Abstract
The central melanocortin system has been implicated in emotional stress-induced anxiety, anorexia and activation of the hypothalamo-pituitary-adrenal (HPA) axis. However, the underlying neural substrates have not been identified. The medial amygdala (MeA) is highly sensitive to emotional stress and expresses high levels of the melanocortin-4 receptor (MC4R). This study investigated the effects of activation and blockade of MC4R in the MeA on anxiety-like behaviour, food intake and corticosterone secretion. We demonstrate that MC4R-expressing neurons in the MeA were activated by acute restraint stress, as indicated by induction of c-fos mRNA expression. Infusion of a selective MC4R agonist into the MeA elicited anxiogenic-like effects in the elevated plus-maze test and decreased food intake. In contrast, local MeA infusion of SHU 9119, a MC4R antagonist, blocked restraint stress-induced anxiogenic and anorectic effects. Moreover, plasma corticosterone levels were increased by intra-MeA infusion of the MC4R agonist under non-stressed conditions and restraint stress-induced elevation of plasma corticosterone levels was attenuated by pretreatment with SHU 9119 in the MeA. Thus, stimulating MC4R in the MeA induces stress-like anxiogenic and anorectic effects as well as activation of the HPA axis, whereas antagonizing MC4R in this region blocks such effects induced by restraint stress. Together, our results implicate MC4R signalling in the MeA in behavioural and endocrine responses to stress.
Keywords: Anorexia, anxiety, corticosterone, medial amygdala, melanocortin-4 receptor, restraint stress
Introduction
The central melanocortin system, which consists of the endogenous melanocortin agonist, α-melanocyte-stimulating hormone (α-MSH) derived from proopiomelanocortin (POMC), the endogenous inverse agonist, agouti-related protein and the melanocortin-4 receptor (MC4R), has been well recognized for its function in the control of eating behaviour (Barsh & Schwartz, 2002; Cone, 2005; Farooqi & O'Rahilly, 2004). Accumulating evidence suggests a functional interaction between the central melanocortin system and the stress system. First, POMC neurons in the arcuate nucleus are rapidly activated by acute emotional stress (Liu et al. 2007). Second, levels of POMC mRNA and its derivative α-MSH increase after exposure to stress (Baubet et al. 1994; Harbuz & Lightman, 1989; Khorram et al. 1985; Sumpter et al. 1986; Yamano et al. 2004). Third, intracerebroventricular (i.c.v.) injection of melanocortin receptor agonists, particularly those selective for MC4R, induces stress-like endocrine and behavioural responses, such as activation of the hypothalamo-pituitary-adrenal (HPA) axis, increased anxiety and reduced food intake (de Barioglio et al. 1991; Gonzalez et al. 1996; Klenerova et al. 2008; Lu et al. 2003; Rao et al. 2003). Finally, i.c.v. injection of MC4R antagonists blocks stress-induced anxiogenic and anorectic effects (Chaki et al. 2003; Kokare et al. 2010; Liu et al. 2007; Vergoni et al. 1999). These findings suggest that activation of the central melanocortin system is involved in endocrine and behavioural responses to stress. However, the neural substrates involved remain to be elucidated.
The amygdala is one candidate brain area that may convey hyper-melanocortinergic tone during stress exposure. This structure is essential for the processing of emotions, including fear and anxiety (Aggleton, 1993; Davis, 1992; LeDoux, 2000). It consists of a group of anatomically and functionally distinct nuclei. Although various sub-regions of the amygdala have been reported to take part in stress responses, the medial amygdala (MeA) is highly sensitive to stressors with apredominantly emotional component, including restraint (Arnold et al. 1992; Cullinan et al. 1995; Dayas et al. 1999, 2001; Windle et al. 2004), immobilization (Imaki et al. 1993; Ma & Morilak, 2004; Roske et al. 2002), forced swim (Cullinan et al. 1995; Dayas et al. 2001), social defeat (Chung et al. 1999; Nikulina et al. 2004) and inescapable foot shock (Rosen et al. 1998). The MeA serves as one component of stress excitatory circuits (Dunn & Whitener, 1986; Matheson et al. 1971; Redgate & Fahringer, 1973). Direct stimulation of MeA increases corticosterone levels (Dunn & Whitener, 1986; Feldman et al. 1990), whereas lesions of the MeA attenuate the HPA axis response to stress (Dayas et al. 1999; Feldman et al. 1994). α-MSH/POMC projections within the amygdala arise from the arcuate nucleus and are particularly abundant in the MeA (Bagnol et al. 1999; O'Donohue et al. 1979 Watson et al. 1978b). The MeA also expresses high levels of MC4R (Kishi et al. 2003; Lu, 2001; Mountjoy et al. 1994). These findings led us to investigate whether MC4R-expressing neurons in the MeA are responsive to acute emotional stress and whether activation or blockade of MC4R in this region modulates emotional and feeding behaviours as well as activity of the HPA axis under basal and stress conditions. Because acute restraint stress activates melanocortinergic neurons (Liu et al. 2007) and induces anxiety and anorexia and activates the HPA axis (Chaki et al. 2003; Dayas et al. 1999; Rybkin et al. 1997; Thorsell et al. 1999), this stressor was used in this study to induce behavioural and endocrine changes.
Method
Animals
Adult male Sprague–Dawley rats (Charles River Laboratories Inc., USA), weighing 250–300 g, were housed in groups of two under conditions of constant temperature and humidity on a 12-h light/dark cycle (lights on 07:00 hours). Food and water were available ad libitum. Animals were allowed to acclimate to these housing conditions for 1 wk before experiments began. All animal procedures were conducted in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Surgery
Rats were anaesthetized with a cocktail (43 mg/kg ketamine, 9 mg/kg xylazine and 1.4 mg/kg acepromazine in saline) and fixed in a stereotaxic apparatus for cannula implantation. Using the stereotaxic coordinates, −2.6 mm posterior to Bregma, ±3.3 mm lateral to midline and −8.0 mm ventral to skull, a permanent 22-gauge stainless steel guide cannula (Plastics One, USA) was implanted into the MeA. The guide cannula was secured in place using dental cement and three stainless steel mounting screws anchored to the skull. The guide cannula was 1 mm above the MeA to minimize tissue damage. A stainless-steel dummy cannula, extending 0.5 mm beyond the guide, was used to seal the guide cannula when not in use. After surgery, the animals were housed individually to avoid damage to guide and dummy cannulae.
In order to collect blood samples, the jugular vein catheterization procedure was performed. At 4 d after cannulation, a catheter was introduced into the jugular vein of deeply anaesthetized rats and sutured to the surrounding muscle to anchor it. After implantation into the vein, the catheter was tunnelled subcutaneously to exit the skin on the back of the rats. The catheter was filled with a sterile 50 U/ml heparin saline solution and plugged with a gold wire. Heparin saline flush solution was used to maintain patency of the indwelling intravenous (i.v.) catheters in blood sampling.
Microinjection
Animals were allowed to recover for 7 d after intra-MeA cannulation. During this period, they were handled daily to minimize stress caused by the microinjection procedure. Melanocortin 3/4 receptor antagonist SHU 9119 (Peninsula Laboratories, USA) and the highly selective MC4R agonist Cyclo (β-Ala-His-D-Phe-Arg-Trp-Glu)-NH2 (Bednarek et al. 2001) (Phoenix Pharmaceuticals, USA) were freshly dissolved in artificial cerebrospinal fluid (aCSF) before use. All intra-MeA microinjections were performed on conscious, unrestrained, freely moving rats in their home cages. Injections were performed over 1 min using a 28-gauge stainless-steel injector connected to a 5-μl syringe (Hamilton Company, USA), which was operated by an infusion pump. The injector was inserted and extended 1 mm beyond the tip of the guide cannula. Drug solution or vehicle was infused in a volume of 0.5 μl delivered over 1 min. An additional minute was allowed for diffusion and prevention of backflow through the needle track before the injector was withdrawn.
