Abstract
The lymphocyte transformation responses to purified preparations of two extracellular products of group A streptococci (blastogen A and nuclease B), to phytohemagglutinin, and to Candida albicans antigen were measured in tonsillar and peripheral blood lymphocytes from patients with rheumatic heart disease (RHD) and suitably matched nonrheumatic (control) subjects.
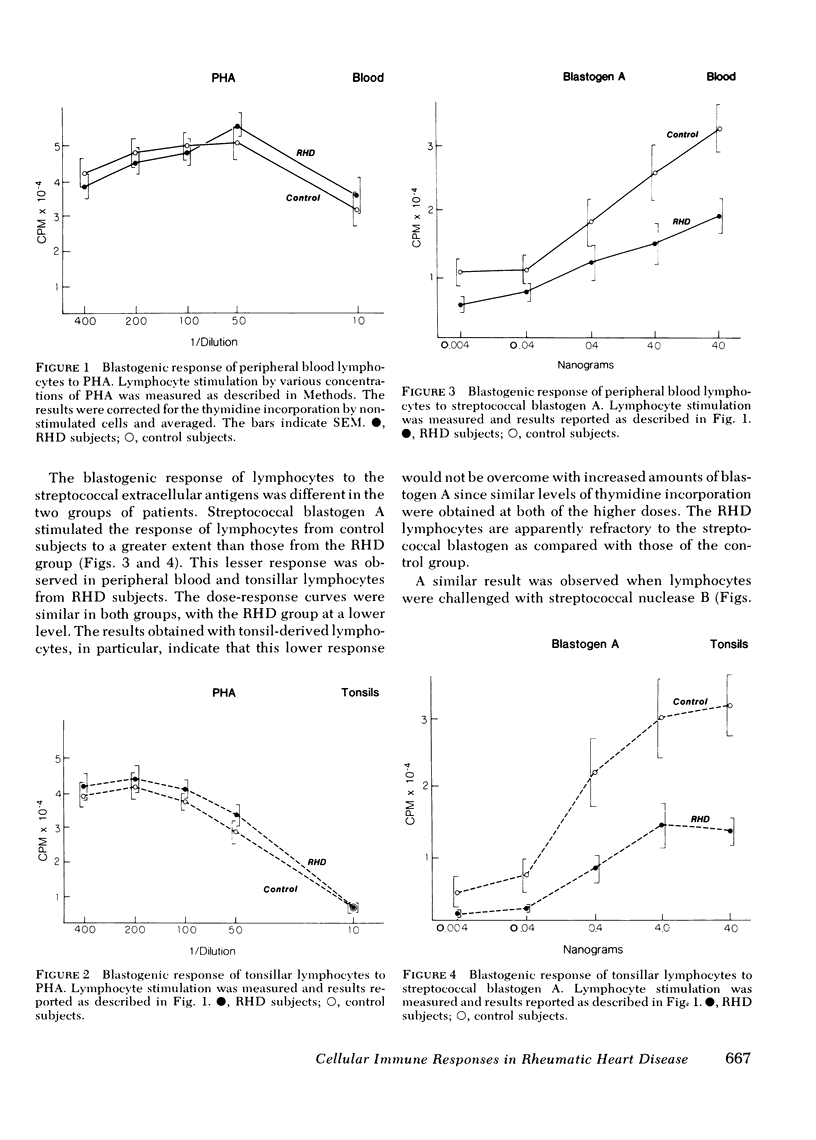
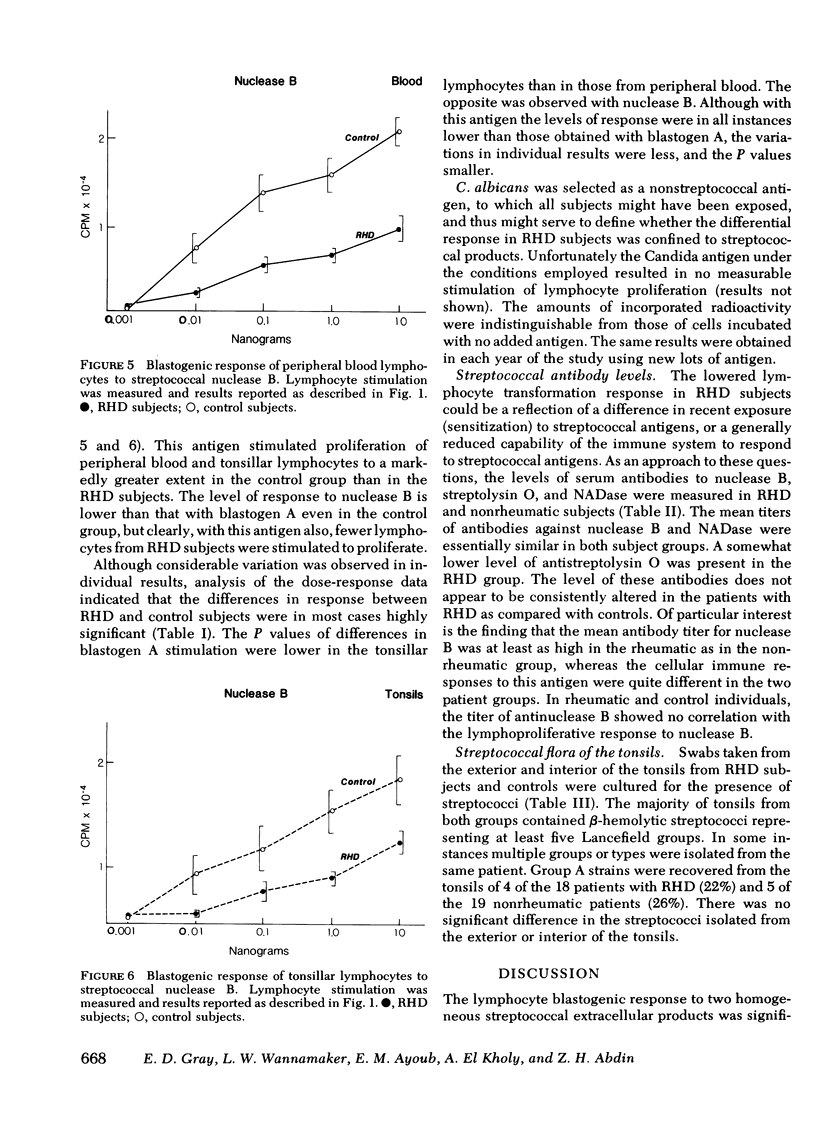
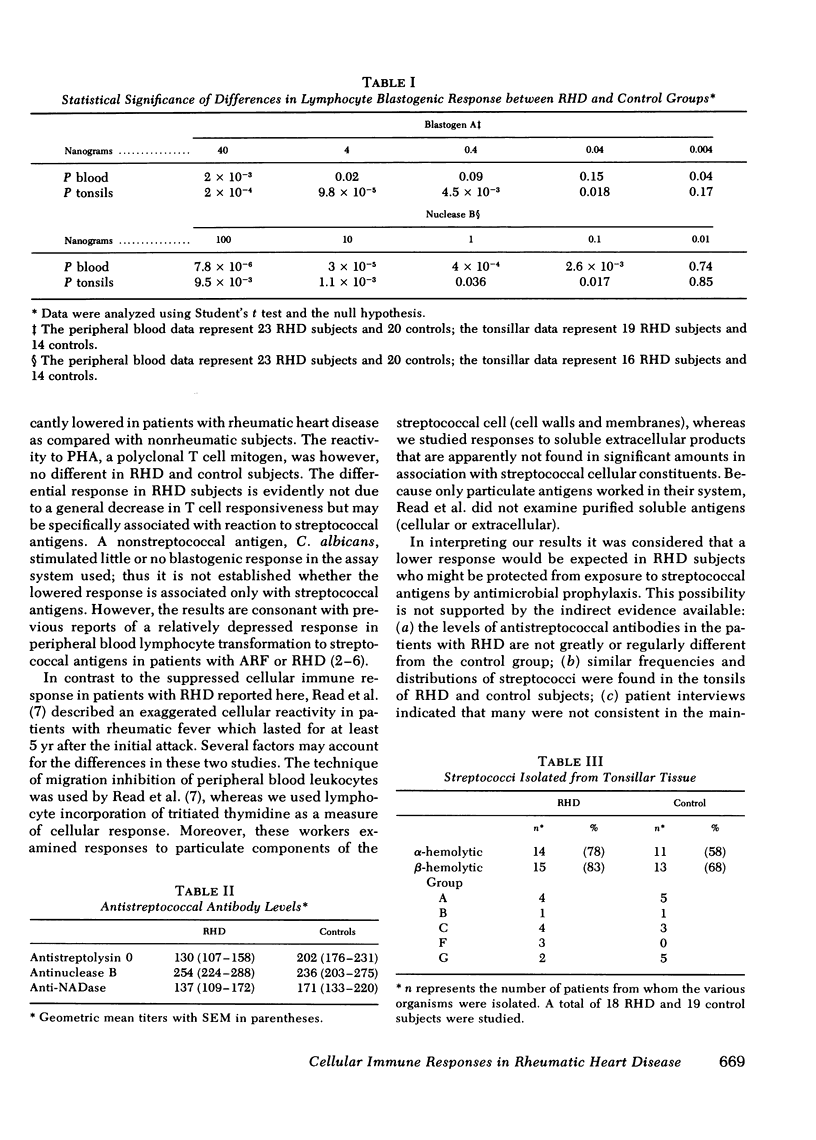
The mean phytohemagglutinin dose responses of tonsillar and peripheral lymphocytes from RHD patients were essentially indistinguishable from those of controls. In contrast, the responses of tonsillar and peripheral blood lymphocytes to the two extracellular products of group A streptococci were significantly lower in RHD patients than in nonrheumatic control subjects. Candida antigen produced very little stimulation of lymphocytes in any of the subjects.
The geometric means of antibody levels against streptolysin O, nuclease B, and nicotinamide adenine dinucleotidase showed no consistent differences between the control group and the group of RHD subjects. Group A streptococci were isolated from the tonsils of ∼25% of both groups of subjects.
The RHD patients clearly had a depressed cellular immune response to the two purified streptococcal extracellular antigens. The equal frequency in recovery of group A streptococci from tonsils and the absence of consistent difference in titers of humoral antibodies to streptococcal extracellular antigens, particularly nuclease B, suggest that this differential response is not due to a lower level of stimulation by repeated exposure to group A streptococcal products.