Abstract
Diethylenetriaminepentaacetic acid (DTPA) is a chelating agent that is used to facilitate the elimination of radionuclides, such as americium, from contaminated individuals. Its primary site of action is in the blood, where it competes with various biological ligands, including transferrin and albumin, for the binding of radioactive metals. To evaluate the chelation potential of DTPA under these conditions, the competitive binding of 241Am between DTPA and plasma proteins was studied in rat, beagle and human plasma in vitro. Following incubation of DTPA and 241Am in plasma, the 241Am-bound ligands were fractionated by ultrafiltration and ion-exchange chromatography, and each fraction was assayed for 241Am content by gamma scintillation counting. Dose-response curves of DTPA for 241Am binding were established, and these models were used to calculate the 90% maximal effective concentration, or EC90, of DTPA in each plasma system. The EC90 were determined to be 31.4, 15.9 and 10.0 μM in rat, beagle and human plasma, respectively. These values correspond to plasma concentrations of DTPA that maximize 241Am chelation while minimizing excess DTPA. Based on the pharmacokinetic profile of DTPA in humans, after a standard 30 μmol kg−1 intravenous bolus injection, the plasma concentration of DTPA remains above EC90 for approximately 5.6 h. Likewise, the effective duration of DTPA in rat and beagle were determined to be 0.67 and 1.7 h, respectively. These results suggest that species differences must be considered when translating DTPA efficacy data from animals to humans and offer further insights into improving the current DTPA treatment regimen.
Keywords: DTPA, 241Am, chelation, decontamination
Introduction
The growing threat of nuclear terrorism and the heightened awareness of the potential for nuclear accidents have renewed the interest in the development of medical treatments to prevent and treat radiation exposure. Among the drugs of interest is diethylenetriaminepentaacetic acid (DTPA), a chelating agent used as a first-line treatment for individuals that have been contaminated with transuranic radionuclides (Bullard 2012). With a long history as an effective decorporation agent, DTPA is made available in the United States Strategic National Stockpile for use in medical emergencies as the calcium and zinc chelates of DTPA (Ca- and Zn-DTPA). However, the utility of Ca- and Zn- DTPA is constrained by the need for intravenous (IV) administration, which requires trained medical personnel for treatment and limits their accessibility in mass-casualty situations. To overcome this limitation, alternative formulations and delivery strategies with DTPA are being sought (Phan et al. 2005; Reddy et al. 2012; Sadgrove et al. 2012; Shankar et al. 2012), with the potential to expand the use of DTPA under the United States Food and Drug Administration (FDA) Animal Rule.
The FDA Animal Rule permits the approval of drugs using animal efficacy data when human efficacy trials are neither ethical nor feasible. This is an important policy for the development of decorporation agents such as Ca- and Zn-DTPA, since human trials are restricted to subjects of accidental contamination for ethical reasons. Despite the long history of DTPA for use in radionuclide decorporation, its proven efficacy is still mostly limited to studies conducted in animal models. Of the animal models, rat and dog (beagle) represent the most commonly used species, and an IV bolus dose of 30 μmol kg−1 has been established as the standard efficacious dose (Stradling et al. 2000a, 2000b). The same weight-normalized dose is also used in humans, yet an understanding of the optimal dose and blood levels in humans for therapeutic effect has not been clearly established (Breustedt et al. 2009). An empirical analysis of existing data has been used to suggest that a DTPA:actinide molar ratio of 106:1 in plasma may be required for effective decorporation (Taylor et al. 2007). However, as existing human efficacy data are from uncontrolled studies, uncertainties in the physicochemical form of the radionuclide, route of exposure, and extent of contamination make it difficult to fully characterize the effect of the chelation therapy (Wood et al. 2000). While the use of the animal models has played a pivotal role in demonstrating the potential of DTPA for chelation therapy, further work is needed to evaluate the relationship between the animal models and humans for optimizing efficacy.
