGrel was released in adult and mixed cultures than in juvenile ones. B) Lactate was significantly lower in medium from adult and mixed cultures than from juvenile ones.
Figure 2.
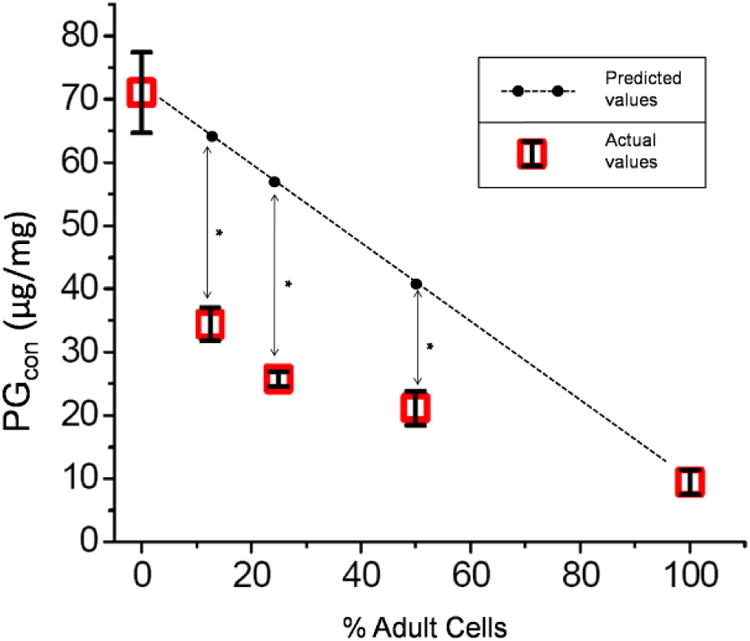
PG content (PGcon) at 6 weeks. Cultures consisted of varying proportion of cells from 100% juvenile to 100% adult. PGcon was highest in juvenile cultures and, when increasing the proportion of adult cells, declined logarithmically, rather than linearly.
Table 1.
Summary of the results for Part 1 of the study.
| Composition | Proteoglican content μg/mg | Titration Saf -O positive cells | Bernscore (from 0 to 9) | ||||
|---|---|---|---|---|---|---|---|
| Adult | Juvenile | Mean | SD | Mean | SD | Mean | SD |
| 1 | 0 | 9.39 | 1.95 | 4% | 2.24 | 2 | 0 |
| 1/2 | 1/2 | 21.13 | 2.66 | 8% | 2.79 | 2.67 | 0.52 |
| 1/4 | 3/4 | 25.74 | 1.18 | 48.80% | 6.49 | 5 | 0.63 |
| 1/8 | 7/8 | 34.41 | 2.65 | 59.70% | 6.21 | 5.67 | 0.52 |
| 0 | 1 | 71.03 | 6.39 | 85.40% | 1.26 | 6.83 | 0.4 |
The PGrel/PGcon ratios for mixed cultures (0.18) and adult cultures (0.38) were relatively high compared to juvenile cultures (0.10), indicating that in mixed cultures much of PG produced was lost to the medium and the matrix destabilized.
Histological analysis
Histology showed that juvenile cells produced more proteoglycans, link protein and type II collagen than their adult counterparts (Figure 3). The percentage of safranin-O positive cells in the isolated juvenile cultures was significantly higher compared to isolated adult and mixed cultures (p<0.001), supporting the findings encountered with the PGcon assay (Table 1 and Figure 4).,.
Figure 3.
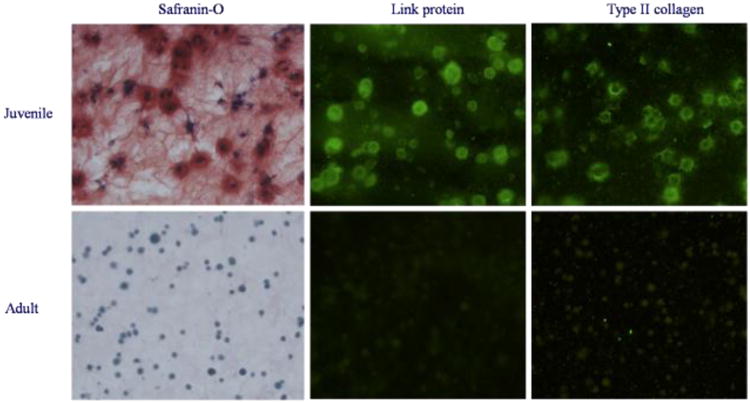
Juvenile and adult chondrocytes cultured separately were compared after 6 weeks of incubation. Shown are typical safranin-O stains for PG and immunofluorescence stains for link protein, collagen type II. Vigorous cartilage matrix production was evident in the culture of juvenile cells, but not in the culture of adult cells.
