Abstract
Toll-like receptors (TLR) expressed on inflammatory cells play a key role in host defense against pathogens, benefiting the host. TLR are also expressed on tumor cells. To evaluate the role of TLR in tumor cells, we investigated TLR4 signaling effects on human head and neck squamous cell carcinoma (HNSCC). Tumor tissues were obtained from 27 patients with laryngeal and 12 with oral cavity cancers. Normal mucosa was obtained from 10 patients with nonneoplastic disorders. Smears for bacteria were taken from all patients during surgery. TLR4 expression in tumors and HNSCC cell lines (PCI-1, PCI-13, and PCI-30) was detected by reverse transcription-PCR and immunohistochemistry. Cell growth, apoptosis, nuclear factor-κB (NF-κB) translocation, and MyD88 and IRAK-4 expression, as well as Akt phosphorylation were measured following tumor cell exposure to the TLR4 ligand lipopolysaccharide (LPS). Tumor cell sensitivity to NK-92–mediated lysis was evaluated in 4-hour 51Cr-release assays. Cytokine levels in HNSCC supernatants were measured in Luminex-based assays. TLR4 was expressed in all tumors, HNSCC cell lines, and normal mucosa. The TLR4 expression intensity correlated with tumor grade. LPS binding to TLR4 on tumor cells enhanced proliferation, activated phosphatidylinositol 3-kinase/Akt pathway, up-regulated IRAK-4 expression, induced nuclear NF-κB translocation, and increased production (P < 0.05) of interleukin (IL)-6, IL-8, vascular endothelial growth factor, and granulocyte macrophage colony-stimulating factor. TLR4 triggering protected tumor cells from lysis mediated by NK-92 cells. TLR4 ligation on tumor cells supports HNSCC progression.
Introduction
Toll-like receptors (TLR) expressed on immune cells play a critical role in immune responses against invading pathogens (1–4). TLR are members of the evolutionary conserved interleukin (IL)-1R receptor family that are broadly represented on immune cells, especially on antigen-presenting cells. At least 11 mammalian TLR have been identified and are involved in the recognition by immune and nonimmune cells of pathogen-associated molecular patterns, such as lipopolysaccharide (LPS), viral double-stranded RNA, and unmethylated CpG islands (5–7). TLR can also recognize some host molecules, which are released during cellular stress or cell death (8). TLR triggering induces a release of inflammatory cytokines, activation of adaptive immunity, and maturation of dendritic cells (9).
Due to the pivotal role of TLR in immune responses to pathogens, most research aimed at understanding the TLR biology has been focused on immune cells. In patients with cancer, who are often immunosuppressed (10, 11), TLR-mediated activation of innate or adaptive immunity could be of considerable benefit. It has been known for a long time that microbial compounds can be used as efficient adjuvants in antitumor vaccine formulations, and numerous animal tumor models clearly indicate the potency of different TLR agonists in enhancing antitumor immune responses (12–14). However, TLR are also expressed on endothelial and epithelial cells, including tumor cells (15–18). To date, little is known about the function and biological importance of TLR expressed on tumor cells. Preliminary evidence suggests that TLR expressed on tumor cells may play an important role in the tumor development. For example, ligation of TLR4 expressed on tumor cells can induce chronic inflammation, which promotes tumor growth (19). Triggering of TLR9 on prostate cancer cells has also been shown to promote tumor cell proliferation (20). In contrast, Wang and colleagues (21) showed that TLR9 stimulation on lung cancer cells sensitized tumor cells to apoptosis, leading to the arrest of tumor growth. Thus, controversial information exists about the role of various TLR on tumor progression. In this study, we test the hypothesis that signaling via the TLR4 expressed on human tumor cells induces tumor growth and facilitates tumor escape from immune surveillance.
Materials and Methods
Patients
Primary tumor samples of the larynx and oral cavity were obtained from 39 patients with histopathologically confirmed head and neck squamous cell carcinoma (HNSCC) who underwent surgery in the University Otolaryngology Clinic in Poznan, Poland. Smears for culture of microbes present in the tumor were taken from all patients during surgery. As controls, mucosal tissue samples were obtained from 10 patients undergoing surgery for nonneoplastic and noninflammatory disorder, the sleep apnea syndrome. The study was approved by the Ethics Committee at the University of Medical Sciences in Poznan and all patients signed informed consent forms. The clinicopathologic characteristics of the HNSCC patients included in this study are presented in Table 1.
Table 1.
Clinicopathologic characterization of the HNSCC patients included in this study
| Characteristics | Patients (n = 39) | Healthy controls (n = 10) |
|---|---|---|
| Sex | ||
| Male | 34 | 3 |
| Female | 5 | 7 |
| Age | ||
| Range | 50–77 | 32–59 |
| Median | 60 | 51 |
| Tumor site | ||
| Oral cavity | 12 | — |
| Larynx | 27 | |
| Tumor differentiation | ||
| Well | 8 | — |
| Moderate | 19 | |
| Poor | 12 | |
| Tumor stage | ||
| T1 | 0 | — |
| T2 | 0 | |
| T3 | 27 | |
| T4 | 12 | |
| Nodal status | ||
| N0 | 18 | |
| N1 | 9 | |
| N2 | 11 | — |
| N3 | 1 | |
| Distant metastases | ||
| M0 | 36 | — |
| M1 | 3 | |
| Bacteria and fungi recovered from the tumor* | ||
| Bacteria | 36 | — |
| Fungi | 6 | |
NOTE: All HNSCC patients included in this study underwent surgery; none received radiotherapy or chemotherapy before surgery for tumor removal.
At the time of surgery, samples for bacterial/fungal cultures were obtained from tumor tissues.