Experimental protocols
Animals were randomly assigned to either control or acute restraint stress groups. The stress procedure was performed in a separate procedure room. On the experimental day, animals were first moved to the procedure room for 2 h without any disturbance. All acute stress and microinjections were performed in the late light cycle.
Induction of c-fos mRNA in MC4R-expressing neurons in the MeA by acute restraint stress
For the exposure to acute restraint stress, rats were confined in white flexible plastic wrappers (19×25 cm) enclosed in a 20.5×7.5×7.5-cm clear Plexiglas open frame for 30 min. Holes at both ends of the open frame allowed free air circulation. At the end of restraint stress, animals were immediately killed by decapitation. The unstressed control animals were kept in the home cage without disturbance until decapitated. Brains were removed and quickly frozen in an isopentane-dry ice bath adjusted to −35 °C. Brain sections (20 μm) were cut on a Leica cryostat (Leica Microsystems GmbH, Germany) through the amygdala, thaw mounted onto polylysine-coated slides and stored at −80 °C until processing for double-labelling fluorescence in situ hybridization to detect the co-localization of c-fos mRNA and MC4R mRNA in the MeA.
Effects of activation of MC4R in the MeA on food intake and anxiety-like behaviour
To examine the effect of activation of MC4R in the MeA on anxiety-like behaviour, the MC4R agonist (0, 0.1 and 1.0 nmol) was directly infused into the MeA 1 h before the elevated plus-maze test. The elevated plus-maze was made of black acrylic, with four arms (45-cm long and 12-cm wide) arranged in the shape of a ‘plus’ sign and elevated to a height of 70 cm above the floor. Two arms opposite each other have no side or end walls (open arms) and the other two arms have side walls and end walls (45-cm high) but are open on top (closed arms). A central 12×12 cm square platform provides access to all arms. Rats were placed in the centre square facing the corner between a closed arm and an open arm and were allowed to explore the elevated plus-maze for 5 min. Their activity on the elevated plus-maze was recorded by a digital CCD camera and analysed using an EthoVision video tracking system (Noldus Information Technology Inc., USA). After each test, the maze was thoroughly cleaned with 20% alcohol to eliminate the odour and trace of the previously tested animal. The time spent on the open and closed arms and the number of entries made into each arm were measured. Entry was defined as all four paws being positioned within one arm. The degree of anxiety was assessed by calculating the percentage of open arm entries (entries into the open arms/total entries into all arms) and percentage of open arm time (time spent in the open arms/total time spent in all arms).
To investigate the effect of activation of MC4R in the MeA on food intake, rats were weighed and counterbalanced into different treatment groups prior to the experiment. The MC4R agonist (0, 0.1 and 1.0 nmol) was directly infused into the MeA 1 h before the dark cycle (18:00 hours). A pre-weighed chow hopper was placed in the home cage of each rat at the onset of the dark cycle (19:00 hours). Food intake was measured by weighing the remaining pellets and the spillage for 30 min, 120 min and 12 h. A red light was provided during the measurement of food consumption in the dark cycle. To minimize disruption of food accessibility, two sets of containers were used to provide pre-weighed food to each animal. Food intake was calculated by subtracting the weight of remaining food from the initial weight.
Effects of blockade of MC4R in the MeA on restraint stress-induced anxiety and anorexia
To determine the effects of blockade of MC4R in MeA on stress-induced anxiety-like behaviour, rats received an intra-MeA microinjection of a MC4R antagonist, SHU 9119 (0, 0.5 and 1 nmol). After intra-MeA injection (30 min later), rats were subjected to either no stress (control) or 30-min restraint stress. Rats were tested in the elevated plus-maze 30 min after the onset of restraint exposure. The elevated plus-maze test was performed as described above and elsewhere (Liu et al. 2007).
To examine the effect of blockade of MC4R in the MeA on stress-induced anorexia, rats were weighed and counterbalanced into different treatment groups prior to the experiment. The SHU 9119 (0, 0.5 and 1 nmol) was infused into the MeA 1 h before the dark cycle (18:00 hours). Animals were subjected to either no stress or 30-min restraint stress at 30 min after intra-MeA injection (18:30 hours). A pre-weighed chow hopper was placed in the home cage of each rat at the onset of the dark cycle (19:00 hours). Food intake was measured by weighing the remaining pellets and the spillage for 30 min, 120 min and 12 h, as described above.
Effects of activation or blockade of MC4R in the MeA on restraint stress-induced stimulation of the HPA axis
To investigate the effect of MC4R signalling in the MeA on HPA axis activity, the experiments were conducted in the early light cycle when basal corticosterone levels are low and the HPA axis is highly sensitive to stress and pharmacological stimulation (Dallman et al. 1994). To determine the effect of the MC4R agonist on corticosterone levels in the plasma, a baseline blood sample was drawn through the indwelling jugular vein catheter prior to intra-MeA injection of the MC4R agonist (1.0 nmol) and vehicle. Following microinjection, blood samples were collected every 15 min for 60 min.
To examine the effect of blockade of MC4R on corticosterone levels under basal and stressed conditions, rats received an intra-MeA injection of SHU 9119 (0.1 and 1.0 nmol) or vehicle after a baseline blood sample was collected. Fifteen minutes after the intra-MeA injection, animals were subjected to non-stressed control condition or restraint stress for 30 min. Blood samples were taken through the jugular vein catheter at 0, 15, 30, 45 and 60 min after microinjection. Animals were released from the restrainer at the end of 30-min restraint stress.
Histological verification of cannula placement
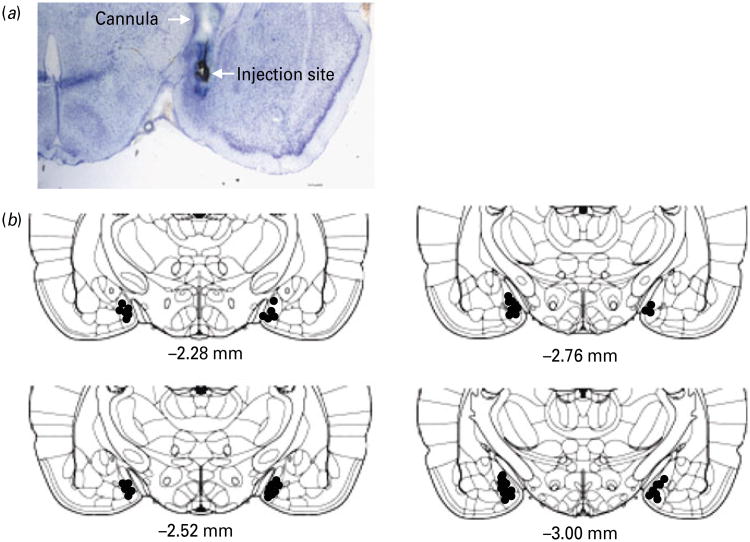
Histological verification of the intra-MeA cannula was performed at the end of the experiments. Rats were injected with 0.5 μl India ink via an injector under anaesthesia and decapitated. Brains were then removed and frozen in a dry ice-isopentane bath (−35 °C). Brain sections at 20 μm were cut in the coronal plane with a cryostat. The sections were mounted on poly-lysine-coated slides and stained with Toluidine Blue. The cannula placement was determined according to the rat brain atlas (Paxinos & Watson, 1998) (Fig. 8).
Fig. 8.
(a) Representative image showing dye diffusion at the injection site; (b) schematic drawings of coronal brain sections through the amygdala showing placements of microinjection sites in the medial amygdala. The drawings of coronal sections were derived from the atlas of Paxinos & Watson (1998).
Determination of corticosterone concentrations in plasma
Plasma corticosterone levels were determined using a highly specific corticosterone antibody (Chemicon International, USA). Briefly, 10 μl duplicate samples of plasma were heated at 70 °C for 30 min to denature corticosterone-binding protein and incubated overnight with corticosterone antibody and [3H]corticosterone (PerkinElmer, USA). Free and bound corticosterone were separated by incubation with charcoal for 15 min.