Figure 4.
Safranin-O stained sections of juvenile cells alone, adult cells alone and mixed juvenile/adult cells (1:1).
The Bern score was significantly higher (p<0.001) in the isolated juvenile cultures compared to isolated adult and mixed cultures (Table 1).
Part 2
Biochemical analysis
The PG content, DNA content and PG/DNA ratio at 2 and 6 weeks have been summarized in Table 2. At 2 weeks, the PG/DNA ratio was significantly higher in juvenile monocultures, than in adult monocultures (p=0.02). No other significant differences were noted between the other groups (i.e. co-cultures vs adult monocultures, and agarose vs Hyaff)
Table 2.
Part 2 of the study; summary of the results (PG content, DNA content, PG/DNA ratio, and titration of safranin-O positive cells) for each sample at 2 and 6 weeks.
| 2 weeks | 6 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | PG μg Mean (SD) | DNA ng Mean (SD) | PG/DNA ratio Mean (SD) | PG μg Mean (SD) | DNA ng Mean (SD) | PG/DNA ratio Mean (SD) | Increment (n times) of PG/DNA ratio (6ww/2ww) | % of Saf-O positive cells Mean (SD) | Bern score (range 0-9) Mean (SD) |
| DD + A | 4.97 (0.22) | 106.74 (0.95) | 0.04 (0.0001) | 345.47 (56.08) | 66.9 (0.74) | 5.13 (0.22) | 128.25 | 22.69 (2.98) | 4.67 (0.52) |
| GC + A | 6.84 (0.6) | 35.24 (0.30) | 0.17 (0.0012) | 611.48 (71.75) | 98.81 (0.79) | 6.23 (0.59) | 36.65 | 46.33 (2.87) | 5.17 (0.41) |
| WE + A | 7.5 (0.57) | 57.38 (0.19) | 0.14 (0.0008) | 483.41 (90.32) | 68.15 (3.08) | 7.05 (0.47) | 50.36 | 48.24 (12.49) | 5.5 (0.55) |
| DD + H | 4.72 (0.16) | 88.36 (0.57) | 0.05 (0.0001) | 141.77 (45.08) | 61.27 (2.19) | 2.35 (0.23) | 47 | 6.92 (4.38) | 4.33 (0.52) |
| GC + H | 6.09 (0.35) | 72.93 (1.31) | 0.08 (0.0002) | 165.72 (9.07) | 80.07 (1.51) | 2.02 (0.19) | 25.25 | 6.48 (4.44) | 4.5 (0.54) |
| WE + H | 9.78 (1.71) | 90.42 (0.46) | 0.09 (0.0002) | 260.87 (46.28) | 66.62 (2.11) | 3.96 (0.44) | 44 | 9.78 (3.64) | 4.67 (0.52) |
| DD + J + A | 15.44 (1.01) | 95.42 (0.37) | 0.16 (0.0011) | 895.01 (190.75) | 86.88 (0.41) | 10.34 (0.66) | 64.63 | 50.12 (9.12) | 6.67 (0.52) |
| GC + J + A | 11.86 (0.9) | 62.79 (0.23) | 0.18 (0.0001) | 1047.42 (244.93) | 84.39 (0.50) | 12.33 (1.11) | 68.5 | 51.48 (10.1) | 6.83 (0.41) |
| WE + J + A | 10.68 (1.21) | 82.92 (1.81) | 0.13 (0.0002) | 1728.44 (139.72) | 66.72 (0.93) | 25.83 (0.89) | 198.69 | 92.57 (11.19) | 8.67 (0.51) |
| DD + J + H | 17.32 (1.57) | 162.78 (0.69) | 0.1 (0.0021) | 611.94 (176.1) | 66.72 (0.65) | 9.02 (0.35) | 90.2 | 49.38 (7.65) | 6.5 (0.55) |
| GC + J + H | 17.58 (1.47) | 99.48 (0.63) | 0.18 (0.0019) | 662.47 (163.97) | 68.9 (0.94) | 9.66 (0.18) | 53.67 | 50.93 (7.46) | 6.67 (0.52) |
| WE + J + H | 5.48 (0.49) | 147.44 (0.74) | 0.04 (0.0008) | 989.36 (269.53) | 61.61 (0.53) | 16.01 (0.90) | 400.25 | 90.65 (8.31) | 8.5 (0.55) |
| J + A | 14.57 (1.01) | 66.9 (0.25) | 0.21 (0.0038) | 712.58 (118.43) | 66.81 (0.72) | 10.73 (0.55) | 51.1 | 51.89 (4.83) | 4.67 (0.52) |
| J + H | 22.82 (1.8) | 125.78 (0.84) | 0.18 (0.0017) | 641.62 (170.38) | 71.59 (0.72) | 9.03 (0.62) | 50.17 | 50.97 (8.56) | 6.5 (0.55) |
WE, DD, GC=adult cartilage sources (see text); J=juvenile cartilage fragments; A=agarose; H=Hyaluronic Acid scaffold.