Cell lines
HNSCC cell lines (PCI-1, PCI-13, and PCI-30) were established at the University of Pittsburgh Cancer Institute and maintained as previously described (22). PCI-1 and PCI-30 cell lines originated from the well-differentiated tumors of the larynx or oral cavity, respectively, and PCI-13 from a poorly differentiated oral cavity carcinoma (23, 24). Cells were cultured in RPMI 1640 supplemented with 10% (v/v) FCS, l-glutamine, and antibiotics (all from Invitrogen). Cells used for our experiments were in the log phase of growth and were negative for Mycoplasma and endotoxin, as confirmed by PCR (Mycoplasma Tissue Culture Detection kit, Gen-Probe) and Limulus Amebocyte Lysate assay (Cambrex), respectively.
Treatment of tumor cells with LPS and cisplatin
Cells were seeded in wells of 6- or 24-well plates (5 × 105/mL). LPS and cisplatin (both purchased from Sigma-Aldrich) were added to tumor cells at the concentrations of 0.1 to 10 μg/mL and 10 μmol/L, respectively. As controls, tumor cells were cultured in medium alone. In some experiments, tumor cells silenced for TLR4 with small interfering RNA (siRNA) were stimulated with LPS. Plates were incubated at 37°C for 6 to 48 h. Supernatants were collected and stored frozen at –20°C for cytokine analyses.
Reverse transcription-PCR
To determine TLR expression at the mRNA level, reverse transcription-PCR (RT-PCR) was performed. Tumor cells (2 × 106) were harvested, washed in PBS, cryopreserved, and stored in –80°C. After cell thawing and washing, total RNA was isolated using the Trizol reagent (Invitrogen). RT-PCR was performed according to the manufacturer's instructions. Reverse transcription was performed with random primers using Omniscript RT kit (Qiagen). The set of primers used to detect TLR1 to TLR10 were previously described by Lauzon and colleagues (25) or designed by us: TLR1, 5′-CTTATAAGTGTGACTACCCGG-3′ (forward) and 5′-CCACAATGCTCTTGCCAGG-3′ (reverse); TLR2, 5′-GTTAACAATCCGGAGGCTGC-3′ (forward) and 5′-TTGGGAATGCAGCCTGTTAC-3′ (reverse); TLR3, 5′-GTGCCAGAAACTTCCCATGT-3′ (forward) and 5′-CTTCCAATTGCGTGAAAACA-3′ (reverse); TLR4, 5′-CTGCAATGGATCAAGGACCA-3′ (forward) and 5′-TCCCACTCCAGGTAAGTGTT-3′ (reverse); TLR5, 5′-TGGGGGAACTTTACAGTTCG-3′ (forward) and 5′-CTGGGATTCTCTGAAGGGG-3′ (reverse); TLR6, 5′-GGGTTGAGAGTATAGTGGTG-3′ (forward) and 5′-GTAGATGCAGAGGGAGGTC-3′ (reverse); TLR7, 5′-CCTCAGCCACAACCAACTG-3′ (forward) and 5′-TTGTGTGCTCCTGGCCCC-3′ (reverse); TLR8, 5′-AAACTTGAGCCACAACAACATTT-3′ (forward) and 5′-ATCTCCAATGTCACAGGTGC-3′ (reverse); TLR9, 5′-AACTGGCTGTTCCTGAAGTC-3′ (forward) and 5′-TGCCGTCCATGAATAGGAAG-3′ (reverse); and TLR10, 5′-AAAACTCTAAATGCGGGAAGAAA-3′ (forward) and 5′-GAAATAAATGCGTGGAATCGGA-3′ (reverse). The PCR was performed using an Expand High Fidelity PCR System (Roche) under the following conditions: denaturation temperature of 95°C for 45 s, annealing temperature of 60°C for 45 s, and extension temperature of 72°C for 1 min.
Flow cytometry
TLR4 expression on PCI cells was evaluated by flow cytometry as described (26) using the Alexa Fluor 488–labeled mouse anti-human TLR4 antibody, HTA-125 clone (eBioscience).
Immunostaining
The following primary antibodies were used for immunostaining: polyclonal rabbit anti-human TLR4 (Abcam), polyclonal goat anti-human TLR4 (Santa Cruz Biotechnology), or appropriate isotype control IgG. Donkey anti-goat FITC labeled was used as the secondary antibody at 1:500 dilution (Jackson ImmunoResearch). Mouse anti-human pancytokeratin antibody (Dako) was used to confirm the epithelial origin of tumor cells. Antibodies were used at working concentrations of 2 to 5 μg/mL. For immunofluorescence, tumor cells were seeded on glass slides, air dried, washed in PBS, fixed in 2% (w/v) paraformaldehyde for 15 min, permabilized with 0.1% Triton X, and blocked with 2% bovine serum albumin (BSA) in PBS for 45 min. Sections were incubated with the goat primary antibody for TLR4 for 1 h at room temperature in a moist chamber and then with a secondary antibody. In controls, primary antibody was replaced by 0.5% BSA or isotype IgG. Sections were mounted in a medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories) to trace cell nuclei. Paraffin sections of tumor tissues or normal mucosa were cut and stained as previously described (18). After standard deparaffinization, the EnVision+ System (Dako) was used for staining according to the manufacturer's instructions. In short, after an overnight incubation with the rabbit anti-TLR4 antibody, sections were first incubated with labeled polymer-horseradish peroxidase (HRP) anti-rabbit antibody and then with 3,3′-diaminobenzidine. To eliminate nonspecific binding of the secondary antibody, tissue sections were incubated with a serum-free protein blocker before adding the goat anti-TLR4 antibody. Sections were counterstained with Meyer's hematoxylin and mounted in glycerol jelly.
Slides were evaluated in a light microscope (×400 magnification) or in an inverted Olympus FluoView 1000 laser scanning confocal microscope under an oil immersion objective (Center for Biologic Imaging Core Facility, University of Pittsburgh). For digital image analysis, the software Adobe Photoshop version 7.0 was used. Results were scored by two independent investigators (M.J.S. and M.H.) as positive, heterogeneous, or negative, when the percentage of stained tumor cells in each section was >75%, between 25% and 75%, and <25%, respectively. The two scores were averaged. The level of staining intensity was recorded as none, weak, moderate, or strong.