In an effort to better understand the suitability of animal models for human efficacy predictions, in vitro experiments were conducted to characterize the binding of 241Am by DTPA in the plasma of rat, beagle, and human. DTPA forms stable chelates with 241Am, possessing a high stability constant of 1026.2 M−1 (Ansoborlo et al. 2006). Plasma represents the primary site of action for DTPA and contains endogenous ligands that can compete with DTPA for the formation of 241Am complexes. In blood, greater than 90% of the 241Am resides in the plasma component (whole blood minus blood cells), with approximately 30% bound to the iron-transport protein, transferrin, and the remainder to albumin, globulins, and various low molecular weight ligands (Taylor 1998; Turner and Taylor 1968). Internalized 241Am is transported in blood before depositing in the skeleton and liver, from where it is excreted very slowly with an elimination half-life of 50 and 2.5 years, respectively (Agency for Toxic Substances and Disease Registry 2004). Intercepting the 241Am in the blood prior to its deposition in tissues has been proven to be an effective treatment approach in both animals and human. A better understanding of the chelation potential of DTPA in blood could be useful to improve the dosing regimen and to support the development of alternative DTPA products within the framework of the FDA Animal Rule.
Materials and Methods
The competition between plasma proteins and DTPA for the binding of 241Am in blood was characterized by in vitro experimentation. Various concentrations of DTPA were reacted with 241Am in rat, beagle, and human plasma before separation and assay. All samples were prepared using fixed volumes of the constituents by combining 385 μL of plasma, 8 μL of DTPA solution, and 7 μL of 241Am solution. The concentrations of DTPA and 241Am were altered by adjusting their stock concentrations. Plasma with sodium heparin (Lampire Biological Laboratories, Pipersville, PA) were filtered through a 0.45μm pore size polypropylene membrane with a borosilicate glass fiber pre-filter before use. The DTPA stock solutions were prepared by dissolving crystalline DTPA (Thermo Fisher Scientific, Morris Plains, NJ) in 10 mM sodium phosphate buffer at pH 7.4, and diluting with de-ionized water as needed. Following its addition to plasma, the final concentrations of DTPA were 1.67–53.4 μM in rat plasma, 1.67–26.7 μM in beagle plasma, and 0.84–26.7 μM in human plasma. Commercially available Ca- and Zn-DTPA (Heyl Chemisch-Pharmazeutische Fabrik GmbH & Co. KG, Berlin, Germany) were used and diluted in a similar manner with de-ionized water before use. The 241Am stock solutions were prepared by diluting 241AmCl3 in 1 M hydrochloric acid (Eckert & Ziegler Isotope Products, Valencia, CA) with 1% citrate acid solution. The addition of 241Am citrate to the mixture of DTPA and plasma in the final addition step yielded a concentration of 3 nM in the reaction mixture. In cases where lower 241Am levels were used, the 241Am citrate stock solution was diluted with de-ionized water before addition to the reaction mixture.
Immediately after combining the components, the samples were incubated for 0.5 h on a heating block at 37 °C. The solutions were then transferred to Amicon Ultra centrifugal filter devices fitted with an Ultracel 3K membrane (Millipore, Billerica, MA) and centrifuged at 14,000 ×g for 0.5 h to extract protein-bound 241Am. A bicinchoninic (BCA) assay was used to confirm that ultrafiltration successfully removed the proteins in the filtrates during preliminary work. Filtrates from the filter devices were transferred to Pierce Strong Cation Exchange Spin Columns (Thermo Fisher Scientific, Rockford, IL) that had been pre-conditioned with 0.8 M ammonium hydroxide and water to separate positively charged 241Am species from negatively charged DTPA-bound 241Am. After centrifuging the spin columns at 2,000 ×g for 5 min, the initial eluents were collected as the DTPA-bound 241Am fraction. The positively charged 241Am species retained on the column were subsequently eluted with two 400 μL aliquots of 1 M nitric acid by centrifuging at 2,000 ×g for 5 min each. This separation method was validated by replacing plasma with 25 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) or carbonate buffers at pH 7.4 and performing the chromatography in the presence and absence of DTPA. The 241Am content in each sample fraction was measured by gamma scintillation counting (Perkin Elmer Model 2470 Gamma Counter). Background-corrected radioactivity was quantified over 30 min using a 40–80 keV energy window to measure the 59.7 keV photon emission associated with 241Am decay.