Double-labelling fluorescence in situ hybridization
To examine the co-localization of c-fos mRNA with MC4R mRNA in the MeA, double-labelling fluorescence in situ hybridization was performed. Antisense and sense cRNA probes for c-fos mRNA and MC4R mRNA were labelled by fluorescein-12-UTP or digoxigenin-11-UTP (Roche Diagnostics, USA) using a standard transcription method. Brain sections were hybridized with a mixture of c-fos and MC4R cRNA probes for 18 h at 55 °C. The following day, brain sections were washed with sodium citrate buffer (SSC) and treated with RNase a (200 μg/ml) for 1 h at 37 °C. After the final wash in 0.1×SSC at 68 °C for 1 h, sections were then transferred to the 0.05 M phosphate-buffered saline (PBS) and processed to visualize fluorescein or digoxigenin-labelled mRNA using the TSA Plus Fluorescence System kit (PerkinElmer). Briefly, brain sections were incubated in 2% H2O2 in PBS for 30 min. After rinsing in Tris buffered saline, the sections were treated with a blocking buffer (PerkinElmer) for 1 h and then incubated with an anti-fluorescein antibody conjugated to horseradish peroxidase (HRP; Roche Applied Science) for 1 h. After three washes, the sections were incubated with the fluorescein tyramide amplification reagent (PerkinElmer) for 15 min to reveal c-fos signalling. To visualize MC4R mRNA, the sections were incubated in 2% H2O2 for 30 min, followed by sheep anti-digoxigenin antibody (Roche Diagnostics) overnight. After three washes, the sections were then incubated with anti-sheep antibody conjugated to HRP (Sigma, USA). The sections were washed and incubated with the cyanine 3 tyramide amplification reagent (PerkinElmer) for 15 min for visualization of MC4R mRNA. After rinsing, the slides were coverslipped using ProLong® Gold antifade reagent (Invitrogen, USA). The MeA neurons that were positive for c-fos mRNA and MC4R mRNA were identified with an Olympus BX52 fluorescent microscope (Olympus Corporation, Japan).
Data analysis
Results are expressed as means±S.E.M. Statistical analyses were performed by using one-way analysis of variance (ANOVA) on the elevated plus-maze test, one-way ANOVA with repeated measures on food intake and corticosterone secretion and two-way ANOVA with repeated measures on the effects of SHU 9119 and restraint stress on corticosterone levels, followed by a post-hoc Bonferroni/Dunn or Tukey/Kramer (for unequal n) test.
Results
Stress-induced c-fos mRNA expression in MC4R-expressing neurons in the MeA
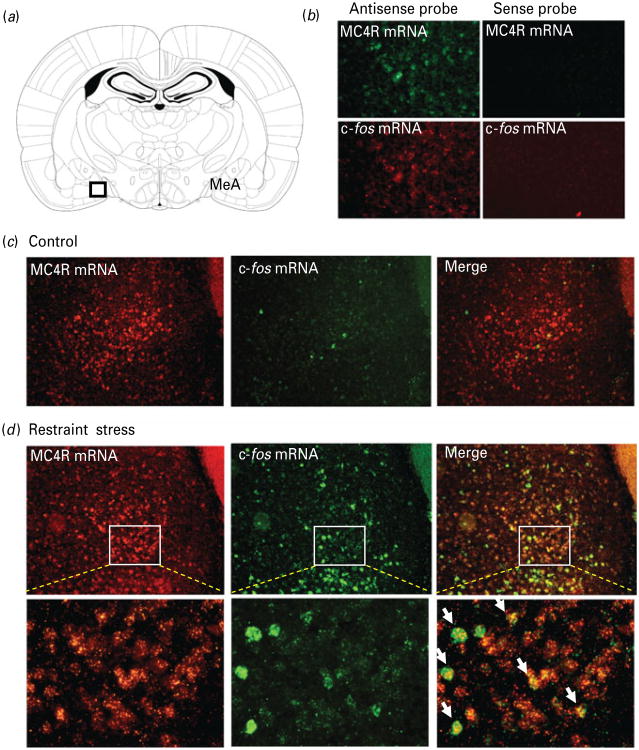
Within the amygdala, MC4R mRNA displayed moderate expression in the MeA with a high-density of signal in the anteroventral MeA (Kishi et al. 2003; Lu, 2001). c-fos is widely used as a marker of neuronal activation in response to stress. To determine if restraint stress activates MC4R-expressing neurons in the MeA, double-labelling fluorescent in situ hybridization was used to reveal the co-localization of c-fos mRNA and MC4R mRNA in the MeA. As shown in Fig. 1, while non-stressed control rats exhibited negligible c-fos mRNA expression in MeA, 30-min restraint stress induced robust c-fos mRNA expression, suggesting that the MeA is highly sensitive to emotional stress. This is consistent with previous findings reported by us and others (Cullinan et al. 1995; Dayas et al. 2001; Liu et al. 2007). Moreover, we found that a majority (>60%) of c-fos mRNA expressing neurons were also positive for MC4R mRNA (Fig. 1c). No signal was detected using sense probes for c-fos and MC4R mRNA (Fig. 1b). These results suggest that MC4R-expressing neurons in the MeA are responsive to stress.
Fig. 1. Acute restraint stress activates melanocortin-4 receptor (MC4R)-expressing neurons in the medial amygdala.
Double-labelling fluorescent in situ hybridization showing the co-localization of c-fos mRNA (green) with MC4R mRNA (red) in the medial amygdala (MeA). (a) Schematic diagram of coronal brain section through the amygdala (Paxinos & Watson, 1998); (b) double-labelling fluorescent in situ hybridization using sense and anti-sense cRNA probes to detect expression of MC4R and c-fos mRNA in rat MeA; (c) representative images showing c-fos mRNA and MC4R mRNA expression in the MeA from a naive rat; (d) representative images showing the co-localization of c-fos mRNA and MC4R mRNA expression in the MeA from a rat exposed to 30-min restraint stress. The white arrowheads indicate cells double-labelled for c-fos mRNA and MC4R mRNA.
Effects of activation of MC4R in the MeA on anxiety levels and food intake in non-stressed rats
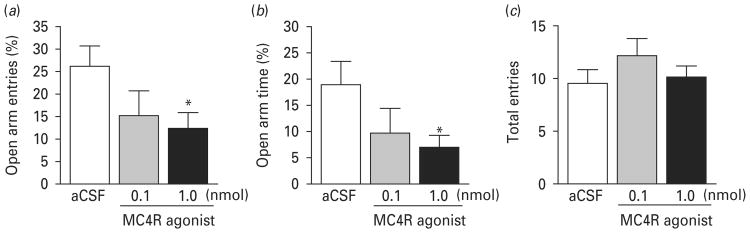
The effects of activation of MC4R in the MeA by local infusion of the MC4R agonist on anxiety-like behaviour were evaluated in the elevated plus-maze test. This test has been widely used to evaluate anxiety levels in rodents and is sensitive to anxiolytic and anxiogenic drugs (Griebel et al. 1996; Pellow et al. 1985). The percentage of open arm entries and time spent in the open arms in the elevated plus-maze test has been validated as measures of anxiety (Pellow et al. 1985). Rats received an intra-MeA microinjection of the MC4R agonist (0, 0.1, or 1 nmol) 1 h before testing in the elevated plus-maze. ANOVA revealed a significant main effect of drug treatment. The MC4R agonist decreased the percentage of open arm entries (F2,21 =5.508, p < 0.05) and the percentage of time spent in the open arms (F2,21 =4.157, p<0.05), but had no significant effect on total arm entries (F2,21 = 0.816, p = 0.46). Post-hoc analysis indicated that MC4R agonist at the dose of 1.0 nmol significantly reduced the percentage of open arm entries and the time spent in the open arm compared to vehicle treatment (p<0.05) (Fig. 2a, b).
Fig. 2.
