Abstract
Oxidative stress has been the object of considerable biological and biochemical investigation. Quantification has been difficult although the quantitative level of products of biological oxidations in tissues and tissue products has emerged as a widely used technique. The relationship between these products and the amount of oxidative stress is less clear. Imaging oxidative stress with electron paramagnetic resonance related magnetic resonance imaging, while not addressing the specific issue of quantification of initiating events, focuses on the anatomic specific location of the oxidative stress. Moreover, the relative quantification of oxidative stress of one location against another is possible, sharpening our understanding of oxidative stress. This promises to improve our understanding of oxidative stress and its deleterious consequences and enhance our understanding of the effectiveness of interventions to modulate oxidative stress and its consequences.
ASSESSMENT OF OXIDATIVE STRESS IN BIOLOGICAL SYSTEMS
Reactive oxygen species (ROS) are generated constantly in living cells as a byproduct of oxidative metabolism. Their deleterious effects on cell components are determined by the rate of generation of ROS in the cell and the concentration of low-molecular-weight antioxidants together with activities of enzymatic antioxidants. Factors increasing the rate of ROS generation (i.e. ionizing radiation or metal ions) as well as factors decreasing antioxidant capacity lead to oxidative stress, causing enhanced oxidative damage of cellular components. Oxidative stress usually leads to a decrease in antioxidant defense capacities or depletion of reducing ability of exposed tissue. Closely connected to oxidative stress is tissue redox environment, or redox state, understood widely as bioreductive capacity of the system, or, more precisely, as redox buffer capacity (1). Stronger oxidative stress produces greater changes in the redox state. Both terms are convenient operational concepts and still await the complete understanding of spatial and kinetic factors involved.
Oxidative stress is implicated in pathology of many diseases, such as diabetes, intoxication, neurological disorders and ischemia. Estimation of level of the oxidative stress in tissues is useful to determine the mechanisms and the role of ROS in these pathologies as well as the extent and significance of antioxidant therapies. What is more, both initial redox state and oxidative stress generated might differ considerably among tissues and are influenced by other spatially differentiated factors, such as hypoxia. Therefore, 3D spatial mapping of oxidative stress is particularly informative in investigating the mechanisms of oxidative stress and related pathologies.
To confirm the presence of the oxidative stress, most often indirect detection of the oxidation products is used. Oxidative modifications of various cell components may serve as indicators of the oxidative stress, such as e.g. DNA/RNA damage, oxidative protein products or lipid peroxidation of lipid membrane components (2). In addition, measurement of the antioxidant defense system components, such as catalase, SOD or glutathione can be performed. Direct measurements of reactive oxygen species is possible using several methods, including EPR (3). A method most widely used in cellular studies employs probes that fluoresce when exposed to oxidation, such as dichlorofluorescein (4). These measurements are generally nonspecific and rely on the transformation of a chemical species that is added to the biological system. The transformation takes place in response to the presence of ROS, but they may react with other reducing or oxidizing biological components as well. Other free radicals, such as intermediate probe radicals, as well as ROS can be generated (5). Recently, fluorescent probes responding to more specific oxidative agents such as mitochondrial superoxide have become available (6). In the in vivo setting, the context of animal measurements, however, fluorescence at optical frequencies can be measured only from surface tissues no more than a few millimeters deep due to the limited tissue penetration of light. This significant absorption is dependent on various aspects of the tissues (e.g. skin pigmentation) that compromise quantification of the signal. Electron paramagnetic resonance (EPR) imaging, particularly at frequencies of 1 to 1.5 GHz or lower, can overcome these limitations and provides quantitative noninvasive, three-dimensional images of oxidative stress in living animals.
Imaging of the oxidative stress using EPR is based on the monitoring the signal of the paramagnetic redox-sensitive probe in a whole organism or a chosen part of the organism and analyzing the time-dependent decrease of this signal. The most commonly used redox-sensitive spin probes are nitroxides, which interact with many biological redox-active compounds, such as ascorbate, glutathione, flavins, redox enzymes, etc. Administration of specific inhibitors or enhancers of ROS will modify this decay and thus could provide more detailed insight into the redox state of the system. This approach was first studied spectroscopically in vitro in a wide variety of settings (7–10), then transferred into in vivo studies, and finally applied in imaging of oxidative stress.
Besides monitoring the redox state of tissues in an indirect way, EPR also enables the detection of specific free radicals directly, such as superoxide ions in biological systems by using spin trapping (11, 12). However, due to the usual low concentration of ROS in cellular environments, this technique is available as spectroscopy only and can presently be used for imaging only to a limited degree (13–16). When the concentration of the free radical is high enough, e.g. after irradiation, in vivo detection of generated hydroxyl radical is possible (17).