Western blot analysis
Tumor cells were coincubated with LPS for the indicated periods of time at 37°C, and Western blots were performed as previously described (27). Briefly, cells were lysed in equal volumes of ice-cold lysis buffer and a protease inhibitor cocktail (Pierce Chemical Co.). Cell homogenates were boiled for 5 min in 5× Laemmli sample buffer and proteins were separated by SDS-PAGE. Polyvinylidene difluoride membranes were blocked in 5% fat-free milk or 5% BSA in 0.05% Tween 20 in TBS. After an overnight incubation at 4°C with monoclonal or polyclonal rabbit anti–phosphorylated Akt (pAkt), anti-Akt, anti–IRAK-4, or anti-MyD88 antibodies (all from Cell Signaling Technology), membranes were incubated with the HRP-conjugated secondary antibody at 1:150,000 dilution (Pierce Chemical) for 1 h at room temperature and developed with a SuperSignal chemiluminescent detection system (Pierce Chemical). To inhibit phosphatidylinositol 3-kinase (PI3K) activity, LY294002 (Cell Signaling Technology) inhibitor was used at the concentration of 10 μmol/L (28).
Nuclear factor-κB assays
Aliquots of tumor cells (1 × 104) to be used for confocal microscopy were seeded on glass slides placed in Petri dishes overnight at 37°C in 5% of CO2 in air. LPS was added to the cells, and after 12 h of incubation, cells were stained as described above using the rabbit anti-p65 antibody. Translocation of a nuclear factor-κB (NF-κB) p65 subunit to nuclei was observed. The percentages of positive cells were determined by examining at least 200 tumor cells for each treatment condition. To measure the p65 subunit binding activity, nuclear and cytoplasmic extracts were prepared using a TransFactor extraction kit (Clontech). DNA binding by p65 was detected by ELISA using a TransFactor Profiling kit (Clontech; ref. 29). 1-Pyrrolidinecarbodithioic acid (PDTC; Calbiochem) was used at the concentration of 15 μmol/L for blocking of NF-κB activity (30).
Tumor cell proliferation
Tumor cells plated overnight in wells of six-well plates at the density of 10 × 103 per well were incubated with a fresh medium or medium supplemented with LPS at various working concentrations. The viability and numbers of tumor cells were determined by microscope counts in the presence of a trypan blue dye using tumor cells harvested after treatment with TripLE Select solution (Invitrogen) on days 3 and 5 of culture.
7-Amino-actinomycin-D assay
Apoptosis was analyzed by flow cytometry as previously described (31). Tumor cells were treated with cisplatin (10 μmol/L) for 24 h in the presence or absence of LPS (1 μg/mL). Then, the cells were harvested and washed in PBS, resuspended in prediluted binding buffer, and stained with 7-amino-actinomycin-D (7-AAD; Becton Dickinson) for 15 min on ice, protected from light. The cells were washed and resuspended in 7-AAD–binding buffer, and apoptosis of tumor cells was immediately evaluated by flow cytometry.
Luminex-based assay
Supernatants of tumor cells (plated at 5 × 105/mL) were collected after 48 h of coincubation with TLR ligands. The levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-α1), fibroblast growth factor (FGF), eotaxin, MCP-1, MIP-1α, RANTES, and IP-10 were measured using immunobead-based 16-plex assays (Luminex). Panels of capture antibody-coated beads and labeled detection antibodies were purchased from Biosource. The assay sensitivity varied from 5 to 15 pg/mL, depending on the analyte. The assays were performed using the Bio-Plex System (Bio-Rad).
Natural killer cell cytotoxicity assays
The 4-h 51Cr-release assays were performed using the PCI cells as targets and NK-92 cells as effectors (32). Controls included targets incubated in medium alone (spontaneous release) and targets in 5% (v/v) Triton X-100 in PBS (maximum release). The radioactivity was measured using Wallac Wizard 1470 Automatic Gamma Counter. The percentages of cytotoxic activity and lytic units were calculated using the Coggins program.
Silencing TLR4
Expression of TLR4 in the cell lines was temporarily silenced by using siRNA (20, 33, 34). Briefly, 2 × 105 tumor cells were seeded in wells of six-well plates and cultured in RPMI 1640. At confluence, cells were collected and resuspended in the transfection medium containing the transfection reagent and human TLR4 siRNA (Santa Cruz Biotechnology) at different concentrations (4, 8, 10, or 12 μL). Negative control siRNA, which had no homology to known sequences from humans (i.e., nontargeting 20- to 25-nucleotide siRNA), was used as control. Transfection was performed as recommended by the siRNA manufacturer. To assess efficiency of the transfection process, nontargeting siRNA labeled with FITC was used in parallel to experimental cultures. Cultures with the transfection efficiency of >90% as assessed by fluorescence microscopy were used for further studies. Cells were incubated in the RPMI 1640 complete medium for 6 h at 37°C in an atmosphere of CO2 in air and, after a medium change, for additional 24 h in the medium supplemented with LPS. The viability of tumor cells was tested by a trypan blue dye exclusion, and the expression of targeted genes in tumor cells was tested on days 4, 8, and 12 by RT-PCR and Western blots. Assessments of tumor cell proliferation, cytokine production, and translocation of p65 subunit of NF-κB were also performed.
Statistical analysis
Data were summarized by descriptive statistics (the mean and SD for continuous variables; the frequency and percentage for categorical variables). The paired Student's t test was used to evaluate differences between treated versus untreated pairs of cell lines. Statistical analyses of differences in more than two parameters were performed using the ANOVA test. The P values of <0.05 were considered significant.