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub E. M., Ferretti J. J. Use of bisulfite in the streptococcal anti-nicotinamide adenine dinucleotidase test. Appl Microbiol. 1966 May;14(3):391–393. doi: 10.1128/am.14.3.391-393.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B., Kapp J. A., Debré P., Pierce C. W., de la Croix F. The stimulation of specific suppressor T cells in genetic non-responder mice by linear random copolymers of L-amino acids. Transplant Rev. 1975;26:21–38. doi: 10.1111/j.1600-065x.1975.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Chernata L. I., Vershigora A. E. Nebnye mindaliny i immunitet. Soobshchenie IV. Blasttransformatsiia limfotsitov razlichnykh limfoidnykh organov zhivotnykh, immunizirovannykh mikrobnymi antigenami. Zh Mikrobiol Epidemiol Immunobiol. 1975 May;(5):123–127. [PubMed] [Google Scholar]
- Cuppari G., Quagliata F., Ieri A., Taranta A. Lymphocyte transformation with streptolysin S preparations and inhibition of streptolysin S by serum in rheumatic fever and other rheumatic diseases. J Lab Clin Med. 1972 Aug;80(2):165–178. [PubMed] [Google Scholar]
- Drucker M. M., Agatsuma Y., Drucker I., Neter E., Bernstein J., Ogra P. L. Cell-mediated immune response to bacterial products in human tonsils and peripheral blood lymphocytes. Infect Immun. 1979 Feb;23(2):347–352. doi: 10.1128/iai.23.2.347-352.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E. A. Protocol for micro antistreptolysin O determinations. J Bacteriol. 1964 May;87(5):1254–1255. doi: 10.1128/jb.87.5.1254-1255.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T. C., Oppenheim J. J. Impaired lymphocyte stimulation by some streptococcal antigens in patients with recurrent aphthous stomatitis and rheumatic heart disease. Clin Exp Immunol. 1970 Apr;6(4):573–586. [PMC free article] [PubMed] [Google Scholar]