Activation of melanocortin-4 receptor (MC4R) in the medial amygdala induces anxiogenic-like effects. Rats received intra-MeA microinjection of the MC4R agonist (0, 0.1 or 1 nmol) 1 h before the elevated plus-maze test. The number of entries made into the open arms and closed arms and time spent on the open arms and closed arms were measured. (a) Percentage of open arm entries = number of entries made onto open arms/total number of entries made onto both open and closed arms ×100; (b) percentage of open arm time = time spent on open arms/total time spent on both open and closed arms ×100; (c) total number of entries made onto both open and closed arms. Vehicle [artificial cerebrospinal fluid (aCSF)] treatment, n =11; 0.1 nmol MC4R agonist, n=6; 1.0 nmol MC4R agonist, n=7. Data are expressed as means±S.E.M. * p<0.05 compared to aCSF controls.
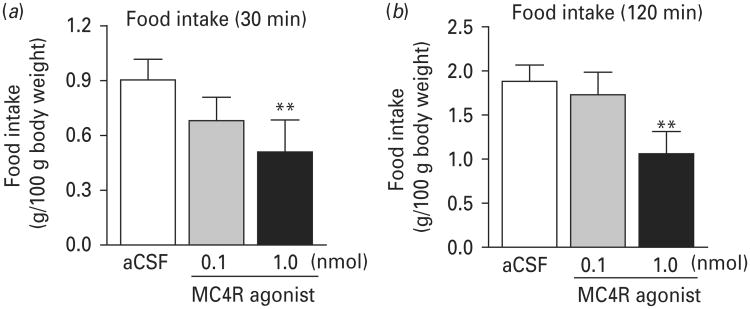
The effects of activation of MC4R in MeA on spontaneous food intake were determined in non-fasted rats after intra-MeA infusion of two doses (0.1 and 1.0 nmol) of the MC4R agonist. Intra-MeA infusion was performed 1 h before the dark cycle. Food was provided at the onset of the dark cycle and food intake was measured 30 min, 120 min and 12 h after food was provided. One-way ANOVA with repeated measures revealed a main effect of treatment (F2,18=5.983, p<0.01). Post-hoc analysis showed that MC4R agonist at the dose of 1.0 nmol significantly decreased spontaneous food intake within 30 and 120 min after food was provided when compared to the vehicle-treated group (p<0.01 for both time points) (Fig. 3). The lower dose of the MC4R agonist (0.1 nmol) did not significantly affect food intake measured at either 30 or 120 min (Fig. 3). There was no difference in 12 h food intake between three treatment groups (aCSF: 6.83±0.45 g; 0.1 nmol MC4R agonist: 6.59±0.42 g; 1.0 nmol MC4R agonist: 7.14±0.30 g; food intake was adjusted by 100 g body weight).
Fig. 3.
Activation of melanocortin-4 receptor (MC4R) in the medial amygdala induces anorectic effects. The MC4R agonist (0, 0.1 or 1 nmol) was infused into the medial amygdala 1 h before the dark cycle. Food was provided at the onset of dark cycle. Food consumption was measured 30 min (a) and 120 min (b) after the onset of dark cycle. Data are expressed as mean±S.E.M. Vehicle [artificial cerebrospinal fluid (aCSF)] treatment, n=8; 0.1 nmol MC4R agonist, n=6; 1.0 nmol MC4R agonist, n=7. **p<0.01 compared to aCSF controls.
Effects of blockade of MC4R in the MeA on acute stress-induced anxiety-like behaviour and anorexia
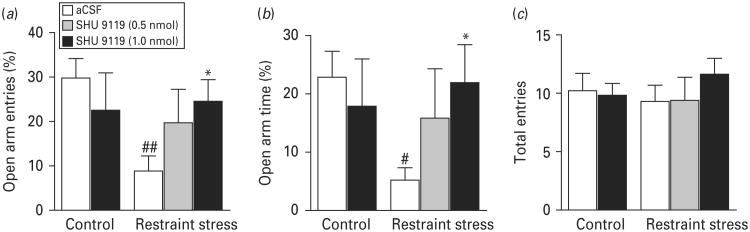
Acute restraint stress induces anxiogenic effects (Ebner et al. 2004; Liu et al. 2007 Moller et al. 1997; Thorsell et al. 1999). To determine whether activation of MC4R contributes to restraint stress-induced anxiety-like behaviour, rats received an intra-MeA infusion of the MC4R antagonist SHU 9119 (0, 0.5 or 1.0 nmol), followed by exposure to 30-min restraint stress. Animals were tested in the elevated plus-maze right after restraint stress. ANOVA revealed significant effects of treatment on the percentage of open arm entries (F4,36=2.523, p=0.05) and an approaching significant effect of time spent on the open arms (F4,36=1.802, p=0.10) but not on total arm entries (F4,36=0.658, p=0.63). Post-hoc analysis showed that intra-MeA injection of 1 nmol SHU 9119 significantly reversed the anxiogenic effect of restraint stress measured on the elevated-plus maze (Fig. 4, p<0.05).
Fig. 4.
Blockade of melanocortin-4 receptor (MC4R) in the medial amygdala attenuates restraint stress-induced anxiogenic effects. SHU 9110 (0, 0.5 or 1.0 nmol) was infused into the medial amygdala 30 min prior to exposure to restraint stress. The elevated plus-maze test was performed following 30-min restraint stress. (a) Percentage of open arm entries = number of entries made onto open arms/total number of entries made onto both open and closed arms × 100; (b) percentage of open arm time = time spent on open arms/total time spent on both open and closed arms × 100; (c) total number of entries made onto both open and closed arms. Vehicle treatment without stress, n=9; 1.0 nmol SHU 9110 without stress, n=6; vehicle treatment with stress, n=10. 0.5 nmol SHU 9110 with stress, n=5; 1.0 nmol SHU 9110 with stress, n=11. Data are expressed as means ± S.E.M. # p<0.05, ## p<0.01 compared to vehicle-treated controls without stress. * p<0.05 compared to vehicle-treated controls with stress.
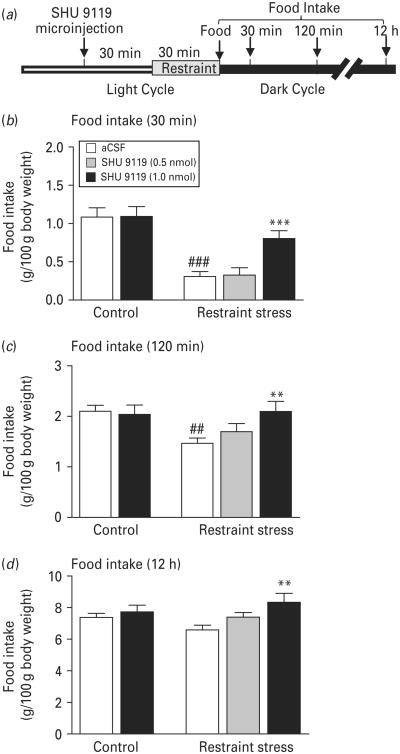
We and others have previously shown that acute restraint stress results in short-term anorexia (Chaki et al. 2003; Liu et al. 2007; Rybkin et al. 1997). To examine the role of MC4R signalling in the MeA in stress-induced anorexia, the MC4R antagonist SHU 9119 (0, 0.5 or 1.0 nmol) was infused into the MeA 1 h before the onset of the dark cycle, followed by exposure to 30-min restraint stress in non-fasted rats. Food was provided at the onset of the dark cycle. ANOVA with repeated measures revealed a significant main effect of treatment on spontaneous food intake (F4,96=5.779, p<0.01). Food intake was significantly reduced at 30 min (Fig. 5a, p<0.001) and 120 min (Fig. 5b, p<0.01) after restraint stress. The cumulative food intake at 12 h after exposure to restraint stress exhibited no difference from the non-stressed control condition (Fig. 5c, p=0.12). The intra-MeA injection of 1 nmol SHU 9119 alone had no significant effect on food intake (Fig. 5), but reversed restraint stress-induced reduction of food intake at 30 and 20 min after stress exposure (30-min food intake: p<0.001; 120-min food intake: p<0.01). The lower dose of SHU 9110 (0.5 nmol) did not elicit a significant effect on stress-induced suppression of food intake at any time point measured (Fig. 5).
Fig. 5.