NITROXIDES AS REDOX-SENSITIVE PROBES IN BIOLOGICAL SYSTEMS
Redox-sensitive spin probes (nitroxides, aminoxyl radicals) are free radicals that are stable in solution. In the presence of many biological redox-active compounds and enzymes, such as ascorbate, glutathione, superoxide and others, they participate in redox reactions (7, 10, 18–20). Acceptance of an electron (reduction) leads to a hydroxyl-amine and to giving an electron (oxidation) to N-oxo ammonium salts (Fig. 1). In living cells or tissues both reduction and oxidation reactions can occur, and all three forms can exist (21). Nitroxide/hydroxylamine pairs acts as a cycling antioxidant (22–24), and nitroxide/oxoammonium cation mimics the action of superoxide dismutase (25). These antioxidant properties of nitroxides make them good radioprotectors (26, 27). After administration of nitroxide into the cell or tissue, a decay in paramagnetic signal is observed, mainly due to reduction to hydroxylamines (7, 18). This decay is faster in hypoxic tissues (28–30). Hydroxylamine may be also reoxidized back to the nitroxide, and after in vivo administration of EPR-silent hydroxylamine, EPR signal reaches a similar level as after administering its nitroxide counterpart (31). Beside redox reactions, other pharmacokinetical mechanisms of nitroxide decay have to be considered, such as washout from the tissue of interest. For example, it has been shown that in the muscle the rate of nitroxide washout is 1–20 times higher than the rate of reduction (32). Effects such as diffusion through the tissue and recirculation by the blood were negligible (32). Therefore, a careful estimation of all the factors responsible for the decay in a particular model is required before drawing conclusions as to the redox state. A confirmation of the redox-sensitive role for nitroxides came from an MRI study, where pharmacokinetic images of these nitroxides in different tissues in mice were obtained simultaneously, as well as oxidized plus reduced forms measured in tissue extracts by EPR. These results verified that for cell membrane-permeable nitroxides, their metabolism, measured as reduction rate, reflects the intracellular redox status (33).
FIG. 1.
Reactions of nitroxides in biological systems.
The discovery of the dependence of nitroxide paramagnetism decay on reduction-oxidation reactions in biological systems, suggested early on the possible application of these compounds to study redox environment (10). The ratio between the EPR-visible nitroxide form and the EPR-silent hydroxylamine form depends on the redox environment (34). Mid-range redox potential of this redox couple [E½ = 0.17–0.42 V compared to Ag/AgCl (19)], and the resulting large extent of redox reactions influencing the nitroxide/hydroxylamine ratio makes them a good marker of cellular redox state.
Oxidative stress causes an increase in the rate of nitroxide bioreduction, whereas the presence of antioxidants leads to a slower rate of nitroxide bioreduction. Therefore, the assessment of redox state using nitroxides might also be used for evaluation of oxidative stress. Usually a comparison of the nitroxide decay is made under oxidative stress conditions, and in the absence of oxidative factors, or alternatively, some scavengers of oxidative species can be applied. These relationships were studied first by EPR spectroscopy in vivo (35–37) before being applied to EPR redox state imaging.
RESONANCE TECHNIQUES FOR REDOX STATE IMAGING
There are three basic instrumental approaches to the resonance imaging of redox state in vivo: EPR alone, Overhauser enhanced MRI (OMRI) or MRI with nitroxides as contrast agents. Direct EPR imaging with time resolution allowing registering spin probe decay provides 2D or 3D oxidative stress images of tissues such as tumors, heart or brain in vivo (38–41). Spatial resolution in redox imaging with EPR is affected by linewidth of nitroxides (typically ~1 gauss) and their relatively short half-life in vivo. The half-life of nitroxides in most tissues is of the order of minutes (19), and such a time window allows probing of the tissue redox environment, as the experiments described below confirm. Prolonging the half-life of nitroxides in vivo is possible, but it might affect their function as a redox-sensitive probe. This severely limits the sensitivity of the method and spatial resolution of redox mapping using direct EPR imaging of nitroxides.
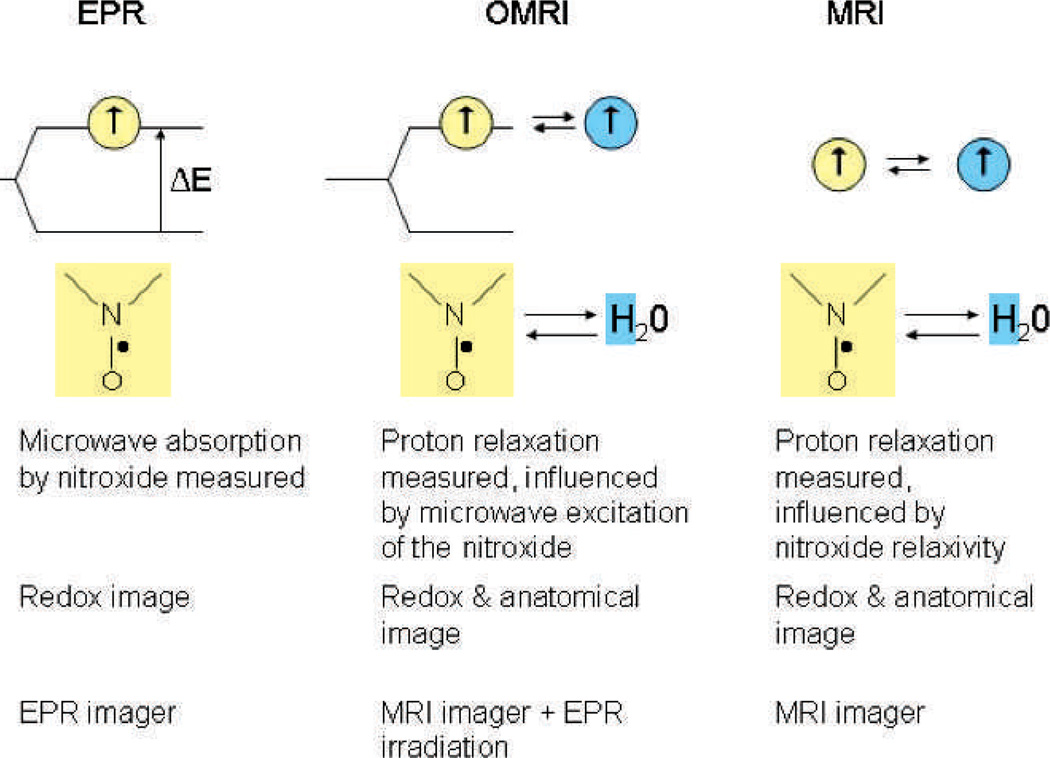
To improve spatial resolution, OMRI and MRI are used. The schematic comparison of the three approaches is shown in Fig. 2. All three techniques are based on the sensitivity of nitroxides to the redox state of the imaged tissue, but employing MRI provides more spatial resolution and anatomical information in addition to the redox state. OMRI is a double resonance technique based on the Overhauser effect. A nitroxide is injected in the animal and the nitroxide electron is irradiated, changing the electron spin population. This stimulates changes in the water proton spin population through the Overhauser effect, enhancing the water proton signal in the sample. Water proton MRI is a readout of the nitroxide concentration and is used to obtain the image. Multiple MRIs obtained in this fashon show the reduction in enhancement in time and provides the high spatial resolution images of the nitroxide signal decay. In contrast, redox state imaging using MRI alone explores the use of nitroxides as T1-contrast agents (42, 43). This technique monitors the change in T1-weighted enhancement induced by the nitroxide in the images. The rate of decrease in this effect depends on the local transformation of nitroxide to hydroxylamine. Excellent reviews have been recently published describing redox imaging of tissues using MRI alone (44, 45); therefore, in this paper, we focus on EPR and OMRI techniques only.