At 6 weeks, co-cultures performed significantly better than isolated adult cultures in terms of PG/DNA ratio (p=0.01).. The PG/DNA ratio was higher in co-cultures than in isolated juvenile cultures, even if not significantly. These data seem to support the theory of a reciprocal stimulation between adult and juvenile chondral fragments. Surprisingly, the cartilage harvested from the donor with most severe knee arthritis (WE), in the co-cultures, showed higher PG/DNA ratio compared to the other co-cultures, even if not significantly. No significant differences were noted between agarose and Hyaluronic Acid scaffold.
In terms of increment of PG/DNA ratio from week 2 to week 6, no significant differences were observed between the different groups.
Confocal microscopic evaluation
At 4 weeks Confocal evaluation showed the migration of chondrocytes outside the fragments and the creation of bridges between the cartilage fragments in every culture (Figure 5).
Figure 5.

At 4 weeks Confocal microscopic evaluation showed migration of chondrocytes outside the fragments and creation of bridges between the cartilage fragments in every culture, even though more evident in isolated juvenile and mixed cultures.
Histological analysis
At 2 weeks, no neo-cartilage was present outside of the chondral fragments and the histological analysis was not conducted.
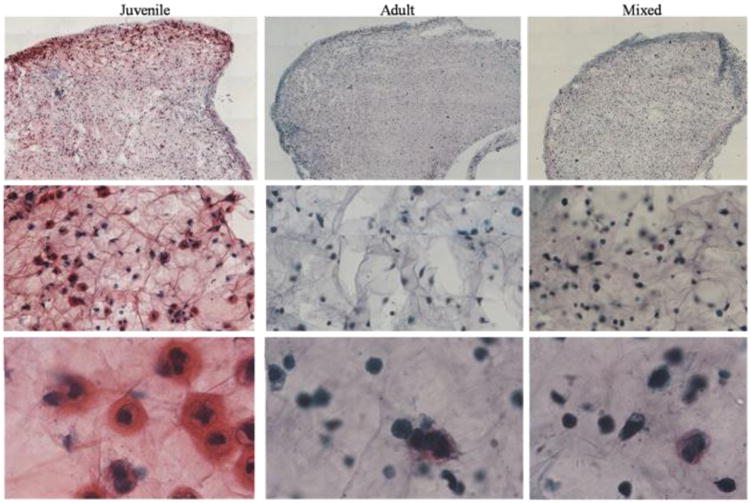
The results of the safranin-O positive cells titration and the Bern scoring at 6 weeks are summarized in Table 2. Co-cultures showed significantly higher, safranin-O positive cells (p=0.01), Bern score (p=0.001) and production of Collagen type II, compared to isolated adult cultures (Figure 6,7). In addition, co-cultures performed better than isolated juvenile cultures, in terms of safranin-O positive cells titration, Bern score and production of Collagen type II, even if not significantly (Figure 6,7). Surprisingly, the cartilage harvested from the adult donor with more severe knee arthritis (WE), in the co-cultures, showed better properties compared to the other co-cultures, in terms of safranin-O positive cells, Bern score and collagen type II production, even if not significantly. No other significant differences were noted between the groups.
Figure 6.