Dose-response curves were constructed by calculating the fraction of total 241Am that was bound to either DTPA or plasma proteins. The sigmoidal regression profiles were generated by Gauss-Newton non-linear least squares regression analysis using Statistica 8.0 (StatSoft, Tulsa, OK). The regression applied the logistic equation (Hoehler 1995),
where a describes the slope as the curve approaches the asymptote, b is the inflection point, c is the asymptote, and x is the concentration of DTPA that corresponds to y, the fraction of bound 241Am. The EC90 values were determined by setting y equal to 90% of the c term and calculating for x. The 90% level was chosen since it represented a point along the dose-response curve that was approaching the asymptote and did not appear to be distinguishable from the asymptote based on the variability of the data. The same regression analysis was applied to the data for 241Am-protein binding; however, a transformation was applied to yprotein such that,
where y′ was used for the regression analysis. Subsequent reversal of the transformation produced the final binding curves.
Results
The suitability of the separation method was confirmed during preliminary work as follows. The ultrafiltration step was used to isolate the protein-bound 241Am species and cation-exchange chromatography was used to separate the negatively charged 241Am-DTPA complexes from positively charged 241Am species. Specifically, a BCA assay showed that only 0.6% of the proteins were present in the filtrate after ultrafiltration of the plasma mixture, confirming the successful removal of proteins from the plasma. To evaluate the separation of the 241Am species by cation-exchange chromatography, similar binding experiments were performed in the presence and absence of DTPA while replacing plasma with HEPES and bicarbonate buffers. In the absence of DTPA, only 2% of the input 241Am was detected in the initial eluent from the chromatographic separation. In contrast, when DTPA was present, approximately 85% of the 241Am was measured in the eluent. 241Am in solution exists in the +3 oxidation state (Runde and Shulz 2006), and the effect of DTPA on the elution of 241Am is consistent with the expectation that 241Am forms a negatively charged complex with DTPA at physiological pH. These preliminary results confirmed that the separation method could adequately separate 241Am bound by proteins from those chelated with DTPA.
The dose-response curves for the binding of 241Am by DTPA and proteins in rat, beagle, and human plasma are shown in Fig. 1a–c. DTPA and plasma proteins exhibit opposing sigmoidal 241Am binding profiles, indicative of a competitive binding relationship. The logistic equation parameters for the DTPA regression models are summarized in Table 1. The models achieved goodness of fit (R2) of greater than 0.92 in all cases. While some residual loss of sample occurred during the separation and transfer steps, greater than 90% of the total input 241Am was recovered. Of the total 241Am recovered, less than 10% corresponded to the low molecular weight, positively charged 241Am species. These were considered to be associated with the small organic and inorganic ligands of low 241Am affinity that are present in blood, such as chlorides, carbonates, citrates, aspartates, and glutamates (Ansoborlo et al. 2006). The least-squares linear regression fits on these data exhibited slopes approaching zero, indicating that they essentially remained unchanged with increasing DTPA concentration.
Fig. 1.
The competitive binding of 3 nM 241Am by DTPA (●, solid line) and plasma proteins (○, dotted line) after 0.5 h incubation at 37 °C in (a) rat, (b) beagle, and (c) human plasma.
Table 1.
Logistic equation parameters and EC90 of DTPA for 241Am binding
| Plasma | Parameters for logistic equation (Estimate ± 95% Conf. Interval) | R2 | EC90 (μM) | ||
|---|---|---|---|---|---|
| a | b | c | |||
| Rat | 0.18 ± 0.07 | 19.1 ± 2.9 | 64.8 ± 8.9 | 0.922 | 31.4 |
| Beagle | 0.36 ± 0.07 | 9.80 ± 0.57 | 58.3 ± 2.7 | 0.986 | 15.9 |
| Human | 0.40 ± 0.13 | 4.53 ± 0.95 | 72.0 ± 6.3 | 0.921 | 10.0 |
A comparison of the dose-response curves in rat, beagle, and human plasma reveals species differences in the efficiency of DTPA to bind 241Am. A simple comparison of the dose response curves in Fig. 1a–c reveals that higher concentrations of DTPA are required in rat and beagle plasma than in human plasma to achieve equivalent levels of 241Am chelation. To more precisely define the species differences, the EC90 of DTPA in each plasma system were determined. From the dose-response curves, it can be noted that approximately 60–70% of the total 241Am is maximally bound by DTPA in all species. The EC90 corresponds to the DTPA concentration at which the 241Am binding by DTPA is approaching this maximum. In rat plasma, the EC90 of DTPA was calculated to be 31.4 μM. In beagle plasma, the EC90 was determined to be 15.9 μM, or approximately two-fold lower than in rat plasma. In human plasma, the EC90 was even lower at 10.0 μM. While EC90 values reflect DTPA concentrations that maximize 241Am chelation in plasma, these values also correspond to the concentrations at which excess DTPA is minimized, thereby reducing the likelihood of binding endogenous metal ions, the primary cause of toxicity in DTPA therapies (Catsch et al. 1964). Thus, in a practical sense, the EC90 represents the optimal concentration of DTPA in plasma for maximizing chelation while minimizing toxicity.