FIG. 2.
Comparison of oxidative stress measurement using nitroxides as a redox-sensitive probe between EPR, OMRI and MRI. Redox state of the tissue is reflected by the ratio of paramagnetic form of the nitroxide and its reduced non-paramagnetic form, hydroxylamine. All three techniques are based on monitoring the kinetics of nitroxide decay in the tissue. EPR signal measures the absorption of microwave energy by nitroxide, directly reflecting the amount of paramagnetic form present in the sample. OMRI detects changes in the proton relaxation induced by excited nitroxide spins. MRI also measures the proton relaxation, but nitroxide spins act here as contrast agents.
DEVELOPMENT OF EPR REDOX STATE SPECTROSCOPY AND IMAGING
Intense development of imaging techniques in EPR in the 1980s developed in parallel with applications to biological objects. The first 1D2 EPR image of a cross section of a celery stalk, obtained at L-band, was published in 1985 (46, 47). This technique was soon extended to a tumor in a living mouse. One-dimensional images (4 projections at 45° angles) of a tumor in a living mouse loaded with nitroxide were collected at L-band (48).
Small biological samples could be visualized at X-band. This approach was applied to study tumor cell spheroids (cellular aggregates of 100–500 µm in diameter). Two-dimensional images consisting of 32–64 projections were acquired within 6–12 min using a 90 G/cm gradient. An approximately 200-µm rim of live cells could be discerned in these images (49). However, studies in the living animals require bigger sample volumes than X-band allows, and further studies were pursued with lower frequencies.3 Nitroxide distributions were shown in a 3D image of a rat tail (50, 51) and 2D whole rat images (52). The same laboratory has also shown sequential 2D images of coronal plane of a rat abdomen, obtained in 5 min, with a spatial resolution of 8 mm (53). Nitroxide 3D distributions in a rat head (54) and 2D images of a whole mouse were performed using spin labeled dextran (55). Spectral-spatial images in living mice were collected using four different nitroxides, and their pharmacokinetics have been compared (56).
Animal organs excised and studied ex vivo, especially an isolated beating heart, were a useful model for developing the imaging techniques. Three-dimensional spatial EPR images of a rat heart using nitroxide have shown spatially defined differences in the rate of radical clearance (57). The same paper also presented 3D spectral-spatial images of rabbit aortas, where the aorta wall was clearly seen. This required 64 projections that were taken within 12 min using a 49 G/cm gradient (57). The main limiting factor in obtaining images of better resolution was the stability of nitroxides. For example, the resolution of images of isolated rat hearts obtained with a single line glucose char probe was much better (0.2 mm) than that obtained with nitroxides (1– 2 mm) (58, 59). A solution to this problem used polynitroxyl-albumin of prolonged half-live, which allowed 3D imaging of a rat heart with submillimeter resolution. The image contained 144 projections collected in 18 min using a 20 G/cm gradient (60). To avoid artifacts caused by the motion of a beating heart, image acquisition was gated and synchronized with the heartbeat (61, 62). Imaging of larger animal specimens with EPR was accomplished with longitudinally detected ESR/EPR (LODESR) (63, 64).
EPR REDOX STATE IMAGING–STUDIES IN VIVO
Spatial Nitroxide Distribution Imaging
Several studies demonstrated the feasibility of redox state imaging using nitroxides in different organs. For example, a 2D image of rat cross section in the kidney region using OMRI technique was shown (65). Two-dimensional images of the whole body of a mouse were collected at S-band (2–4 GHz) every 6 min. This allowed for analysis of the signal decay from different organs. MRI has been used for identification of anatomical structure (66). Another study from the same group demonstrated 1D spatial imaging in human skin using a surface coil. Nitroxide (15N-PDT) was applied topically, and then the rate of penetration into the skin was estimated with a single gradient perpendicular to the skin. Two disctinct bands of nitroxide distribution along the depth of the skin were observed (67, 68). The surface coil approach was also used to generate 2D images of a slice of rat kidney. The images were taken every 2 min, and differences in the reduction of the signal from parenchyma and pelvis were detected (69).
Development of the imaging techniques was a stimulus that accelerated spin probe design. A new ester derivative for brain studies was introduced that was stable and reached high levels in the brain (70, 71). 3-Hydroxymethyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl was also applied to brain imaging as a BBB-permeable spin probe. Two-dimensional slices of images of rat brain showing the distribution of the nitroxide were presented (72). Aceto-methoxy esters that are both persistent and concentrate in the intracellular environment have recently been developed (73).