Results
Patients and controls
Table 1 summarizes clinicopathologic characteristics of the patients and normal controls included in this study. The group of normal controls comprised seven men and three women (age range, 32–59 years) who underwent laryngological surgery for nonmalignant diseases. All 39 HNSCC patients had advanced primary tumors (stages T3 and T4), and 21 had nodal involvement. The patient group included 34 males and 5 females (age range, 50–77 years). All had histopathologically confirmed squamous cell carcinomas. Cultures of samples taken from the tumor at the time of surgery included Gram-negative (Citrobacter diversus, Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus mirabilis) and/or Gram-positive (Streptococcus viridans, Streptococcus hemophilus, Streptococcus epidermidis, Staphylococcus aureus, and Actinobacteria) bacteria and/or fungi (Candida albicans).
TLRs are expressed by tumor cells
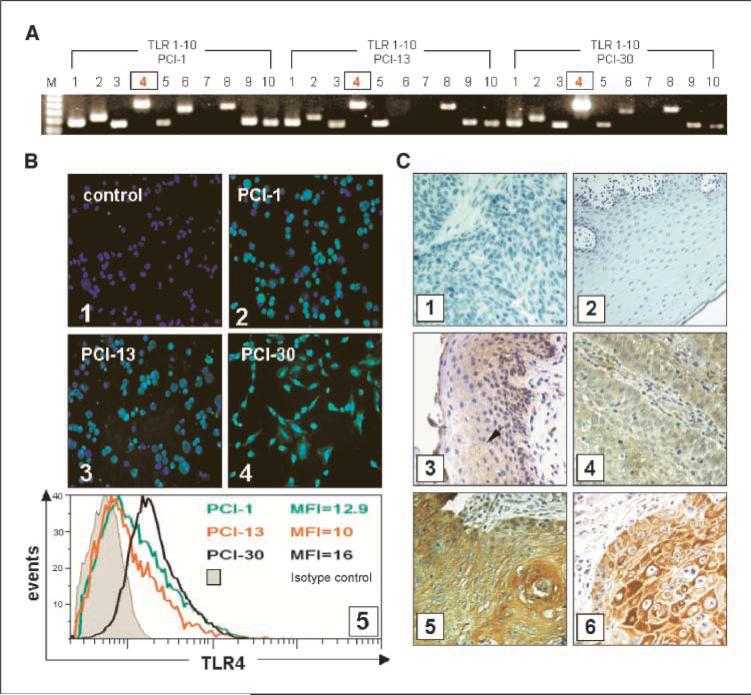
Using RT-PCR and immunostaining, we showed that TLRs are expressed on tumor cells (Fig. 1). All three tested tumor cell lines showed high levels of TLR4 expression. The highest level of mRNA for TLR4 was detected in the PCI-30 cell line (Fig. 1A). Immunofluorescence confirmed TLR4 protein expression in all tumor cell lines (Fig. 1B). TLR4 was also detected in tumor tissues, as previously reported (17). In tumor cells, TLR4 was localized in the cytoplasm and the cell membrane, and its expression ranged from weak to strong (Fig. 1C). TLR4 was also detected in 9 of 10 specimens of the normal mucosa, although the cytoplasmic expression of TLR4 was weak relative to that seen in the tumor. The epithelial origin of tumor cells in tissue sections and normal mucosa was confirmed by the staining for cytokeratin (Fig. 1C).
Figure 1.
TLR expression in tumors. TLR expression at the mRNA and protein levels in tumor cell lines, tumor, or normal mucosa. A, expression of TLR1 to TLR10 mRNA in the three HNSCC cell lines. B, expression of TLR4 protein in the three HNSCC cell lines. Magnification, ×400. 1, negative staining for TLR4 using isotype control IgG; 2, TLR4 in PCI-1; 3, TLR4 in PCI-13; 4, TLR4 in PCI-30 cells; 5, mean fluorescence intensity of TLR4 as determined by flow cytometry in the three cell lines. C, immunohistochemistry for TLR4 in tissue sections. Magnification, ×400. 1, isotype control in the tumor; 2, isotype control in the normal mucosa; 3, several TLR4+ cells in the normal mucosa (arrow); 4, a poorly differentiated HNSCC (G3) showing a weak positive reaction for TLR4; 5, a well-differentiated HNSCC (G1) showing strong positive reaction; 6, a representative HNSCC stained for cytokeratin.
A strong positive reaction for TLR4 was characteristic for well-differentiated or moderately well-differentiated tumors (G1 or G2) relative to moderate or weak staining intensity in poorly differentiated tumors (G3; Fig. 1C). These tissue staining results correspond to expression of TLR4 on the HNSCC cell lines (i.e., PCI-30 cells), which originated from a well-differentiated tumor and also showed the highest expression of TLR4. In the tumor microenvironment, a moderate to large number of TLR4+ inflammatory cells were observed (data not shown). We did not find any correlation between expression of TLR4 on tumor cells and the number of tumor-infiltrating leukocytes.
LPS promotes tumor cell proliferation
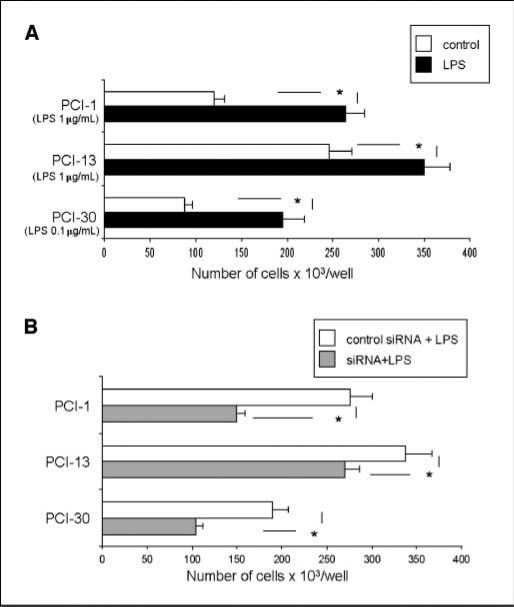
Effects of LPS on tumor cell proliferation were studied in all three PCI cell lines, which were either exposed to various concentrations of LPS (0.1–10 μg/mL) or pretreated with siRNA specific for TLR4 before LPS addition. As shown in Fig. 2A, LPS significantly enhanced tumor cell proliferation of (P < 0.05). The proliferation was decreased (P < 0.05) in the presence of siRNA specific for TLR4 followed by stimulation with LPS (Fig. 2B).