Blockade of melanocortin-4 receptor (MC4R) in the medial amygdala attenuates restraint stress-induced anorectic effects. (a) Timeline of the experimental procedure. SHU 9110 (0, 0.5 or 1.0 nmol) was infused into the medial amygdala 1 h before the dark cycle. Restraint stress was applied to animals 30 min after microinjection. Food was provided at the onset of dark cycle. Food consumption was measured 30 min (b), 120 min (c) and 12 h (d) after food was provided. Vehicle treatment without stress, n=12; 1.0 nmol SHU 9110 without stress, n=10; vehicle treatment with stress, n=14; 0.5 nmol SHU 9110 with stress, n=6; 1.0 nmol SHU 9110 with stress, n=11. Data are expressed as means±S.E.M. ## p<0.01, ### p<0.001 compared to vehicle-treated, non-stressed controls. ** p<0.01, *** p<0.001 compared to vehicle-treated controls with the same stress exposure.
Effects of activation of MC4R in the MeA on plasma corticosterone levels
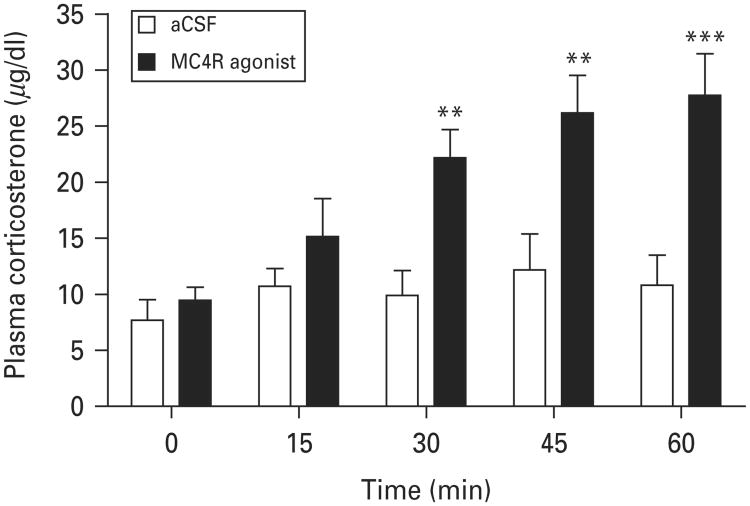
To determine the effects of activation of MC4R in the MeA on the HPA axis, we measured plasma corticosterone levels in response to an intra-MeA injection of MC4R agonist. Blood samples were taken through an indwelling jugular vein catheter immediately before the intra-MeA injection (baseline) and at 15, 30, 45 and 60 min after the intra-MeA injection of 1.0 nmol MC4R agonist. ANOVA analysis with repeated measures revealed a significant effect of treatment on plasma corticosterone levels (F1,52=23.556; p<0.001). Post-hoc analyses indicated that MC4R agonist at the dose of 1.0 nmol significantly increased plasma corticosterone levels at 15, 30, 45 and 60 min post-injection compared to vehicle treatment (Fig. 6). These results indicate that activation of MC4R in the MeA is sufficient to stimulate HPA activity under non-stressed conditions.
Fig. 6.
Effects of activation of melanocortin-4 receptor (MC4R) in the medial amygdala on the time course of plasma corticosterone levels. Blood samples were collected from the jugular vein before (baseline) and after infusion of the MC4R agonist (1.0 nmol) or vehicle [artificial cerebrospinal fluid (aCSF)] into the medial amygdalda at 15-min intervals. Vehicle, n=8; MC4R agonist, n=7. ** p<0.01, *** p<0.001 compared to vehicle controls.
Effects of blockade of MC4R in the MeA on restraint stress-induced elevation of corticosterone levels
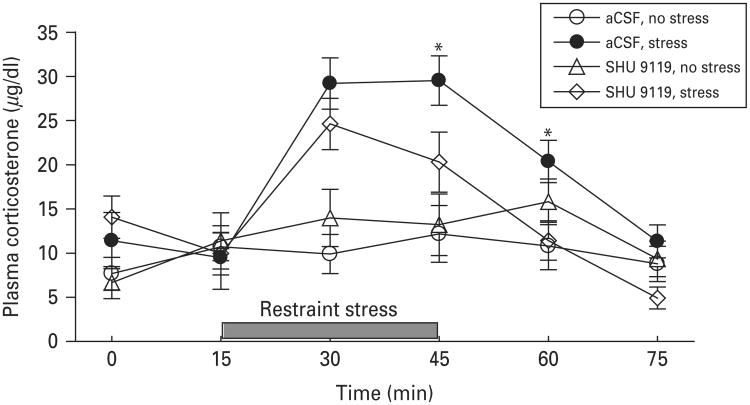
To examine the role of MC4R signalling in the MeA in stress-induced stimulation of the HPA axis, rats were pretreated with SHU 9119 (1.0 nmol) through the implanted intra-MeA cannula, followed by 30-min restraint stress. Blood samples were taken through the jugular vein catheter at 0, 15, 30, 45, 60 and 75 min after the intra-MeA microinjection. Two-way ANOVA with repeated measures revealed a main effect of stress (F1,92=8.216; p<0.01) but not treatment (F1,92=0.816; p<0.38). There was a significant interaction between stress and treatment (F1,92=4.357; p<0.05). Corticosterone levels rapidly increased after the onset of restraint stress and declined to basal levels after the termination of restraint stress. SHU 9119 treatment alone had no effect on plasma corticosterone levels, but significantly attenuated restraint stress-induced corticosterone secretion (F1,68=6.316, p<0.01). Post-hoc analysis indicated that SHU 9119 (1.0 nmol) decreased restraint stress-induced corticosterone secretion at 30 min after the onset of restraint stress (p<0.01) and 15 min after the termination of restraint stress (p<0.05) (Fig. 7). These findings suggest that MC4R signalling in the MeA is involved in stress-induced HPA activation.
Fig. 7.
Effects of blockade of melanocortin-4 receptor (MC4R) in the medial amygdala on the time course of restraint stress-induced increase in plasma corticosterone levels. Blood samples were collected from the jugular vein before and after infusion of SHU 9110 or vehicle into the medial amygdala. Rats were subjected to 30-min restraint stress and blood samples were collected immediately before and at 15 min intervals after the initiation of restraint stress. Animals that received microinjection of vehicle or SHU 9110 (1.0 nmol) into the medial amygdala without exposure to restraint stress served as non-stressed controls. Vehicle treatment without stress, n=8; 1.0 nmol SHU 9110 without stress, n=4; vehicle treatment with stress, n=7; 1.0 nmol SHU 9110 with stress, n=9. Data are expressed as mean±S.E.M. * p<0.05 compared to SHU 9110 treatment with restraint stress.
Discussion
The present study demonstrated that MC4R-expressing neurons in MeA are activated by acute restraint stress, accompanied by anxiety-like behaviour, reduction of food intake and stimulation of the HPA axis. Infusion of a MC4R agonist to the MeA induces stress-like anxiogenic and anorectic effects and elevation of plasma corticosterone levels. Blockade of MC4R in this brain region attenuated behavioural and endocrine responses to restraint stress. These results provide evidence that the MeA is an important neural substrate involved in mediating the effects of MC4R signalling in stress responses.