Redox Imaging of Tumors using EPR
Demonstrating differences in the redox state of tissue requires measurement of nitroxide reduction rate, i.e. time resolution of the imaging technique of the order of minutes. Such a functional approach has been successful in many experimental setups.
Two-dimensional sequential images of RIF-1 tumors taken every 2 min confirmed a more rapid reduction of nitroxide in tumor tissue than in muscle (74, 75). In the same tumor type, heterogeneity in redox maps was observed as well as a reduction of the rate with decrease in GSH or an increase in oxygenation. Spatial resolution was 0.2 × 0.2 × 5 mm (38, 76, 77). An example of redox mapping of tumors in animals breathing air and carbogen is shown in Fig. 3. Similarly, nitroxide reduction rate images from tumors on day 5, 7, 12 and 14 of tumor growth show heterogeneity in redox state and changes with time. In animals with depleted thiols, the reduction rate was 24 and 36% slower in normal tissue and tumor, respectively (78).
FIG. 3.
Redox mapping of tumor. After tail vein infusion of 3-CP, a series of 2D images of the nitroxide from tumors of air- or carbogen-breathing mice were measured using the L-band EPR imaging method. The image data were acquired using a magnetic-field gradient of 150 mT/m at 16 orientations in the 2D plane. Two-dimensional spatial mapping of nitroxide distribution, pseudo-first-order rate constant, and frequency plot of the nitroxide decay constant in the tumor tissue of air-breathing (a1–a3) and carbogen-breathing (b1–b3) mice are shown in the figure. From Ilangovan et al. (77) with permission.
A differentiation of normal and tumor tissue was shown in a model of chemically induced gastric cancer in rat. The induction was correlated with generation of a high level of oxidative stress. Three-dimensional images showed a faster rate of reduction nitroxide in the tumor in comparison with normal gastric mucosa. These images were obtained using a surface coil that was introduced into the peritoneal cavity through a small incision (39).
Pulsed EPRI was used to obtain the spatial distribution of 15N-PDT nitroxide in a murine tumor. The signal decayed rapidly below the noise level, preventing kinetics measurements, but a 3D nitroxide spatial distribution map was obtained and an oxygen map was calculated from the linewidths of the nitroxide (79).
Nontumor Oxidative Stress EPR Imaging
Because oxidative stress leads to a faster reduction rate of nitroxides, time-resolved nitroxide imaging allows visualization of oxidative stress in vivo. This approach has been employed in many types of tissues in the context of numerous important pathologies, such as diabetes, ischemia-reperfusion or intoxication.
Two-dimensional images of living mice with carbon-tetrachloride damaged livers have shown much a slower reduction rate in the liver region than in untreated mice (80). An interesting study was performed using 1D imaging of nitroxide reduction in the skin of a living rat. Diverse bioreduction rates of Tempo were shown in different layers of the skin with an image resolution about 0.1 mm. The reduction rate was decreased after UVB irradiation (67, 81).
Oxidative stress was also imaged in the diabetic mouse kidney. The nitroxide reduction rate was much faster in diabetic mice than in the control animals. After treatment with an angiotensin II type 1 receptor blocker, the reduction returned to control levels, confirming the antioxidant properties of this drug (82).
In the heart, myocardial ischemia is a known oxidative stress factor. An infarct region in the rat heart was detected using 3D nitroxide imaging. Faster nitroxide reduction was observed in the risk region (40, 83).
Free Radical Scavengers Clarify the Mechanisms of Oxidative Stress
More in-depth information about the mechanism of oxidative stress and related pathologies can be obtained by applying various free radical or ROS scavengers. Coadministration of specific radical scavengers with nitroxide has served to elucidate the role of specific components of oxidative stress (84). For example, in a diesel exhaust particle (DEP) inhalation model, intratracheal nitroxide reduction was significantly enhanced by DEP in thoracic region (85). The enhanced signal decay of the nitroxide was completely suppressed when radical scavengers were administered along with nitroxides such as dimethylthiourea, dimethylsulfoxide and mannitol. An iron chelator, desferrioxamine also suppressed the enhanced decay. Simultaneous administration of SOD accelerated the enhanced signal decay, while administration of catalase suppressed the acceleration, suggesting that intratracheal exposure to DEP produced hydroxyl radical in the lung through an iron-catalyzed reaction.
Another study employing the same approach to elucidate oxidative mechanisms was performed in a gastric ulcer model in rats (86). In the stomachs of NH4OH-treated rats, nitroxide reduction was faster than that in the stomachs of saline-treated rats after intragastric administration. Coadministration of the hydroxyl radical scavengers mannitol or catalase suppressed the enhanced reduction of nitroxide in a dose-dependent manner.
Even though both of the above studies were performed spectroscopically, they illustrate well the potential scope of the oxidative stress EPRI for studying the mechanisms involved in oxidative stress.
Most in vivo redox state images have been acquired in mice and rats. Most images in rats have been in portions of rats, although Overhauser MRI experiments are carried out with radiofrequencies high enough to visualize human size subjects (87).
Redox Imaging of the Brain
Extensive redox imaging studies were conducted in the rat brain. Early studies focused on the distribution of nitroxides in the rat head (88–90). To obtain high levels of nitroxide in the brain, a continuous infusion was used (91). In time, new blood-brain barrier-permeable nitroxides were developed, and their rates of reduction were compared in the rat cerebral cortex, striatum and hippocampus area (92).