Figure 2.
Proliferation of tumor cells in response to LPS. A, PCI cell lines were incubated with 0.1 to 1.0 μg/mL of LPS. Each line was incubated with the LPS dose, which gives optimal tumor cell proliferation. Cells were counted on day 5 of culture. Control cultures contained no LPS. B, tumor cells were treated with TLR4-specific siRNA or with nonrelevant siRNA. A and B, columns, mean cell counts from three independent experiments; bars, SD. *, significant differences (P < 0.05) between experimental and control cultures.
LPS protects tumor cells from drug-induced apoptosis
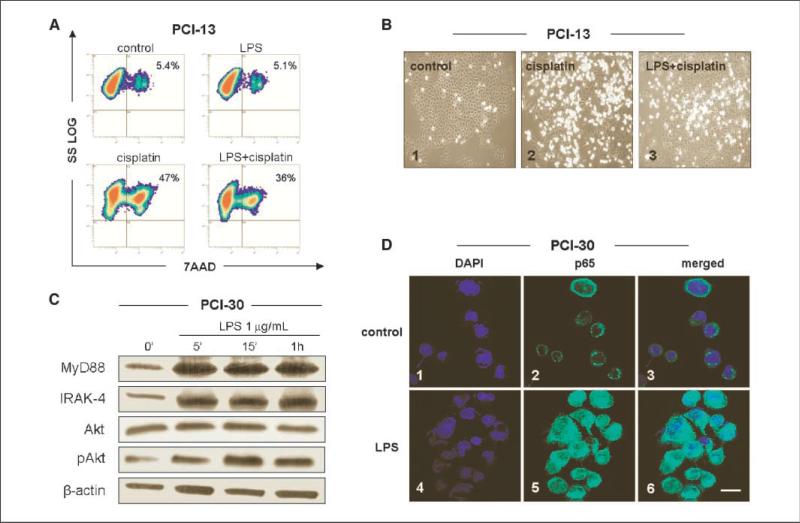
In the control cultures, spontaneous apoptosis ranged from 3.8% to 7.6%. After stimulation with LPS alone, apoptosis levels remained low (3–8%). Cisplatin induced apoptosis in PCI-1, PCI-13, and PCI-30 cells (mean ± SD = 36 ± 4%, 44 ± 3%, and 33 ± 4%, respectively) after 24-hour incubation. The 6-hour preincubation of tumor cells with LPS decreased the sensitivity to cisplatin-induced apoptosis to 36% in PCI-13 cells (Fig. 3A). For all three tumor cell lines, this decrease was 28 ± 5%, 36 ± 3%, and 22 ± 4.5% in PCI-1, PCI-13, and PCI-30, respectively (P < 0.05; Supplementary Fig. S1). As shown in Fig. 3B, PCI-13 cells treated with cisplatin showed an altered morphology, with many cells undergoing apoptosis. However, the effect of the drug was partially abrogated when tumor cells were preincubated with LPS (0.1–10 μg/mL). These data suggested that LPS protected TLR4+ tumor cells from apoptosis.
Figure 3.
Effects of LPS pretreatment on cisplatin-induced apoptosis and NF-κB activation in cancer cells. PCI cell lines were treated with 1 μg/mL LPS. A, LPS pretreatment protects tumor cells from apoptosis induced by cisplatin. Cell lines were first incubated ± LPS for 12 h and then with 10 μmol/L cisplatin for 24 h, stained with 7-AAD, and examined by flow cytometry. Results of a representative experiment of three performed are shown. B, morphologic changes in culture of PCI-13 cells incubated ± cisplatin or + LPS and cisplatin. Magnification, ×200. C, Western blots of PCI-30 cells incubated ± LPS show activation of the PI3K/Akt survival pathway. Increased levels of MyD88 and IRAK-4 in cells stimulated with LPS are accompanied by nuclear localization of the p65 subunit of NF-κB in LPS-treated PCI-30 cells. D, tumor cells plated overnight were stimulated with LPS (0.1 μg/mL) for 12 h and then stained as described in Materials and Methods. 1 to 3, control cells were untreated; 1, 3, 4, and 6, nuclei are stained blue; 2 and 5, the p65 subunit of NF-κB is stained green; 3, an overlay of 1 and 2; 6, an overlay of 4 and 5. At least 200 cells were randomly counted. Bar, 20 μm. Results are representative of three independent experiments.
TLR4 signaling in tumor cells engages MyD88, phosphatidylinositol 3-kinase, and IRAK pathways
To evaluate molecular pathways engaged in the induction of tumor cell resistance or sensitivity to apoptosis on stimulation with LPS, components of the phosphatidylinositol 3-kinase (PI3K)/Akt survival pathway were examined. MyD88, IRAK-4, and pAkt were constitutively expressed in all three PCI cell lines. The highest level of baseline pAkt was observed in PCI-30 (Fig. 3C) and PCI-1 (data not shown). After stimulation with LPS for 5 to 15 minutes, the level of pAkt increased, as did levels of MyD88 and IRAK-4 (Fig. 3C). When the PCI-30 cell line was incubated with LY294002, phosphorylation of Akt was blocked (Supplementary Fig. S2A) and this corresponded to the partial abrogation of the protective effects of LPS from cisplatin-induced apoptosis (Supplementary Fig. S2C). The data suggest that LPS-mediated protection of TLR4+ tumor cells from drug-induced apoptosis is mediated through the phosphorylation of Akt.