Additional studies were conducted in human plasma to evaluate the effects of variables such as 241Am concentration, incubation time, and DTPA complex. When lower concentrations of 241Am (0.8 nM) were used, the results did not appear to be significantly different from those reported with 3 nM 241Am. This was not unexpected as the concentrations of both DTPA and plasma proteins were in great excess relative to 241Am under these conditions. In another study, the reaction mixtures were processed after 1.5 and 18 h incubations. Despite the longer reaction times, the results did not exhibit differences from data after 0.5 h incubation, suggesting that the 241Am-DTPA complexation approaches equilibrium rapidly.
The input DTPA was also replaced with commercially available Ca- and Zn-DTPA preparations in the human plasma system. Both calcium and zinc are endogenous metal ions that are present in plasma at concentrations of approximately 10−3 and 10−5 M, respectively, and exhibit affinity for DTPA with stability constants of 1010.9 and 1018.6 M−1, respectively (Seidel 1973). However, both DTPA complexes exhibit very rapid metal exchange rates in favor of 241Am (Seidel 1973). Thus, as expected, the use of these forms of DTPA did not significantly alter the 241Am binding behavior based on the comparison of dose-response curves. The lack of differences is likely the result of the significantly higher affinity of DTPA for 241Am, 1026.2 M−1, over calcium and zinc ions.
Discussion
The efficacy of DTPA for radionuclide decorporation in humans has been proven mostly through controlled studies in rat and beagle animal models. However, the data presented here suggests that different plasma concentrations of DTPA are required in rat, beagle and human to effectively chelate 241Am. The results show that DTPA is most effective in human plasma, requiring the lowest concentration to maximize the chelation of 241Am. In contrast, DTPA is least effective in rat plasma, requiring the highest concentration to effectively bind 241Am. This work suggests that species differences in the effectiveness of DTPA must be considered when using rat and beagle animal models to predict the efficacy of DTPA for 241Am decorporation in humans.
The species differences in the effective concentration of DTPA are presumably due to differences in their plasma composition; however, the mechanisms underlying these species differences remain unknown. Plasma is a complex matrix containing numerous endogenous ligands and metals that can interfere with 241Am-DTPA complexation. Transferrin and albumin are considered to be the two major endogenous ligands that can compete with DTPA for 241Am (Taylor 1998; Turner and Taylor 1968), while endogenous metals, such as iron, may compete with 241Am for the binding site of DTPA. Since these components are known to differ in plasma concentration depending on species (Durbin 2006), additional studies were conducted to explore the effects of these components on the dose-response curves. In one study, plasma was replaced with aqueous buffers containing isolated transferrin and albumin from rat and human serum at physiologically-relevant concentrations to compare the species-specific plasma protein affinity for 241Am in the presence of DTPA. As an alternative mechanism, the effect of iron as a competing metal for 241Am was investigated by adding ferric citrate or ferric chloride to human plasma at various concentrations. However, these studies did not reveal significant shifts in the distribution of 241Am that account for the large differences that were observed in the different plasma systems. Taking into consideration the complexity of plasma composition and the collective effects that the endogenous ligands and metals can have on interaction between DTPA and 241Am, these univariate approaches may be inadequate and developing a mechanistic understanding is proving to be challenging.