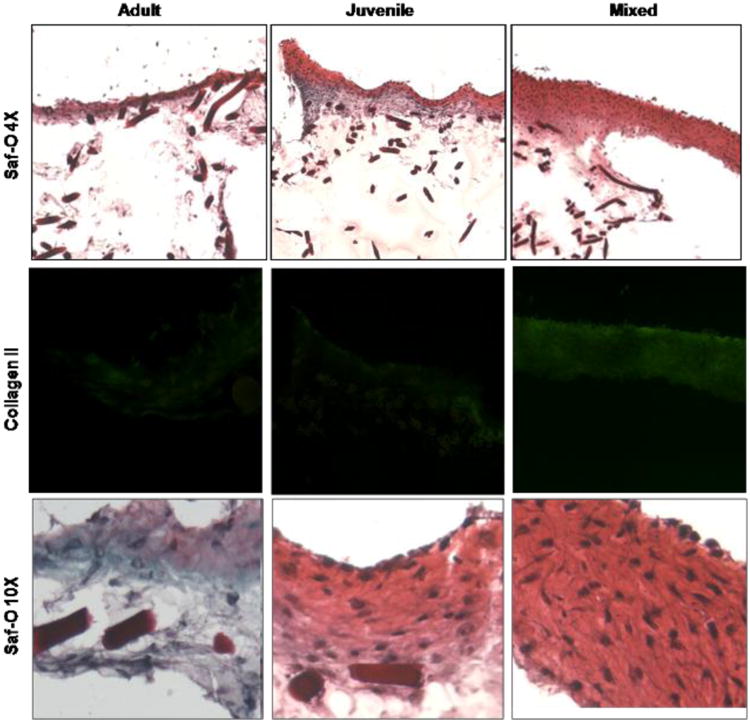
Histology of juvenile, adult and mixed samples cultured with Hyaff (dark bars). Safranin-O (Saf-O) positive cells and collagen type II immuno-staining are higher in juvenile and mixed cultures than in adult alone. The mixed culture expresses higher cellularity and more intense immunostaining compared to both adult and juvenile cultures. 4×, 10×=microscope magnification.
Figure 7.

Histology of juvenile, adult (WE is the source) and mixed (WE is the source of adult cartilage) samples cultured with agarose. Safranin-O (Saf-O) positive cells and collagen type II immunostaining are higher in juvenile and mixed cultures than in adult alone. The co-culture expresses higher cellularity and more intense immunostaining compared to both adult and juvenile cultures. 4×, 10×=microscope magnification.
Discussion
In part 1 of the present study, we tested the null hypothesis that there would have been no interaction between juvenile and adult chondrocytes in terms of chondrogenic activity in agarose cultures. Instead, we found that PG content, safranin-O positive cells, and lactate production declined non-linearly with increasing proportions of adult cells, indicating a negative interaction. This was not due simply to dilution effects, as varying the density of juvenile chondrocytes in agarose without adult cells lead to a linear decline in matrix accumulation (data not shown). The increased release of PG to the medium observed in co-cultures suggests enzymatic degradation of aggrecan, or failure to entrap newly synthesized aggrecan in the matrix. On the other hand, the decrease in lactate production in co-cultures compared with juvenile cultures is an indication that glycolytic activity was suppressed. Thus, the data suggest that increased catabolic activity as well as decreased anabolic activity contributed to the failure of matrix production in chondrocyte co-cultures.
In part 2 of the study we tested again the null hypothesis that no interaction would have occurred between juvenile and adult cartilage fragments with respect to chondrogenic activity in co-cultures. Instead, a positive interaction between juvenile and adult chondral fragments was found, with a stimulus to extracellular matrix production.
The chondrogenic potential of juvenile chondrocytes has been previously described1,2, as well as the use of autologous chondral fragments to repair cartilage defects.3,13,18,19
Adkisson et al. showed that juvenile chondrocytes, when maintained in static culture under defined serum-free conditions, deposited an extracellular matrix that accumulated in the form of tissue disks.1 Electron microscopic evaluation of neocartilage disks revealed collagenous matrices characteristic of articular cartilage from human infants.