Our previous studies have shown that POMC neurons in the arcuate nucleus are activated by restraint stress (Liu et al. 2007). POMC neurons in the arcuate nucleus provide melanocortinergic input to the amygdala (Bagnol et al. 1999; O'Donohue et al. 1979 Watson et al. 1978b). Arcuate lesions almost completely eliminate α-MSH immunoreactivity in the amygdala (O'Donohue et al. 1979). Among the different amygdaloid nuclei, the MeA contains the most abundant α-MSH immunoreactive fibres (Bagnol et al. 1999; Jacobowitz & O'Donohue, 1978; O'Donohue et al. 1979 Watson et al. 1978a, b). This area also expresses high levels of MC4R (Kishi et al. 2003; Lu, 2001; Mountjoy et al. 1994). A number of studies have reported that neurons in the MeA are highly sensitive to emotional stress, including restraint stress (Arnold et al. 1992; Cullinan et al. 1995; Dayas et al. 1999, 2001; Windle et al. 2004). The present study confirmed neuronal activation in the MeA following 30-min restraint stress. Furthermore, using double-labelling fluorescent in situ hybridization, we demonstrated that restraint stress induced activation of MC4R-expressing neurons, as indicated by c-fos mRNA induction. This suggests that melanocortin target neurons in this area are one component of stress excitatory circuits. Given the findings of rapid activation of arcuate POMC neurons by acute stress (Liu et al. 2007) and the arcuate-MeA melanocortin projections (O'Donohue et al. 1979), enhanced arcuate melanocortinergic input into the MeA during stress may contribute to neuronal activation in the MeA.
We have previously shown that acute restraint stress-induced activation of POMC neurons is accompanied by anxiogenic-like behaviours and this was attenuated by blockade of melanocortin receptors via i.c.v. injection of SHU 9119, a MC4R antagonist (Liu et al. 2007). The neural circuits mediating the central effects of melanocortin signalling in stress-induced anxiety behaviours are poorly understood. Several lines of evidence support a role for the MeA in modulating anxiety-related behaviours. Various pharmacological manipulations in this region have been shown to modulate anxiety states (Duxon et al. 1997; Ebner et al. 2004; Forestiero et al. 2006; Kokare et al. 2005). Additionally, electrical stimulation of the MeA produces anxiogenic-like effects (Adamec & Shallow, 2000; Morgan et al. 1999), whereas lesions of the MeA caused anxiolytic-like effects (Blanchard & Blanchard, 1972; Luiten et al. 1985). In the present study, we showed that direct infusion of a MC4R agonist into the MeA caused anxiogenic effects, as indicated by decreased entries into open arms and time spent on the open arms in the elevated plus-maze test. In contrast, an intra-MeA injection of SHU 9110 blocked restraint stress-induced anxiogenic effects. These findings, together with the observation that MC4R neurons are activated by restraint stress, imply that enhanced MC4R signalling in the MeA may represent an underlying mechanism by which restraint stress exerts its anxiogenic effects.
The role of the central melanocortin system in controlling food intake and body weight is well documented (Barsh & Schwartz, 2002; Cone, 2005; Farooqi & O'Rahilly, 2004). A number of studies have largely focused on feeding effects of melanocortin signalling under basal, non-stressed conditions. We have shown that melanocortin signalling participates in emotional stress-induced anorectic effects in rodents (Liu et al. 2007). An i.c.v. injection of SHU 9119 blocks the reduction of food intake induced by restraint stress (Liu et al. 2007). It has been shown that electrical stimulation of the MeA suppresses food intake in food-deprived rats (White & Fisher, 1969). Conversely, lesions in the MeA induce excessive weight gain (Coscina et al. 2000; King et al. 2003). These findings support that the MeA exerts a regulatory effect on food intake. In the present study, we found that activation of M4R in the MeA decreased spontaneous food intake for up to 2 h and blockade of MC4R in this area attenuated restraint stress-induced anorectic effects, suggesting that MC4R signalling in this region regulates feeding. Of note, intra-MeA injection of SHU 9119 alone had no effect on food intake, which is in contrast to the orexigenic effects of SHU 9119 elicited by i.c.v. injection or intra-paraventricular nucleus (PVN) injection (Blevins et al. 2009; Garza et al. 2008; Giraudo et al. 1998; Liu et al. 2007; Seeley et al. 1997). These results suggest that MC4R signals in the MeA have a minimal effect on normal feeding but convey anorectic signals during stress.
Both anxiety and anorexia have been linked to abnormal HPA axis function (Licinio et al. 1996; Pego et al. 2010). Previous work has demonstrated that activation of MC4R via intra-PVN injection or i.c.v. administration of α-MSH and its analogues stimulates ACTH secretion and elevates plasma corticosterone levels (Dhillo et al. 2002; Lu et al. 2003; Ludwig et al. 1998; von Frijtag et al. 1998). In this study, we found that infusion of the MC4R agonist into the MeA increased corticosterone levels, suggesting that MC4R activation in the MeA is sufficient to stimulate the HPA axis. Blockade of MC4R by intra-MeA injection of SHU 9119 had no effect on plasma corticosterone levels under non-stressed conditions. However, SHU 9119 in the MeA attenuated restraint stress-induced increase in corticosterone levels. The significant effects of SHU 9119 were observed after 30-min exposure to restraint stress and continued for 15 min after the cessation of stress exposure. This could imply that blockade of melanocortin signalling in the MeA has little effect on the peak effect of acute restraint stress on the HPA axis, but rather accelerates the termination of an activated HPA axis.
One of the important questions that remain to be answered is what could be the downstream substrates of MC4R that mediates its behavioural and endocrine effects in the MeA. Several lines of evidence suggest that the corticotrophin-releasing hormone (CRH) is one of the candidates that relay central MC4R signals. CRH plays a critical role in mediating anxiety, feeding and HPA activation in response to stress (Bale et al. 2002; Vale et al. 1981), via activation of two receptor subtypes, CRHR1 and CRHR2 (Chen et al. 1993; Lovenberg et al. 1995; Perrin et al. 1995). Central melanocortin signalling has been shown to functionally interact with the CRH/CRH receptor system in modulating anxiety, feeding and HPA responses to stress. First, central administration of α-MSH up-regulates CRH mRNA levels and increases CRH release from hypothalamic explants (Fekete et al. 2000; Lu et al. 2003). Second, the anorectic effects and HPA stimulation induced by MC4R agonists can be blocked by pretreatment with α-helical-CRH9-41, a non-selective CRHR antagonist (Kamisoyama et al. 2009; Lu et al. 2003). Moreover, i.c.v. injection of CRH antiserum attenuates the anxiogenic effect induced by α-MSH (Vecsernyes et al. 2000). The next question that arose is how MC4R in the MeA interacts with the CRH/CRHR system. Because of the absence of CRH neurons in the MeA, MC4R signals in the MeA must be conveyed to CRH neurons via the efferents of the MeA. One of the target regions of the MeA efferents is to the bed nucleus of stria terminalis (BNST), which contains CRH neurons (Canteras et al. 1995; Coolen & Wood, 1998; Roozendaal et al. 2009). A number of studies have suggested that the BNST is involved in stress-elicited emotional responses and HPA activation (Choi et al. 2007; Forray & Gysling, 2004; Hammack et al. 2007; Herman & Cullinan, 1997; Herman et al. 2005; Lee & Davis, 1997; Walker et al. 2003, 2009). Stress activates CRH neurons in the BNST (Chappell et al. 1986; Stout et al. 2000), which can, in turn, excite CRH neurons in the PVN via a monosynaptic pathway (Dong et al. 2001). Moreover, activation of CRH receptors in the BNST induces anxiety-like behaviour (Lee & Davis, 1997; Sahuque et al. 2006) and blockade of CRH receptors in this area suppresses stress-induced anxiety (Cooper & Huhman, 2005). In addition, stress-induced inhibition of feeding behaviour has been linked to the BNST (Ciccocioppo et al. 2003; Ohata & Shibasaki, 2011). These findings suggest that CRH neurons in the BNST may function as a downstream neural substrate of MeA MC4R signals in the regulation of behavioural and endocrine responses to stress. The MeA also sends efferent projections to the immediate area surrounding the PVN, an area containing GABAergic interneurons (Herman et al. 1996). Given the direct synaptic connection between GABAergic inputs and CRH neurons in the PVN (Cullinan, 2000; Miklos & Kovacs, 2002), MC4R signalling in the MeA may influence the HPA output via interneuronal projections to CRH neurons in the PVN. CRH neurons in the PVN are not only essential to regulate the HPA axis function, but also affect food intake (Bale et al. 2002; Brady et al. 1990; Menzaghi et al. 1993; Suemaru et al. 1986; Vale et al. 1981).