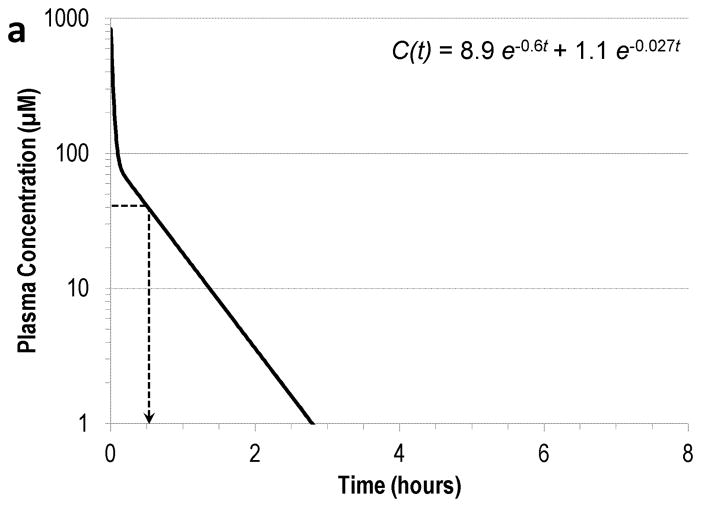
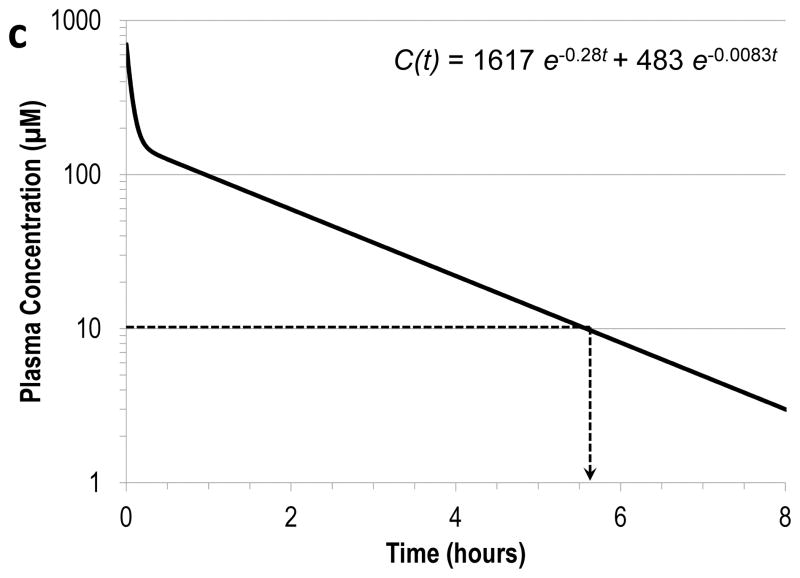
The effective plasma concentration of DTPA established in this work may be of further use to understand the effect of the current dosing regimen in 241Am decorporation. Since chelation is dictated by the concentrations of the binding components and plasma represents the primary site of action for DTPA, the plasma concentration of DTPA was assumed to be a reliable indicator of decorporation efficacy. With this assumption, the practical significance of the EC90 of DTPA was examined in the context of the previously established pharmacokinetic profiles in the different animal models. Following an IV bolus injection of DTPA in humans, the plasma concentration profile is expressed as the sum of two exponential components with rate constants of 0.28 and 0.0083 min−1 (Durbin and Schmidt 1989). At the current standard dose of 30 μmol kg−1 in a 70 kg human subject with a plasma volume of 3 L, the plasma concentration of DTPA is sustained above EC90 for approximately 5.6 h after an IV bolus injection. This interpretation is graphically depicted in Fig. 2a (Durbin and Schmidt 1989). Similar assessments for the animal models estimated the effective duration of DTPA to be 40 min in rats and 1.7 h in beagles, also shown in Fig. 2b–c (Durbin and Schmidt 1989). Beyond these times, the DTPA concentration is too low to overcome the competition with endogenous ligands for 241Am binding, and the effectiveness of DTPA is significantly reduced. This suggests that the optimal treatment regimen is unique to each species and that increasing the dosing frequency from the current once-daily IV bolus injection may be beneficial to sustain optimal DTPA blood levels. Consistent with this proposal, the use of multiple injections and infusion therapies with DTPA has been demonstrated to improve the therapeutic benefit in animal models (Guilmette and Muggenburg 1988; Stradling et al. 1989).
Fig. 2.
The plasma concentration of DTPA after a 30 μmol kg−1 IV bolus injection (solid line) and the effective duration (dotted line) in (a) rat, (b) beagle, and (c) human (Data adapted from Durbin and Schmidt 1989).