A differential response of hippocampus and cerebral cortex to kainic-acid induced seizure was detected using EPR redox imaging (89). The ability of the intrahippocampus to reduce the nitroxide radical was impaired in the epileptic rats and was intact in the cerebral cortex. These findings were confirmed using an acyl-protected hydroxyl-amine, which was oxidized to form a nitroxide (93). The results showed that the oxidative stress in the hippocampus and striatum was enhanced and not changed relative to that in the control animals in the cerebral cortex.
In another study from the same research center, rats were treated with neuroleptics, which are known to cause oxidative stress in the brain (94). EPR imaging using nitroxides demonstrated that the reducing abilities of the striatum and cerebral cortex decreased in the rats treated with neuroleptics.
Ischemia in the murine brain was found to increase with nitroxide half-life (95). Likewise, in neonatal rats with ischemic-reperfusion injury, the reducing ability of cortex and striatum was diminished, and treatment with an antioxidant agent caused the reduction to return to control level (96). The effects of neonatal stress and acute stress on the redox state of the adult rat brain were also studied using EPR redox imaging. It turned out that the intracerebral reducing ability was significantly increased in rats exposed to neonatal stress and significantly depleted in the same rats that had been subjected to acute stress (41).
REDOX IMAGING USING OMRI
OMRI is a dynamic nuclear polarization technique for imaging free radicals based on MRI (97). It uses the Overhauser effect, in which saturation of electron spin resonance of a free radical causes a polarized nuclear spin state (98).
By applying EPR RF to the two-spin system, which contains electron spin from nitroxide and proton spin of water, relative populations of the four states are modified through dipole-dipole interaction for a nitroxide radical solution (99, 100). This EPR saturation thus results in enhanced proton nuclear magnetic resonance of water protons in the solution compared with thermal equilibrium without EPR RF. In this way, it is possible to image nitroxide content indirectly from changes in proton intensity. The advantage of OMRI approach in free radical imaging is in its high spatial resolution.
Figure 4 shows typical OMRI images after intravenous administration of a blood-brain-permeable nitroxide (101). The spatial resolution of the redox-sensitive nitroxide was 0.5 mm. After 24 h reperfusion after 1 h ischemia, the nitroxide reduction in rat brain was imaged. The images showed that OMRI image intensity decreased significantly slower over time in the ischemic hemisphere than the contralateral hemisphere, although amounts of the nitroxide initially in both hemispheres were the same.
FIG. 4.
Nitroxide reduction in rat brain. Panel A: Time-dependent OMRI image of methoxycarbonyl-PROXYL in the head region and image showing the decay rates. Panel B: Semi-logarithmic plot of image intensities of the contralateral hemisphere (○) and ischemic hemisphere (●) images. Panel C: The signal decay rates of the contralateral hemisphere (open bar) and ischemic hemisphere (closed bar). Panel D: Amount of total methoxycarbonyl-PROXYL in the contralateral hemisphere (open bar) and ischemic hemisphere (closed bar) determined using X-band EPR. Each value represents the mean ± SD of four rats. *P < 0.05 compared with the contralateral hemisphere. From Yamato et al. (101) with permission.
Imaging studies using OMRI/nitroxides demonstrated that redox status can be imaged with high resolution in living animals, both in the pharmacodynamics of nitroxides and analysis of disease models: diverse distribution and bioreduction of nitroxides in living mice (102, 103), heterogeneous redox capacity in tumor bearing mice (104), and decreased brain antioxidant levels and mitochondrial dysfunction in an ischemia-reperfusion mouse model (101). In combination with conventional MRI, a detailed redox map was obtained and used to examine the oxidative mechanisms involved.
A very interesting approach was reported by Utsumi et al. using OMRI and different isotope-labeled nitroxides that could be used for redox molecular imaging (105). Nitroxides with either lipophilic and lipophobic characteristics were labeled with 14N and 15N isotopes and administered to the mouse either intragastrically or intravenously. Both nitroxide distributions in a mouse were imaged with OMRI by excitation of the specific magnetic resonance absorption of each isotopes, resulting in simultaneous separate images of the two nitroxides.
Along with organic synthesis of nitroxides for location-and reaction-specific detection, the OMRI technique appears to be promising for imaging redox related diseases models and the therapeutic effects of redox-related drugs to the diseases.
POTENTIAL PROBLEMS WITH REDOX IMAGING
A number of potential problems have been identified and associated with redox imaging that may interfere with the proper interpretation of these images. These include transport of the nitroxide from the local region, which would confound the interpretation of the diminution of the nitroxide signal as being due to local bioreduction. This concern has largely been allayed by the clever set of experiments comparing an 15N nitroxide whose distribution is largely extracellular and an 14N nitroxide with both the intracellular and extracellular distribution described above (105). These studies allow measurements distinguishing intra- and extracellular processes. The conclusions from experiments comparing both sets of measurements were essentially consistent, allaying concerns about the difficulty with nitroxide transport.
Concern about the effect of broadening of the spectral line by oxygen is a relatively small effect, particularly for nitroxides with natural abundance hydrogen. Hydrogen nuclei cause a peak-to-peak unresolved broadening that is independent of oxygen. This is nearly an order of magnitude larger component of the spectral line width than that caused by oxygen change (106). Similar concerns with self broadening and consequent signal height reduction are otherwise relatively small (107). Finally, these concerns can be alleviated if necessary with spectroscopic imaging with integration over the spectral dimension of the image (108). Other local effects can affect this component (109) but they are, again, small and not likely to substantially affect the bioreduction measurement, where the size of the signal diminishes by factors of three to ten.
Footnotes
The number of spatial dimensions of the EPR image is described as 1, 2 or 3D. Spectral or temporal dimensions are mentioned separately.
L-band (1–2 GHz), 700/750 MHz and 280/250 MHz.