LPS induces activation of NF-κB
Next, translocation of a p65 subunit of NF-κB to cell nuclei and binding activity of p65 to DNA were evaluated after stimulation of tumor cells with LPS (Fig. 3D). The baseline translocation level of p65 subunit was detected in each PCI cell line, and the translocation occurred in the similar proportion of cells: 28 ± 10%, 22 ± 8%, and 19 ± 12% (mean ± SD) for PCI-1, PCI-13, and PCI-30 cells, respectively. We observed significant increases in the percent of cells, showing the p65 subunit translocation after treatment with LPS: 78 ± 14%, 72 ± 15%, and 90 ± 9% for PCI-1, PCI-13, and PCI-30 cells, respectively (P < 0.05). The highest frequency of positive cells and the highest intensity of fluorescence were observed in PCI-30 cell line (90 ± 9% of positive cells in Fig. 4C). When the PCI-30 cell line was incubated with PDTC, an inhibitor of p65 binding to DNA (30), LPS-induced NF-κB activation was completely inhibited (Supplementary Fig. S2B), and LPS-mediated protection from cisplatin-induced apoptosis was significantly abrogated (Supplementary Fig. S2C). These results further indicate that LPS-mediated protection of TLR4+ tumor cells from apoptosis induced by cisplatin is mediated via the NF-κB pathway and binding of the p65 subunit to DNA.
Figure 4.
Silencing of TLR4 using siRNA specific for TLR4. A, TLR4 was effectively silenced for at least 8 d as confirmed by RT-PCR and Western blot assays. B, the PI3K/Akt pathway in PCI-30 cells treated with control siRNA or siRNA specific for TLR4 and LPS. C, the p65 subunit binding activity or translocation of NF-κB into the nuclei in PCI-30 cells treated with control siRNA or siRNA specific for TLR4 and with LPS. *, significant increase or decrease of p65 binding activity at P < 0.05 in tumor cells stimulated with LPS or silenced for TLR4 expression, respectively.
siRNA-mediated inhibition of TLR4 expression in tumor cell lines
The expression of TLR4 was temporarily silenced using TLR4-specific siRNA. The transfection frequency was determined in a fluorescence microscope, where at least 90% of siRNA-FITC cells were positive (Supplementary Fig. S3). The transfected cells were assessed for the successful silencing of TLR4. Using Western blots (Fig. 4A), the optimal concentration of TLR4 siRNA (8–10 μL) was determined in titration experiments until TLR4 was successfully silenced for at least 8 days. Concentrations of siRNAs higher than 15 μL were found to be toxic to the cells. All tumor cell lines temporarily silenced by siRNA lacked the expression of TLR4 at the mRNA and protein levels for at least 8 days (Fig. 4A), as tested by RT-PCR and Western blot methods on days 4, 8, and 12. The effects of gene silencing on NF-κB translocation, p65 binding activity (Fig. 4C), tumor cell proliferation (Fig. 2B), cytokine production (Fig. 5A), Akt phosphorylation (Fig. 4B), and apoptosis (Supplementary Fig. S1) were also evaluated and consistently showed that siRNA-transfected tumor cells were significantly less sensitive to LPS.
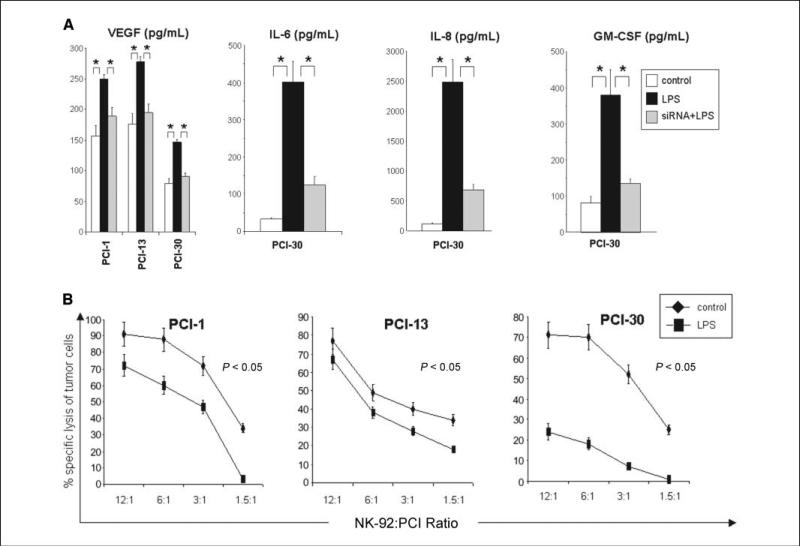
Figure 5.
Levels of cytokines in tumor cell supernatants and sensitivity of tumor to immune effector cells. A, supernatants were collected from tumor cells cultured at a density of 5 × 105/mL after 48 h of stimulation and tested for levels of various cytokines in the presence or absence of siRNA and LPS as described in Materials and Methods. B, results of 51Cr-release assays with tumor cell targets stimulated with LPS (10 μg/mL). NK-92 cells are effector cells. The data are representative from three independent experiments with each tumor cell line used as targets.
LPS-induced production of inflammatory cytokines by tumor cells
The supernatants of each cell line were analyzed for levels of inflammatory cytokines as well as growth factors after 48 hours of culture. Depending on the tumor cell line, supernatants were positive for MCP-1, RANTES, IP-10, TNF-α (data not shown), GM-CSF (detected only in supernatants from PCI-30), IL-6, IL-8, and VEGF (Fig. 5A). The levels of IL-6, IL-8, VEGF, and GM-CSF dramatically increased after stimulation with LPS (10 μg/mL) in supernatants of PCI-30, correlating with the highest expression of TLR4 and NF-κB translocation in this cell line (P < 0.05). For the other cell lines, IL-6 and IL-8 production was variable. Supernatants of all tested tumor cell lines were negative for eotaxin, MIP-1α, MIP-1β, IL-1β, IL-2, IL-4, IL-5, IL-10, IFN-γ, TGF-β1, or FGF. The cytokine production was decreased (P < 0.05) in the presence of siRNA specific for TLR4 followed by stimulation with LPS as shown in Fig. 5A.