A limitation of this type of assessment on the plasma profile of DTPA, however, is that the chelation is assumed to occur only in the plasma compartment. While DTPA is primarily distributed in the extracellular fluids, studies have indicated that DTPA may also gain entry into cells of soft tissues, contributing to delayed or long term decorporation effects (Fritsch et al. 2010). Thus, an assessment of the plasma compartment alone does not account for the complete mechanism of decorporation. A more accurate model for decorporation should also consider the migration of DTPA between the extracellular and intracellular compartments.
In addition to the behavior of DTPA, 241Am and its biokinetics must also be considered when determining an optimal DTPA treatment regimen. Since DTPA is most effective in the blood compartment, the availability of 241Am during translocation will significantly influence the effectiveness of the chelation therapy. It has been shown that DTPA is most effective when the treatment commences as soon as possible after contamination (Lloyd et al. 1979). However, both 241Am and its DTPA chelate follow complex biokinetics, making it difficult to establish predictive models for decorporation (Breustedt et al. 2009). The chemical speciation of internalized 241Am and its route of contamination are also key variables that affect its rate of absorption into the bloodstream (Guilmette and Durbin 2003). The route of contamination can influence the rate at which the radionuclide migrates into the blood; for example, contamination via wound or ingestion can result in slower entry into the systemic circulation than by inhalation or IV injection. Furthermore, in the case of wound contamination, the chemical form dictates the solubility of the 241Am species, thereby affecting the rate of uptake into the blood and its retention at the wound site. A slow or prolonged release of 241Am from the wound site may necessitate the use of more frequent or extended treatments of DTPA to capture the radionuclide during its translocation. While the effective concentration of DTPA in blood is only one aspect of the overall decorporation process, the in vitro assessment presented in this work provides a basis for exploring further improvements in the current DTPA dosing regimen.
Conclusion
This work provides useful insight into improving the current dosing regimens for DTPA and reveals significant species differences in 241Am chelation that may impact decorporation. DTPA is most efficient in human plasma, requiring concentrations that are significantly lower than in rat or beagle plasma. The results may be further interpreted in the context of plasma concentration profiles for DTPA, where the effective duration following a standard treatment dose is longest in humans and shortest in rats. These findings offer important considerations in translating decorporation efficacy from animal models to humans, a potentially significant understanding to support the development of alternative DTPA products under the FDA Animal Rule.
Acknowledgments
The authors thank Drs. Xiuling Lu, Jonathan Fitzsimmons, Dora Babu Madhura, Marina G. D. Leed, and Dhiren Thakker for their helpful discussions in support of this work. This work was funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health, U.S. Department of Health and Human Services under contracts HHSN266200500045C and HHSN272201000030C.
Footnotes
Conflicts of interest and source of funding: No conflict of interest declared; funding provided by the National Institute of Allergy and Infectious Diseases, National Institute of Health, U.S. Department of Health and Human Services under contracts HHSN266200500045C and HHSN272201000030C
References
- Agency for Toxic Substances and Disease Registry. Toxicological profile for americium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2004. [PubMed] [Google Scholar]