In another controlled laboratory study, Adkisson et al. showed that juvenile human chondrocytes had greater potential to restore articular cartilage than adult cells and that allogeneic juvenile chondrocytes did not stimulate an immunologic response in vivo.2
In addition, unlike adult autologous or allogeneic osteochondral grafts, juvenile grafts showed complete integration of cartilage at the graft/host junction in adult animals.31 Comparable findings have been reported in animals with implantation of cell-based constructs prepared using juvenile articular chondrocytes.4,28,29
Albrecht et al. showed rapid chondrocyte proliferation, hyaline-like repair tissue, and alcian blue-positive matrix in osteochondral defects treated with autologous cartilage fragments in a rabbit model.3
Lu et al. demonstrated hyaline-like repair tissue in the treatment with cartilage fragments of critical-sized chondral defects in large weight-bearing animals (eight skeletally mature goats).19
Frisbie et al. showed on a horse model similar arthroscopic, histological, and immunohistochemistry results for ACI and autologous cartilage fragments implantation, in the treatment of chondral defects.13
Lind & Larsen investigated the cartilage repair response of autologous cartilage fragments or chondrocytes in combination with a collagen membrane in a goat full thickness cartilage defect model. No histological or biomechanical differences were found between the two groups.18
To our knowledge, there are no studies in the English literature describing the effects of mixing juvenile and adult chondrocytes or cartilage fragments in co-cultures. The in vitro findings of Part 1 of the study showed that autologous chondrocytes, combined with juvenile cells, is not a favorable strategy because of the inhibitory effects of adult cells. On the other hand, the second part of the present study showed the stimulation of matrix production in fragments co-cultures. In every fragment co-culture, the value of PG/DNA ratio and safranin-O positive cells was significantly superior to monocultures of adult cartilage.
The basis for the positive interaction between juvenile and adult chondral fragments is unknown. However, the increased numbers of migrating chondrocytes seen in co-cultures suggests this might involve increased chemotactic activity. Chopping cartilage is likely to provoke an injury response that includes secretion of chemokines and other factors.11 We speculate that the same injury affects juvenile and adult cartilage differently, with adult cartilage releasing more chemokines. On the other hand, juvenile chondrocytes are more capable of responding to chemotactic factors than adult chondrocytes.25 Thus, in the presence of injured adult cartilage, large numbers of juvenile cells migrate out of the original minced tissue, where they proliferate and differentiate to form neocartilage. Less neocartilage is produced in monocultures of juvenile fragments because of the relative absence of chemotactic factors contributed by adult tissue, whereas monocultures of adult cartilage produce less neocartilage because of the absence of juvenile cells capable of responding vigorously to chemokines.
Limitations of this study include: 1) the small number of adult donors used; and 2) the slightly different methods used in part 1 compared to part 2, because of the different material used (chondrocytes vs chondral fragments).
New studies are required to fully understand the mechanisms underlying the negative interaction between adult and juvenile sources with chondrocyte co-cultures, as well as the positive interaction with chondral fragment co-cultures. The promising results of the second part of the study should also lead to new animal trials combining “in situ” autologous and allogenic juvenile cartilage fragments in a “one-stage” surgical procedure for cartilage repair.
References
- 1.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001;391:S280–94. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 2.Adkisson HD, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht F, Roessner A, Zimmermann E. Closure of osteochondral lesions using chondral fragments and fibrin adhesive. Arch Orthop Trauma Surg. 1983;101:213–217. doi: 10.1007/BF00436773. [DOI] [PubMed] [Google Scholar]
- 4.Aston JE, Bentley G. Repair of articular surfaces by allografts of articular and growth-plate cartilage. J Bone Joint Surg Br. 1986;68:29–35. doi: 10.1302/0301-620X.68B1.3941138. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter JA, Mankin HJ. Articular cartilage I. Tissue design and chondrocyte-matrix interactions. J Bone Joint Surg Am. 1997;79:600–611. [Google Scholar]
- 6.Buckwalter JA, Martin J, Mankin HJ. Synovial joint degeneration and the syndrome of osteoarthritis. Instr Course Lect. 2000;49:481–489. [PubMed] [Google Scholar]