However, MC4R signals in the brain sites other than MeA may also participate in stress responses. Indeed, we noted that the effective dosage of intra-MeA injection of SHU 9119 for attenuating stress-induced anorectic and anxiogenic effects is higher than that of i.c.v. administration used in our previous work (Liu et al. 2007). The i.c.v. injections allow the drugs to access their receptors in multiple brain regions. MC4R in these brain sites may act in concert to regulate stress responses, thus leading to a greater effect than a single brain site. Alternatively, we cannot exclude the possibility that diffusion of SHU 9110 from the MeA injection site into other areas may contribute to the observed effects. Also, the quality of the peptide compounds may vary between different batches or with time. Further studies are required to identify the exact neural circuits and downstream mechanisms underlying MC4R-mediated behavioural and endocrine responses to stress.
Clinical studies have shown that anxiety disorders are common in people with eating disorders, including anorexia nervosa (Bulik et al. 1997; Deep et al. 1995; Godart et al. 2000). Dysregulation of the HPA axis is a common pathological feature of anxiety and eating disorders (Licinio et al. 1996; Pego et al. 2010). Stress can result in an overactive HPA axis and trigger disordered eating and affective disorders such as anxiety. The present study provides evidence that melanocortin signalling in the MeA is involved in the regulation of emotional and feeding behaviours and endocrine responses to stress and suggests that abnormal melanocortinergic activity in the MeA may contribute to the pathophysiology and pathogenesis of anxiety and anorexia nervosa.
Acknowledgments
This work was supported by NIH grants MH073844 and MH076929 and the NARSAD award (X.Y.L).
Footnotes
Statement of Interest: None.
References
- Adamec R, Shallow T. Effects of baseline anxiety on response to kindling of the right medial amygdala. Physiology and Behavior. 2000;70:67–80. doi: 10.1016/s0031-9384(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends in Neurosciences. 1993;16:328–333. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- Arnold FJ, de Lucas Bueno M, Shiers H, Hancock DC, et al. Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience. 1992;51:377–390. doi: 10.1016/0306-4522(92)90322-s. [DOI] [PubMed] [Google Scholar]
- Bagnol D, Lu XY, Kaelin CB, Day HE, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocorti in brain. Journal of Neuroscience. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Lee KF, Vale WW. The role of corticotropin-releasing factor receptors in stress and anxiety. Integrative and Comparative Biology. 2002;42:552–555. doi: 10.1093/icb/42.3.552. [DOI] [PubMed] [Google Scholar]
- Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nature Reviews Genetics. 2002;3:589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- Baubet V, Fevre-Montange M, Gay N, Debilly G, et al. Effects of an acute immobilization stress upon proopiomelanocortin (POMC) mRNA levels in the mediobasal hypothalamus: a quantitative in situ hybridization study. Molecular Brain Research. 1994;26:163–168. doi: 10.1016/0169-328x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Tang R, Kalyani RN, et al. Potent and selective peptide agonists of alpha-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. Biochemical and Biophysical Research Communications. 2001;286:641–645. doi: 10.1006/bbrc.2001.5444. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. Journal of Comparative and Physiological Psychology. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Morton GJ, Williams DL, Caldwell DW, et al. Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2009;296:R476–R484. doi: 10.1152/ajpregu.90544.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52:441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Fear JL, Joyce PR. Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatrica Scandinavica. 1997;96:101–107. doi: 10.1111/j.1600-0447.1997.tb09913.x. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. Journal of Comparative Neurology. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Chaki S, Ogawa S, Toda Y, Funakoshi T, et al. Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. European Journal of Pharmacology. 2003;474:95–101. doi: 10.1016/s0014-2999(03)02033-8. [DOI] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, et al. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. Journal of Neuroscience. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proceedings of the National Academy of Sciences USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, et al. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. Journal of Neuroscience. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Martinez M, Herbert J. Central serotonin depletion modulates the behavioural, endocrine and physiological responses to repeated social stress and subsequent c-fos expression in the brains of male rats. Neuroscience. 1999;92:613–625. doi: 10.1016/s0306-4522(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, et al. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. Journal of Neuroscience. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. Journal of Comparative Neurology. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF-sub-2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2005;119:1042–1051. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- Coscina DV, Currie PJ, Bishop C, Parker GC, et al. Posterodorsal amygdala lesions reduce feeding stimulated by 8-OH-DPAT. Brain Research. 2000;883:243–249. doi: 10.1016/s0006-8993(00)02918-8. [DOI] [PubMed] [Google Scholar]
- Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. Journal of Comparative Neurology. 2000;419:344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, et al. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Levin N, Walker CD, et al. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Annals of the New York Academy of Sciences. 1994;746:22–31. doi: 10.1111/j.1749-6632.1994.tb39206.x. discussion 31–32, 64–67. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, et al. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. European Journal of Neuroscience. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. European Journal of Neuroscience. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- de Barioglio SR, Lezcano N, Celis ME. Alpha MSH-induced excessive grooming behavior involves a GABAergic mechanism. Peptides. 1991;12:203–205. doi: 10.1016/0196-9781(91)90189-v. [DOI] [PubMed] [Google Scholar]
- Deep AL, Nagy LM, Weltzin TE, Rao R, et al. Premorbid onset of psychopathology in long-term recovered anorexia nervosa. International Journal of Eating Disorders. 1995;17:291–297. [PubMed] [Google Scholar]
- Dhillo WS, Small CJ, Seal LJ, Kim MS, et al. The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats. Neuroendocrinology. 2002;75:209–216. doi: 10.1159/000054712. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. Journal of Comparative Neurology. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Whitener J. Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology. 1986;42:211–217. doi: 10.1159/000124442. [DOI] [PubMed] [Google Scholar]
- Duxon MS, Kennett GA, Lightowler S, Blackburn TP, et al. Activation of 5-HT2B receptors in the medial amygdala causes anxiolysis in the social interaction test in the rat. Neuropharmacology. 1997;36:601–608. doi: 10.1016/s0028-3908(97)00042-7. [DOI] [PubMed] [Google Scholar]
- Ebner K, Rupniak NM, Saria A, Singewald N. Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proceedings of the National Academy of Sciences USA. 2004;101:4280–4285. doi: 10.1073/pnas.0400794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S. Monogenic human obesity syndromes. Recent Progress in Hormone Research. 2004;59:409–424. doi: 10.1210/rp.59.1.409. [DOI] [PubMed] [Google Scholar]
- Fekete C, Legradi G, Mihaly E, Tatro JB, et al. alpha-Melanocyte stimulating hormone prevents fasting-induced suppression of corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Neuroscience Letters. 2000;289:152–156. doi: 10.1016/s0304-3940(00)01256-8. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Research. 1994;658:21–26. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Saphier D. The preoptic area and bed nucleus of the stria terminalis are involved in the effects of the amygdala on adrenocortical secretion. Neuroscience. 1990;37:775–779. doi: 10.1016/0306-4522(90)90107-f. [DOI] [PubMed] [Google Scholar]