LPS decreased sensitivity of tumor cells to immune lysis
Unstimulated tumor cells were sensitive to NK-92 cell-mediated lysis as shown in 51Cr-release assays (Fig. 5B). When tumor cells were coincubated for 12 hours with LPS, however, they became resistant to immune lysis, showing significantly decreased lytic units of activity relative to nonstimulated cells. The strongest protective effect of LPS was observed on PCI-30 cells and the weakest on PCI-13 cells.
Discussion
Current evidence indicates that TLR play a pivotal role in the activation of innate immunity against invading pathogens, cytokine production, and development of adaptive immune responses (9). In contrast to the protective role of TLR against pathogen infections, this study suggests that TLR expressed on tumor cells contribute to tumor progression. Consistent with our previous studies (17), we now confirm that HNSCC cells in situ and in culture express nearly all TLR. Using TLR4 as an example, we also show that tumors use TLR for their own benefit. Because the endogenous ligands for TLR4 in tumors are not known, we have used LPS and cisplatin to examine the effects of TLR4 activation and signaling on tumor cell growth. In addition, to prove that exogenous ligands similar to LPS exist in the tumor microenvironment and potentially can signal via TLR4, we obtained and examined tumor specimens for the presence of microbes. We found that bacteria and fungi were invariably present in HNSCC tumors. Therefore, triggering of TLR4 expressed on tumor cells by bacteria-derived products, including LPS, could be involved in promoting tumor growth and in protection of tumors from immune interference in vivo.
Most studies of TLR4 signaling are focused on immune cells and suggest that enhancement of TLR functions promotes dendritic cell maturation and thus is beneficial for the tumor-bearing host. Specifically, TLR4 expression on inflammatory cells has been reported to inhibit tumor growth (12). In contrast, TLR4 expressed on tumor cells has been shown to promote colon cancer progression through immune evasion mediated by tumor-induced inhibition of natural killer (NK) cell and effector T-cell activity (35). Ligation of TLR4 expressed on tumor cells can also induce chronic inflammation in the tumor microenvironment known to be a tumor-promoting factor (19). In our hands, triggering of TLR4 by its ligand, LPS, induced tumor promotion by the induction of proliferation, activation of NF-κB, p65 binding to DNA, and resistance to NK cell–mediated cytotoxicity accompanied by the increased production of proinflammatory cytokines (IL-6 and IL-8), VEGF, and GM-CSF. These factors have been reported to promote not only tumor progression but also the development of myeloid-derived suppressor cells (MDSC; refs. 36–41). In turn, MDSC may induce chronic inflammation and induce immune suppression through activation of regulatory T cells (42). In aggregate, it seems that activation of TLR acts as a double-edged sword, enhancing host immunity against tumor on the one hand and, on the other, promoting tumor progression.
The effects of LPS on tumor cell survival and tumor cell proliferation have been previously reported, although inconsistent results were obtained, depending on the tumor type (43–45). Our observations in HNSCC showed that LPS promotes tumor cell proliferation and induces tumor cell resistance to drug-mediated apoptosis or to NK-92 cell-mediated lysis. This resistance correlated with the NF-κB p65 subunit translocation to nucleus, MyD88 activation, and the up-regulation of IRAK-4 expression in tumor cells. Because activated NF-κB has antiapoptotic properties, high levels of its expression in tumor cells are associated with tumor progression and induction of chronic inflammation in the tumor microenvironment (46).
When the expression levels of TLR4 in situ were correlated with tumor differentiation, it seemed that poorly differentiated tumors expressed little TLR4. In contrast, TLR4 was highly expressed in well-differentiated and moderately well-differentiated tumors and in the PCI-30 cell line. Furthermore, TLR4 triggering in PCI-30 cells showed strong protective and tumor growth–promoting effects. In well-differentiated HNSCC, abundant expression of TLR4 on tumor cells may have important biological and clinical implications. These tumors usually contain bacteria and bacterial products, as shown in our patient cohort (Table 1). Thus, in vivo activation of the tumor-associated TLR4 by bacterial products in patients with well-differentiated tumors would be likely to induce abundant chronic inflammation, promote tumor growth, and protect tumor cells from apoptosis. As sensitivity to chemotherapy is dependent on tumor cell proliferation, we hypothesize that the TLR4 expression in vivo may also have considerable effect on the patients’ response to oncologic therapies. Taken together, our results showing that TLR4 is functionally active on HNSCC cells, and that TLR4 signaling modifies tumor behavior and its resistance/sensitivity to immune and drug interventions, contribute to a better understanding of the role of TLR4 in cancer progression.
Supplementary Material
Acknowledgments
Grant support: NIH grants RO-1 DE13918, RO1 CA112643, and PO-1CA109688 (T.L. Whiteside) and Polish Ministry of Science and Higher Education NN 401183333 (J. Zeromski).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
We thank Benedict Hilldorfer for technical assistance and the Center for Biologic Imaging at the University of Pittsburgh for assistance in performing confocal microscopy.