REFERENCES
- 1.Martinovich GG, Martinovich IV, Cherenkevich SN, Sauer H. Redox buffer capacity of the cell: theoretical and experimental approach. Cell Biochem Biophys. 2010;58:75–83. doi: 10.1007/s12013-010-9090-3. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths HR, Moller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A, et al. Biomarkers. Mol Aspects Med. 2002;23:101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 3.Bartosz G. Use of spectroscopic probes for detection of reactive oxygen species. Clin Chim Acta. 2006;368:53–76. doi: 10.1016/j.cca.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 5.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkin S, Mehlhorn RJ, Hideg K, Hankovsky O, Packer L. Reduction and destruction rates of nitroxide spin probes. Arch Biochem Biophys. 1987;256:232–243. doi: 10.1016/0003-9861(87)90441-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen KY, McLaughlin MG. Differences in the reduction kinetics of incorporated spin labels in undifferentiated and differentiated mouse neuroblastoma cells. Biochim Biophys Acta. 1985;845:189–195. doi: 10.1016/0167-4889(85)90176-4. [DOI] [PubMed] [Google Scholar]
- 9.Lukiewicz S, Cieszka K, Wojcik K, Markowska E, Pajak S, Elas M, et al. In vivo ESR studies on the influence of hyperthermia on biological half life of nitroxides in B16 murine melanoma. Phys Med. 1989;V:315–320. [Google Scholar]
- 10.Swartz HM. Use of nitroxides to measure redox metabolism in cells and tissues. J Chem Soc Faraday Trans. 1987;83:191–202. [Google Scholar]
- 11.Finkelstein E, Rosen GM, Rauckman EJ, Paxton J. Spin trapping of superoxide. Mol Pharmacol. 1979;16:676–685. [PubMed] [Google Scholar]
- 12.Rosen GM, Finkelstein E, Rauckman EJ. A method for the detection of superoxide in biological systems. Arch Biochem Biophys. 1982;215:367–378. doi: 10.1016/0003-9861(82)90097-2. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Utsumi H. Development of separable electron spin resonance-computed tomography imaging for multiple radical species: an application to OH and NO. Biophys J. 2000;79:3341–3349. doi: 10.1016/s0006-3495(00)76565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vikram DS, Rivera BK, Kuppusamy P. In vivo imaging of free radicals and oxygen. Methods Mol Biol. 2010;610:3–27. doi: 10.1007/978-1-60327-029-8_1. [DOI] [PubMed] [Google Scholar]
- 15.Fujii H, Itoh K, Pandian RP, Sakata M, Kuppusamy P, Hirata H. Measuring brain tissue oxygenation under oxidative stress by ESR/MR dual imaging system. Magn Reson Med Sci. 2007;6:83–89. doi: 10.2463/mrms.6.83. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura T, Yokoyama H, Fujii S, Takayama F, Oikawa K, Kamada H. In vivo EPR detection and imaging of endogenous nitric oxide in lipopolysaccharide-treated mice. Nat Biotechnol. 1996;14:992–994. doi: 10.1038/nbt0896-992. [DOI] [PubMed] [Google Scholar]
- 17.Halpern HJ, Yu C, Barth E, Peric M, Rosen GM. In situ detection, by spin trapping, of hydroxyl radical markers produced from ionizing radiation in the tumor of a living mouse. Proc Natl Acad Sci USA. 1995;92:796–800. doi: 10.1073/pnas.92.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swartz HM, Sentjurc M, Morse PD., II Cellular metabolism of water-soluble nitroxides: effect on rate of reduction of cell/nitroxide ratio, oxygen concentrations and permeability of nitroxides. Biochim Biophys Acta. 1986;888:82–90. doi: 10.1016/0167-4889(86)90073-x. [DOI] [PubMed] [Google Scholar]
- 19.Kocherginsky M, Swartz H. Nitroxide spin labels: reactions in biology and chemistry. Boca Raton (FL): CRC Press; 1995. [Google Scholar]
- 20.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, et al. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci USA. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weil JT, Veen van der J, Olcott HS. Stable nitroxides as lipid antioxidants. Nature. 1968;219:168–169. doi: 10.1038/219168a0. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Swartz HM. Oxidation of hydroxylamines to nitroxide spin labels in living cells. Biochim Biophys Acta. 1988;970:270–277. doi: 10.1016/0167-4889(88)90126-7. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson UA, Olsson LI, Carlin G, Bylund-Fellenius AC. Inhibition of lipid peroxidation by spin labels. Relationships between structure and function. J Biol Chem. 1989;264:11131–11135. [PubMed] [Google Scholar]
- 25.Samuni A, Krishna CM, Riesz P, Finlelstein E, Russo A. A novel metal-free low molecular weight superoxide dismutase mimic. J Biol Chem. 1988;263:17921–17924. [PubMed] [Google Scholar]
- 26.Mitchell JB, DeGraff W, Kaufman D, Krishna MC, Samuni A, Filkelstein E, et al. Inhibition of oxygen-dependent radiationinduced damage by the nitroxide superoxide dismutase mimic, Tempol. Arch Biochem Biophys. 1991;289:62–70. doi: 10.1016/0003-9861(91)90442-l. [DOI] [PubMed] [Google Scholar]