- Gery I., Brand-Auraban A., Benezra D., Jacob A., Davies A. M. Transformation of lymphocytes from patients with rheumatic fever by streptolysin S. Clin Exp Immunol. 1968 Sep;3(7):717–723. [PMC free article] [PubMed] [Google Scholar]
- Gray E. D. Purification and properties of an extracellular blastogen produced by group A streptococci. J Exp Med. 1979 Jun 1;149(6):1438–1449. doi: 10.1084/jem.149.6.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg L. J., Gray E. D., Yunis E. J. Association of HL-A 5 and immune responsiveness in vitro to streptococcal antigens. J Exp Med. 1975 May 1;141(5):935–943. doi: 10.1084/jem.141.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCHHORN K., SCHREIBMAN R. R., VERBO S., GRUSKIN R. H. THE ACTION OF STREPTOLYSIN S ON PERIPHERAL LYMPHOCYTES OF NORMAL SUBJECTS AND PATIENTS WITH ACUTE RHEUMATIC FEVER. Proc Natl Acad Sci U S A. 1964 Nov;52:1151–1157. doi: 10.1073/pnas.52.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueker R. D., Williams R. C., Jr Decreased reactivity of lymphocytes in mixed-leukocyte culture from patients with rheumatic fever. Circulation. 1972 Oct;46(4):655–660. doi: 10.1161/01.cir.46.4.655. [DOI] [PubMed] [Google Scholar]
- Meriç N., Berkel A. I. Cellular immunity in children with acute rheumatic fever and rheumatic carditis. Pediatr Res. 1979 Jan;13(1):16–20. doi: 10.1203/00006450-197901000-00004. [DOI] [PubMed] [Google Scholar]
- Nelson J., Ayoub E. M., Wannamaker L. W. Streptococcal anti-desoxyribonuclease B: microtechnique determination. J Lab Clin Med. 1968 May;71(5):867–873. [PubMed] [Google Scholar]