Footnotes
Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Stenzel W, Soltek S, Sanchez-Ruiz M, et al. Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am J Pathol. 2008;172:132–45. doi: 10.2353/ajpath.2008.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier A, Alter G, Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–91. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109:1574–83. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen G, Andresen L, Matthiessen MW, Rask-Madsen J, Brynskov J. Expression of Toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin Exp Immunol. 2005;141:298–306. doi: 10.1111/j.1365-2249.2005.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bouteiller O, Merck E, Hasan UA, et al. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem. 2005;280:38133–45. doi: 10.1074/jbc.M507163200. [DOI] [PubMed] [Google Scholar]
- 8.Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–62. [PubMed] [Google Scholar]
- 11.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 13.Mason KA, Ariga H, Neal R, et al. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin Cancer Res. 2005;11:361–9. [PubMed] [Google Scholar]
- 14.Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–9. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- 16.Szczepanski M, Szyfter W, Jenek R, Wrobel M, Lisewska IM, Zeromski J. Toll-like receptors 2, 3 and 4 (TLR-2, TLR-3 and TLR-4) are expressed in the microenvironment of human acquired cholesteatoma. Eur Arch Otorhinolaryngol. 2006;263:603–7. doi: 10.1007/s00405-006-0030-1. [DOI] [PubMed] [Google Scholar]
- 17.Szczepanski M, Stelmachowska M, Stryczynski L, et al. Assessment of expression of toll-like receptors 2, 3 and 4 in laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2007;264:525–30. doi: 10.1007/s00405-006-0215-7. [DOI] [PubMed] [Google Scholar]
- 18.Mozer-Lisewska I, Sluzewski W, Kaczmarek M, et al. Tissue localization of Toll-like receptors in biopsy specimens of liver from children infected with hepatitis C virus. Scand J Immunol. 2005;62:407–12. doi: 10.1111/j.1365-3083.2005.01670.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Alvero AB, Silasi DA, Mor G. Inflammation, cancer and chemoresistance: taking advantage of the toll-like receptor signaling pathway. Am J Reprod Immunol. 2007;57:93–107. doi: 10.1111/j.1600-0897.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 20.Kundu SD, Lee C, Billips BK, et al. The toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate. 2008;68:223–9. doi: 10.1002/pros.20710. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Rayburn ER, Wang W, Kandimalla ER, Agrawal S, Zhang R. Chemotherapy and chemosensitization of non-small cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9. Mol Cancer Ther. 2006;5:1585–92. doi: 10.1158/1535-7163.MCT-06-0094. [DOI] [PubMed] [Google Scholar]
- 22.Heo DS, Snyderman C, Gollin SM, et al. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 1989;49:5167–75. [PubMed] [Google Scholar]
- 23.Lin CJ, Grandis JR, Carey TE, et al. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007;29:163–88. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 24.Meissner M, Reichert TE, Kunkel M, et al. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–60. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 25.Lauzon NM, Mian F, MacKenzie R, Ashkar AA. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol. 2006;241:102–12. doi: 10.1016/j.cellimm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki S, Kobayashi M, Suzuki T, et al. γ-Interferon enhances expression of CD14/MyD88 and subsequent responsiveness to lipopolysaccharide from Actinobacillus actinomycetemcomitans in human gingival fibroblasts. J Periodontal Res. 2004;39:333–43. doi: 10.1111/j.1600-0765.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- 27.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–73. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- 28.Gagnon V, Van Themsche C, Turner S, Leblanc V, Asselin E. Akt and XIAP regulate the sensitivity of human uterine cancer cells to cisplatin, doxorubicin and taxol. Apoptosis. 2008;13:259–71. doi: 10.1007/s10495-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 29.Bayry J, Lacroix-Desmazes S, Donkova-Petrini V, et al. Natural antibodies sustain differentiation and maturation of human dendritic cells. Proc Natl Acad Sci U S A. 2004;101:14210–5. doi: 10.1073/pnas.0402183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q, Zheng Y, Liu Q, Cao X. Rapamycin reverses TLR4 signaling-triggered tumor apoptosis resistance by disrupting Akt-mediated Bcl-xL upregulation. Int Immunopharmacol. 2008;8:1854–8. doi: 10.1016/j.intimp.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Saito T, Dworacki G, Gooding W, Lotze MT, Whiteside TL. Spontaneous apoptosis of CD8+ T lymphocytes in peripheral blood of patients with advanced melanoma. Clin Cancer Res. 2000;6:1351–64. [PubMed] [Google Scholar]
- 32.Kim GG, Donnenberg VS, Donnenberg AD, Gooding W, Whiteside TL. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. J Immunol Methods. 2007;325:51–66. doi: 10.1016/j.jim.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskan MA, Rose BG, Benakanakere MR, Lee MJ, Kinane DF. Sphingosine 1-phosphate 1 and TLR4 mediate IFN-β expression in human gingival epithelial cells. J Immunol. 2008;180:1818–25. doi: 10.4049/jimmunol.180.3.1818. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Barrett RP, McClellan SA, Hazlett LD. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2005;46:4209–16. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- 35.Huang B, Zhao J, Li H, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–14. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 36.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–73. doi: 10.1016/S1470-2045(01)00556-3. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother. 2004;53:543–50. doi: 10.1007/s00262-003-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdollahi T, Robertson NM, Abdollahi A, Litwack G. Identification of interleukin 8 as an inhibitor of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in the ovarian carcinoma cell line OVCAR3. Cancer Res. 2003;63:4521–6. [PubMed] [Google Scholar]
- 39.Nakashima J, Tachibana M, Horiguchi Y, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–6. [PubMed] [Google Scholar]
- 40.Vredevoe DL, Widawski M, Fonarow GC, Hamilton M, Martinez-Maza O, Gage JR. Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure. Am J Cardiol. 2004;93:1007–11. doi: 10.1016/j.amjcard.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 41.Bronte V, Chappell DB, Apolloni E, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 42.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JH, Manning BJ, Wu QD, Blankson S, Bouchier-Hayes D, Redmond HP. Endotoxin/lipopolysaccharide activates NF-κB and enhances tumor cell adhesion and invasion through a β1 integrin-dependent mechanism. J Immunol. 2003;170:795–804. doi: 10.4049/jimmunol.170.2.795. [DOI] [PubMed] [Google Scholar]
- 44.He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44:2850–9. doi: 10.1016/j.molimm.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 45.Tichomirowa M, Theodoropoulou M, Lohrer P, et al. Bacterial endotoxin (lipopolysaccharide) stimulates interleukin-6 production and inhibits growth of pituitary tumour cells expressing the toll-like receptor 4. J Neuroendocrinol. 2005;17:152–60. doi: 10.1111/j.1365-2826.2005.01286.x. [DOI] [PubMed] [Google Scholar]
- 46.Pikarsky E, Porat RM, Stein I, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.