- 27.Hahn SM, Tochner Z, Krishna CM, Glass J, WIlson L, Samuni A, et al. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992;52:1750–1753. [PubMed] [Google Scholar]
- 28.Chen K, Glockner JF, Morse PD2nd, Swartz HM. Effects of oxygen on the metabolism of nitroxide spin labels in cells. Biochemistry. 1989;28:2496–2501. doi: 10.1021/bi00432a022. [DOI] [PubMed] [Google Scholar]
- 29.Quaresima V, Ursini CL, Gualtieri G, Sotgiu A, Ferrari M. Oxygen-dependent reduction of a nitroxide free radical by electron paramagnetic resonance monitoring of circulating rat blood. Biochim Biophys Acta. 1993;1182:115–118. doi: 10.1016/0925-4439(93)90161-s. [DOI] [PubMed] [Google Scholar]
- 30.Utsumi H, Ichikawa K, Takeshita K. In vivo ESR measurements of free radical reactions in living mice. Toxicol Lett. 1995;82–83:561–565. doi: 10.1016/0378-4274(95)03501-x. [DOI] [PubMed] [Google Scholar]
- 31.Hahn SM, Krishna MC, DeLuca AM, Coffin D, Mitchell JB. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic Biol Med. 2000;28:953–958. doi: 10.1016/s0891-5849(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 32.Gallez B, Bacic G, Goda F, Jiang J, O’Hara JA, Dunn JF, et al. Use of nitroxides for assessing perfusion, oxygenation, and viability of tissues: in vivo EPR and MRI studies. Magn Reson Med. 1996;35:97–106. doi: 10.1002/mrm.1910350113. [DOI] [PubMed] [Google Scholar]
- 33.Hyodo F, Matsumoto K, Matsumoto A, Mitchell JB, Krishna MC. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006;66:9921–9928. doi: 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]
- 34.Swartz HM. Principles of the metabolism of nitroxides and their implications for spin trapping. Free Radic Res Commun. 1990;9:399–405. doi: 10.3109/10715769009145700. [DOI] [PubMed] [Google Scholar]
- 35.Ogata T, Ono M, Fujisawa T, Yoshida E, Kamada H. An example of in vivo analysis by L-band electron-spin-resonance technique using a loop-gap resonator. Chem Lett. 1986:1681–1684. [Google Scholar]
- 36.Miura Y, Hamada A, Utsumi H. In vivo ESR studies of antioxidant activity on free radical reaction in living mice under oxidative stress. Free Radic Res. 1995;22:209–214. doi: 10.3109/10715769509147540. [DOI] [PubMed] [Google Scholar]
- 37.Elas M, Cieszka K, Matuszak Z, Lukiewicz S. Bioreduction of nitroxides in murine tumors with blocked thiols in the light of the in vivo data. Curr Top Biophys. 1996;20:62–66. [Google Scholar]
- 38.Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, et al. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002;62:307–312. [PubMed] [Google Scholar]
- 39.Mikuni T, He G, Petryakov S, Fallouh MM, Deng Y, Ishihara R, et al. In vivo detection of gastric cancer in rats by electron paramagnetic resonance imaging. Cancer Res. 2004;64:6495–6502. doi: 10.1158/0008-5472.CAN-04-0319. [DOI] [PubMed] [Google Scholar]
- 40.Velayutham M, Li H, Kuppusamy P, Zweier JL. Mapping ischemic risk region and necrosis in the isolated heart using EPR imaging. Magn Reson Med. 2003;49:1181–1187. doi: 10.1002/mrm.10473. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama H, Morinobu S, Ueda Y. EPRI to estimate the in vivo intracerebral reducing ability in adolescent rats subjected to neonatal isolation. J Magn Reson Imaging. 2006;23:637–640. doi: 10.1002/jmri.20560. [DOI] [PubMed] [Google Scholar]
- 42.Brasch RC, London DA, Wesbey GE, Tozer TN, Nitecki DE, Williams RD, et al. Work in progress: nuclear magnetic resonance study of a paramagnetic nitroxide contrast agent for enhancement of renal structures in experimental animals. Radiology. 1983;147:773–779. doi: 10.1148/radiology.147.3.6844613. [DOI] [PubMed] [Google Scholar]
- 43.Griffeth LK, Rosen GM, Rauckman EJ, Drayer BP. Pharmacokinetics of nitroxide NMR contrast agents. Magn Reson Med. 1984;1:159–160. doi: 10.1097/00004424-198411000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Hyodo F, Murugesan R, Matsumoto K, Hyodo E, Subramanian S, Mitchell JB, et al. Monitoring redox-sensitive paramagnetic contrast agent by EPRI, OMRI and MRI. J Magn Reson. 2008;190:105–112. doi: 10.1016/j.jmr.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto K, Subramanian S, Murugesan R, Mitchell JB, Krishna MC. Spatially resolved biologic information from in vivo EPRI, OMRI, and MRI. Antioxid Redox Signal. 2007;9:1125–1141. doi: 10.1089/ars.2007.1638. [DOI] [PubMed] [Google Scholar]
- 46.Berliner JL, Fujii H. Magnetic resonance imaging of biological specimens by electron paramagnetic resonance of nitroxide spin labels. Science. 1985;227:517–519. doi: 10.1126/science.2981437. [DOI] [PubMed] [Google Scholar]
- 47.Fujii H, Berliner LJ. One- and two-dimensional EPR imaging studies on phantoms and plant specimens. Magn Reson Med. 1985;2:275–282. doi: 10.1002/mrm.1910020310. [DOI] [PubMed] [Google Scholar]