- Oettgen H. F., Silber R., Miescher P. A., Hirschhorn K. Stimulation of human tonsillar lymphocytes in vitro. Clin Exp Immunol. 1966 Jan;1(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- Opelz G., Terasaki P. I., Hirata A. A. Suppression of lymphocyte transformation by aspirin. Lancet. 1973 Sep 1;2(7827):478–480. doi: 10.1016/s0140-6736(73)92073-4. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol. 1979;19:179–201. doi: 10.1146/annurev.pa.19.040179.001143. [DOI] [PubMed] [Google Scholar]
- Read S. E., Fischetti V. A., Utermohlen V., Falk R. E., Zabriskie J. B. Cellular reactivity studies to streptococcal antigens. Migration inhibition studies in patients with streptococcal infections and rheumatic fever. J Clin Invest. 1974 Aug;54(2):439–450. doi: 10.1172/JCI107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapru R. P., Ganguly N. K., Sharma S., Chandani R. E., Gupta A. K. Cellular reaction to group A beta-haemolytic streptococcal membrane antigen and its relation to complement levels in patients with rheumatic heart disease. Br Med J. 1977 Aug 13;2(6084):422–424. doi: 10.1136/bmj.2.6084.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranta A. Lymphocyte mitogens of staphylococcal origin. Ann N Y Acad Sci. 1974 Jul 31;236(0):362–375. doi: 10.1111/j.1749-6632.1974.tb41503.x. [DOI] [PubMed] [Google Scholar]
- Wannamaker L. W. Differences between streptococcal infections of the throat and of the skin. I. N Engl J Med. 1970 Jan 1;282(1):23–31. doi: 10.1056/NEJM197001012820106. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Zabriskie J. B., Mahros F., Hassaballa F., Abdin Z. H. Lymphocyte surface markers in acute rheumatic fever and post-streptococcal acute glomerulonephritis. Clin Exp Immunol. 1977 Jan;27(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B. The relationship of streptococcal cross-reactive antigens to rheumatic fever. Transplant Proc. 1969 Dec;1(4):968–975. [PubMed] [Google Scholar]