- Ansoborlo E, Prat O, Moisy P, Den Auwer C, Guilbaud P, Carriere M, Gouget B, Duffield J, Doizi D, Vercouter T, Moulin C, Moulin V. Actinide speciation in relation to biological processes. Biochimie. 2006;88:1605–1618. doi: 10.1016/j.biochi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Breustedt B, Blanchardon E, Berard P, Fritsch P, Giussani A, Lopez MA, Luciani A, Nosske D, Piechowski J, Schimmelpfeng J, Serandour AL. Biokinetic modeling of DTPA decorporation therapy: the CONRAD approach. Radiat Protect Dosim. 2009;134(1):38–48. doi: 10.1093/rpd/ncp058. [DOI] [PubMed] [Google Scholar]
- Bullard W. Consequence management: Medical countermeasures. Drug Dev Res. 2012;73:274–280. [Google Scholar]
- Catsch A, Le DK, Chambault D. Evaluation of the efficacy of different metal chelates of DTPA in removing internally-deposited radionuclides. Int J Rad Biol. 1964;8:35–43. doi: 10.1080/09553006414550031. [DOI] [PubMed] [Google Scholar]
- Durbin PW. Actinides in animals and man. In: Morss LR, Edelstein NM, Fuger J, editors. The chemistry of the actinides and transactinide elements. 4. New York: Springer; 2006. pp. 3339–3440. [Google Scholar]
- Durbin PW, Schmidt CT. Predicting the kinetics of chelating agents in man from animal data. Health Phys. 1989;57(Suppl 1):165–174. doi: 10.1097/00004032-198907001-00021. [DOI] [PubMed] [Google Scholar]
- Fritsch P, Serandour AL, Gremy O, Phan G, Tsapis N, Fattal E, Benech H, Deverre JR, Poncy JL. Structure of a single model to describe plutonium and americium decorporation by DTPA treatments. Health Phys. 2010;99(4):553–559. doi: 10.1097/HP.0b013e3181c1cccd. [DOI] [PubMed] [Google Scholar]
- Guilmette RA, Muggenburg BA. Reducing the radiation dose from inhaled americium-241 using continuously administered DTPA therapy. Int J Radiat Biol. 1988;53(2):261–271. doi: 10.1080/09553008814550611. [DOI] [PubMed] [Google Scholar]
- Guilmette RA, Durbin PW. Scientific basis for the development of biokinetic models for radionuclide-contaminated wounds. Radiat Protect Dosim. 2003;105(1–4):213–218. doi: 10.1093/oxfordjournals.rpd.a006225. [DOI] [PubMed] [Google Scholar]
- Hoehler FK. Logistic equations in the analysis of S-shaped curves. Comput Biol Med. 1995;25(3):367–371. doi: 10.1016/0010-4825(95)00013-t. [DOI] [PubMed] [Google Scholar]
- Lloyd RD, Taylor GN, Mays CW, Jones CW, Bruenger FW, Atherton DR. Dependency of chelation efficacy upon time after first DTPA injection. Radiat Res. 1979;78:448–454. [PubMed] [Google Scholar]
- Phan G, Herbert A, Cholet S, Benech H, Deverre JR, Fattal E. Pharmacokinetics of DTPA entrapped in conventional and long-circulating liposomes of different size for plutonium decorporation. J Cont Release. 2005;110:177–188. doi: 10.1016/j.jconrel.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Runde WH, Shulz WW. Americium. In: Morss LR, Edelstein NM, Fuger J, editors. The chemistry of the actinides and transactinide elements. 4. New York: Springer; 2006. pp. 1265–1395. [Google Scholar]
- Seidel A. Comparison of the effectiveness of CaDTPA and ZnDTPA in removing 241Am from the rat. Radiat Res. 1973;54:304–315. [PubMed] [Google Scholar]
- Stradling GN, Stather JW, Gray SA, Moody JC, Ellender M, Hodgson A, Volf V, Taylor DM, Wirth P, Gaskin PW. The efficacies of pure LICAM(C) and DTPA on the retention of plutonium-238 and americium-241 in rats after their inhalation as nitrates and intravenous injection as citrate. Int J Radiat Biol. 1989;56(4):503–514. doi: 10.1080/09553008914551641. [DOI] [PubMed] [Google Scholar]
- Stradling GN, Henge-Napoli MH, Paquet F, Poncy JL, Fritsch P, Taylor DM. Approaches for experimental evaluation of chelating agents. Radiat Protect Dosim. 2000;87:19–27. [Google Scholar]
- Stradling GN, Henge-Napoli MH, Paquet F, Poncy JL, Fritsch P, Taylor DM. Optimum treatment regimens with animals. Radiat Protect Dosim. 2000;87:29–40. [Google Scholar]
- Taylor DM. The bioinorganic chemistry of actinides in blood. J Alloy Compd. 1998;271–273:6–10. [Google Scholar]
- Turner GA, Taylor DM. The transport of plutonium, americium and curium in the blood of rats. Phys Med Biol. 1968;13:535–546. doi: 10.1088/0031-9155/13/4/304. [DOI] [PubMed] [Google Scholar]
- Wood R, Sharp C, Gourmelon P, Guen BL, Stradling GN, Taylor GN, Henge-Napoli MH. Decorporation treatment medical overview. Radiat Protect Dosim. 2000;87(1):51–57. [Google Scholar]