- Forestiero D, Manfrim CM, Guimaraes FS, de Oliveira RM. Anxiolytic-like effects induced by nitric oxide synthase inhibitors microinjected into the medial amygdala of rats. Psychopharmacology. 2006;184:166–172. doi: 10.1007/s00213-005-0270-6. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Research Reviews. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Garza JC, Kim CS, Liu J, Zhang W, et al. Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. Journal of Endocrinology. 2008;197:471–482. doi: 10.1677/JOE-08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Research. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- Godart NT, Flament MF, Lecrubier Y, Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: co-morbidity and chronology of appearance. European Psychiatry. 2000;15:38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Vaziri S, Wilson CA. Behavioral effects of alpha-MSH and MCH after central administration in the female rat. Peptides. 1996;17:171–177. doi: 10.1016/0196-9781(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Griebel G, Sanger DJ, Perrault G. The use of the rat elevated plus-maze to discriminate between non-selective and BZ-1 (omega 1) selective, benzodiazepine receptor ligands. Psychopharmacology. 1996;124:245–254. doi: 10.1007/BF02246664. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. Journal of Neurophysiology. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. Journal of Endocrinology. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Critical Reviews in Neurobiology. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Research. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, O'Donohue TL. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proceedings of the National Academy of Sciences USA. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisoyama H, Honda K, Saneyasu T, Sugahara K, et al. Corticotropin-releasing factor is a downstream mediator of the beta-melanocyte-stimulating hormone-induced anorexigenic pathway in chicks. Neuroscience Letters. 2009;458:102–105. doi: 10.1016/j.neulet.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Khorram O, Bedran de Castro JC, McCann SM. Stress-induced secretion of alpha-melanocyte-stimulating hormone and its physiological role in modulating the secretion of prolactin and luteinizing hormone in the female rat. Endocrinology. 1985;117:2483–2489. doi: 10.1210/endo-117-6-2483. [DOI] [PubMed] [Google Scholar]
- King BM, Rollins BL, Grundmann SJ, Olivier LG. Excessive weight gains in female rats with transections of the stria terminalis. Physiology and Behavior. 2003;78:563–568. doi: 10.1016/s0031-9384(03)00042-8. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. Journal of Comparative Neurology. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, et al. Effects of melanotan II, a melanocortin agonist, on grooming and exploration in rats after repeated restraint/immobilization. Neuroscience Letters. 2008;432:202–205. doi: 10.1016/j.neulet.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Chopde CT, Subhedar N. Interaction between neuropeptide Y and alpha-melanocyte stimulating hormone in amygdala regulates anxiety in rats. Brain Research. 2005;1043:107–114. doi: 10.1016/j.brainres.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Singru PS, Gupta GL, et al. Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology. 2010;58:1009–1018. doi: 10.1016/j.neuropharm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. Journal of Neuroscience. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Wong ML, Gold PW. The hypothalamic-pituitary-adrenal axis in anorexia nervosa. Psychiatry Research. 1996;62:75–83. doi: 10.1016/0165-1781(96)02991-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Truong HV, Henschel J, et al. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–5540. doi: 10.1210/en.2007-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- Lu XY. Role of central melanocortin signaling in eating disorders. Psychopharmacology Bulletin. 2001;35:45–65. [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. Journal of Neuroscience. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Mountjoy KG, Tatro JB, Gillette JA, et al. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. American Journal of Physiology. 1998;274:E627–E633. doi: 10.1152/ajpendo.1998.274.4.E627. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Koolhaas JM, de Boer S, Koopmans SJ. The cortico-medial amygdala in the central nervous system organization of agonistic behavior. Brain Research. 1985;332:283–297. doi: 10.1016/0006-8993(85)90597-9. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar-Kyoto rats compared to Sprague-Dawley rats. Neuroscience. 2004;124:963–972. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Matheson GK, Branch BJ, Taylor AN. Effects of amygdaloid stimulation on pituitary-adrenal activity in conscious cats. Brain Research. 1971;32:151–167. doi: 10.1016/0006-8993(71)90160-0. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Heinrichs SC, Pich EM, Tilders FJ, et al. Functional impairment of hypothalamic corticotropin-releasing factor neurons with immunotargeted toxins enhances food intake induced by neuropeptide Y. Brain Research. 1993;618:76–82. doi: 10.1016/0006-8993(93)90431-l. [DOI] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Watchus JA, Milgram NW, Fleming AS. The long lasting effects of electrical simulation of the medial preoptic area and medial amygdala on maternal behavior in female rats. Behavioural Brain Research. 1999;99:61–73. doi: 10.1016/s0166-4328(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, et al. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, et al. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- O'Donohue TL, Miller RL, Jacobowitz DM. Identification, characterization and stereotaxic mapping of intraneuronal alpha-melanocyte stimulating hormone-like immunoreactive peptides in discrete regions of the rat brain. Brain Research. 1979;176:101–123. doi: 10.1016/0006-8993(79)90873-4. [DOI] [PubMed] [Google Scholar]
- Ohata H, Shibasaki T. Involvement of CRF2 receptor in the brain regions in restraint-induced anorexia. Neuroreport. 2011;22:494–498. doi: 10.1097/WNR.0b013e3283487467. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pego JM, Sousa JC, Almeida OF, Sousa N. Stress and the neuroendocrinology of anxiety disorders. Current Topics in Behavioral Neuroscience. 2010;2:97–117. doi: 10.1007/7854_2009_13. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, et al. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proceedings of the National Academy of Sciences USA. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TL, Kokare DM, Sarkar S, Khisti RT, et al. GABAergic agents prevent alpha-melanocyte stimulating hormone induced anxiety and anorexia in rats. Pharmacology, Biochemistry and Behavior. 2003;76:417–423. doi: 10.1016/j.pbb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Redgate ES, Fahringer EE. A comparison of the pituitary adrenal activity elicited by electrical stimulation of preoptic, amygdaloid and hypothalamic sites in the rat brain. Neuroendocrinology. 1973;12:334–343. doi: 10.1159/000122182. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, et al. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Research. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Roske I, Hughes ME, Newson P, Oehme P, et al. Effect of chronic intermittent immobilization stress on Fos-like immunoreactivity in rat brain and adrenal medulla. Stress. 2002;5:277–283. doi: 10.1080/1025389021000061174. [DOI] [PubMed] [Google Scholar]
- Rybkin II, Zhou Y, Volaufova J, Smagin GN, et al. Effect of restraint stress on food intake and body weight is determined by time of day. American Journal of Physiology. 1997;273:R1612–R1622. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, et al. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the striaterminalis in the rat: role of CRF receptor subtypes. Psychopharmacology. 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Yagaloff KA, Fisher SL, Burn P, et al. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, et al. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. European Journal of Pharmacology. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Suemaru S, Hashimoto K, Hattori T, Inoue H, et al. Starvation-induced changes in rat brain corticotropin-releasing factor (CRF) and pituitary-adrenocortical response. Life Sciences. 1986;39:1161–1166. doi: 10.1016/0024-3205(86)90347-4. [DOI] [PubMed] [Google Scholar]
- Sumpter JP, Dye HM, Benfey TJ. The effects of stress on plasma ACTH, alpha-MSH, and cortisol levels in salmonid fishes. General and Comparative Endocrinology. 1986;62:377–385. doi: 10.1016/0016-6480(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vecsernyes M, Biro E, Gardi J, Julesz J, et al. Involvement of endogenous corticotropin-releasing factor in mediation of neuroendocrine and behavioral effects to alpha-melanocyte-stimulating hormone. Endocrine Research. 2000;26:347–356. doi: 10.3109/07435800009066172. [DOI] [PubMed] [Google Scholar]
- Vergoni AV, Bertolini A, Wikberg JE, Schioth HB. Selective melanocortin MC4 receptor blockage reduces immobilization stress-induced anorexia in rats. European Journal of Pharmacology. 1999;369:11–15. doi: 10.1016/s0014-2999(99)00045-x. [DOI] [PubMed] [Google Scholar]
- von Frijtag JC, Croiset G, Gispen WH, Adan RA, et al. The role of central melanocortin receptors in the activation of the hypothalamus-pituitary-adrenal-axis and the induction of excessive grooming. British Journal of Pharmacology. 1998;123:1503–1508. doi: 10.1038/sj.bjp.0701750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like vs. phasic fear-like responses. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis vs. the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Akil H, Richard CW, III, Barchas JD. Evidence for two separate opiate peptide neuronal systems. Nature. 1978a;275:226–228. doi: 10.1038/275226a0. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Richard CW, III, Barchas JD. Adrenocorticotropin in rat brain: immunocytochemical localization in cells and axons. Science. 1978b;200:1180–1182. doi: 10.1126/science.206967. [DOI] [PubMed] [Google Scholar]
- White NM, Fisher AE. Relationship between amygdala and hypothalamus in the control of eating behavior. Physiology and Behavior. 1969;4:199–202. [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, et al. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. Journal of Neuroscience. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, et al. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. Journal of Veterinary Medical Science. 2004;66:1323–1327. doi: 10.1292/jvms.66.1323. [DOI] [PubMed] [Google Scholar]