- 7.Buckwalter JA, Rosenberg LC. Electron microscopic studies of cartilage proteoglycans. Electron Microsc Rev. 1988;1:87–112. doi: 10.1016/s0892-0354(98)90007-7. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA, Rosenberg LC. Electron microscopic studies of cartilage proteoglycans. Direct evidence for the variable length of the chondroitin sulfate-rich region of proteoglycan subunit core protein. J Biol Chem. 1982;257:9830–9839. [PubMed] [Google Scholar]
- 9.Buckwalter JA, Roughley PJ, Rosenberg LC. Age-related changes in cartilage proteoglycans: quantitative electron microscopic studies. Microsc Res Tech. 1994;28:398–408. doi: 10.1002/jemt.1070280506. [DOI] [PubMed] [Google Scholar]
- 10.Buckwalter JA, Woo SL, Goldberg VM, Hadley EC, Booth F, Oegema TR, et al. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am. 1993;75:1533–1548. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Chan PS, Schlueter AE, Coussens PM, Rosa GJ, Haut RC, Orth MW. Gene expression profile of mechanically impacted bovine articular cartilage explants. J Orthop Res. 2005;23:1146–1151. doi: 10.1016/j.orthres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Feder J, Adkisson HD, Kizer N, Hruska KA, Cheung R, Grodzinsky AJ, et al. In: Tissue Engineering in Musculoskeletal Clinical Practice. Sandell LJ, Grodzinsky AJ, editors. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2004. pp. 219–226. [Google Scholar]
- 13.Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009;37(1):71S–80S. doi: 10.1177/0363546509348478. [DOI] [PubMed] [Google Scholar]
- 14.Grogan SP, Barbero A, Winkelmann V, Rieser F, Fitzsimmons JS, O'Driscoll S, et al. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006;12:2141–2149. doi: 10.1089/ten.2006.12.2141. [DOI] [PubMed] [Google Scholar]
- 15.Hoemann CD, Sun J, Chrzanowski V, Buschmann MD. A multivalent assay to detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Anal Biochem. 2002;300:1–10. doi: 10.1006/abio.2001.5436. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan SP, Skinner JA, Bartlett W, Carrington RW, Flanagan AM, Briggs TW, et al. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88:61–64. doi: 10.1302/0301-620X.88B1.16796. [DOI] [PubMed] [Google Scholar]
- 17.Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81:1229–1235. doi: 10.2106/00004623-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Lind M, Larsen A. Equal cartilage repair response between autologous chondrocytes in a collagen scaffold and minced cartilage under a collagen scaffold: an in vivo study in goats. Connect Tissue Res. 2008;49:437–442. doi: 10.1080/03008200802325037. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–1270. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 20.Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol Biol Sci Med Sci. 2001;56:B172–179. doi: 10.1093/gerona/56.4.b172. [DOI] [PubMed] [Google Scholar]
- 21.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 22.Martin JA, Buckwalter JA. The role of chondrocyte-matrix interactions in maintaining and repairing articular cartilage. Biorheology. 2000;37:129–140. [PubMed] [Google Scholar]
- 23.Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulinlike growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 24.Martin JA, Mitchell CJ, Klingelhutz AJ, Buckwalter JA. Effects of telomerase and viral oncogene expression on the in vitro growth of human chondrocytes. J Gerontol A Biol Sci Med Sci. 2002;57:B48–53. doi: 10.1093/gerona/57.2.b48. [DOI] [PubMed] [Google Scholar]
- 25.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26:1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 26.Nettles DL, Chilkoti A, Setton LA. Early metabolite levels predict long-term matrix accumulation for chondrocytes in elastin-like polypeptide biopolymer scaffolds. Tissue Eng Part A. 2009;15:2113–2121. doi: 10.1089/ten.tea.2008.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peretti GM, Randolph MA, Villa MT, Buragas MS, Yaremchuk MJ. Cell-based tissue-engineered allogeneic implant for cartilage repair. Tissue Eng. 2000;6:567–576. doi: 10.1089/107632700750022206. [DOI] [PubMed] [Google Scholar]
- 28.Perka C, Schultz O, Lindenhayn K, et al. Joint cartilage repair with transplantation of embryonic chondrocytes embedded in collagenfibrin matrices. Clin Exp Rheumatol. 2000;18:13–22. [PubMed] [Google Scholar]
- 29.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 31.Specchia N, Gigante A, Falciglia F, Greco F. Fetal chondral homografts in the repair of articular cartilage defects. Bull Hosp Jt Dis. 1996;54:230–235. [PubMed] [Google Scholar]
- 32.Tamai N, Myoui A, Hirao M, Kaito T, Ochi T, Tanaka J, et al. A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2) Osteoarthritis Cartilage. 2005;13:405–417. doi: 10.1016/j.joca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Tang LH, Buckwalter JA, Rosenberg LC. The effect of link protein concentration on articular cartilage proteoglycan aggregation. J Orthop Res. 1996;14:334–339. doi: 10.1002/jor.1100140225. [DOI] [PubMed] [Google Scholar]
- 34.Tran-Khanh N, Hoemann CD, McKee MD, Henderson JE, Buschmann MD. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J Orthop Res. 2005;23:1354–1362. doi: 10.1016/j.orthres.2005.05.009.1100230617. [DOI] [PubMed] [Google Scholar]