- 48.Berliner LJ, Fujii H, Wan XM, Lukiewicz SJ. Feasibility study of imaging a living murine tumor by electron paramagnetic resonance. Magn Reson Med. 1987;4:380–384. doi: 10.1002/mrm.1910040410. [DOI] [PubMed] [Google Scholar]
- 49.Dobrucki JW, Demsar F, Walczak T, Woods RK, Bacic G, Swartz HM. Electron spin resonance microscopy of an in vitro tumour model. Br J Cancer. 1990;61:221–224. doi: 10.1038/bjc.1990.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alecci M, Colacicchi S, Indovina PL, Momo F, Pavone P, Sotgiu A. Three-dimensional in vivo ESR imaging in rats. Magn Reson Imaging. 1990;8:59–63. doi: 10.1016/0730-725x(90)90213-l. [DOI] [PubMed] [Google Scholar]
- 51.Colacicchi S, Ferrari M, Sotgiu A. In vivo electron paramagnetic resonance spectroscopy/imaging: First experiences, problems, and perspectives. Int J Biochem. 1992;24:205–214. doi: 10.1016/0020-711x(92)90248-y. [DOI] [PubMed] [Google Scholar]
- 52.Quaresima V, Alecci M, Ferrari M, Sotgiu A. Whole rat electron paramagnetic resonance imaging of a nitroxide free radical by a radio frequency (280 MHz) spectrometer. Biochem Biophys Res Commun. 1992;183:829–835. doi: 10.1016/0006-291x(92)90558-3. [DOI] [PubMed] [Google Scholar]
- 53.Alecci M, Ferrari M, Quaresima V, Sotgiu A, Ursini CL. Simultaneous 280 MHz EPR imaging of rat organs during nitroxide free radical clearance. Biophys J. 1994;67:1274–1279. doi: 10.1016/S0006-3495(94)80599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishida S, Matsumoto S, Yokoyama H, Mori N, Kumashiro H, Tsuchihashi N, et al. An ESR-CT imaging of the head of a living rat receiving an administration of a nitroxide radical. Magn Reson Imaging. 1992;10:109–114. doi: 10.1016/0730-725x(92)90379-e. [DOI] [PubMed] [Google Scholar]
- 55.Kazama S, Takashige G, Yoshioka H, Tanizawa H, Ogata T, Koscielniak J, et al. Dynamic electron spin resonance (ESR) imaging of the distribution of spin labeled dextran in a mouse. Magn Reson Med. 1996;36:547–550. doi: 10.1002/mrm.1910360407. [DOI] [PubMed] [Google Scholar]
- 56.Halpern HJ, Peric M, Yu C, Barth ED, Chandramouli GV, Makinen MW, et al. In vivo spin-label murine pharmacodynamics using low-frequency electron paramagnetic resonance imaging. Biophys J. 1996;71:403–409. doi: 10.1016/S0006-3495(96)79241-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuppusamy P, Chzhan M, Vij K, Shteynbuk M, Lefer DJ, Giannella E, et al. Three-dimensional spectral-spatial EPR imaging of free radicals in the heart: a technique for imaging tissue metabolism and oxygenation. Proc Natl Acad Sci USA. 1994;91:3388–3392. doi: 10.1073/pnas.91.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuppusamy P, Wang P, Zweier JL. Three-dimensional spatial EPR imaging of the rat heart. Magn Reson Med. 1995;34:99–105. doi: 10.1002/mrm.1910340115. [DOI] [PubMed] [Google Scholar]
- 59.Kuppusamy P, Chzhan M, Samouilov A, Wang P, Zweier JL. Mapping the spin-density and lineshape distribution of free radicals using 4D spectral-spatial EPR imaging. J Magn Reson B. 1995;107:116–125. doi: 10.1006/jmrb.1995.1067. [DOI] [PubMed] [Google Scholar]
- 60.Kuppusamy P, Wang P, Zweier JL, Krishna MC, Mitchell JB, Ma L, et al. Electron paramagnetic resonance imaging of rat heart with nitroxide and polynitroxyl-albumin. Biochemistry. 1996;35:7051–7057. doi: 10.1021/bi952857s. [DOI] [PubMed] [Google Scholar]
- 61.Zweier JL, Chzhan M, Samouilov A, Kuppusamy P. Electron paramagnetic resonance imaging of the rat heart. Phys Med Biol. 1998;43:1823–1835. doi: 10.1088/0031-9155/43/7/002. [DOI] [PubMed] [Google Scholar]
- 62.Zweier JL, Kuppusamy P. Electron paramagnetic resonance measurements of free radicals in the intact beating heart: a technique for detection and characterization of free radicals in whole biological tissues. Proc Natl Acad Sci USA. 1988;85:5703–5707. doi: 10.1073/pnas.85.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yokoyama H, Sato T, Tsuchihashi N, Ogata T, Ohya-Nishiguchi H, Kamada H. A CT using longitudinally detected ESR (LODESR-CT) of intraperitoneally injected nitroxide radical in a rat’s head. Magn Reson Imaging. 1997;15:701–708. doi: 10.1016/s0730-725x(97)82763-4. [DOI] [PubMed] [Google Scholar]
- 64.McCallum SJ, Nicholson I, Lurie DJ. Multimodality magnetic resonance systems for studying free radicals in vivo. Phys Med Biol. 1998;43:1857–1861. doi: 10.1088/0031-9155/43/7/006. [DOI] [PubMed] [Google Scholar]
- 65.Seimenis I, Foster MA, Lurie DJ, Hutchison JM, Whiting PH, Payne S. The excretion mechanism of the spin label proxyl carboxylic acid (PCA) from the rat monitored by X-band ESR and PEDRI. Magn Reson Med. 1997;37:552–558. doi: 10.1002/mrm.1910370413. [DOI] [PubMed] [Google Scholar]
- 66.He G, Deng Y, Li H, Kuppusamy P, Zweier JL. EPR/NMR coimaging for anatomic registration of free-radical images. Magn Reson Med. 2002;47:571–578. doi: 10.1002/mrm.10077. [DOI] [PubMed] [Google Scholar]
- 67.He G, Samouilov A, Kuppusamy P, Zweier JL. In vivo EPR imaging of the distribution and metabolism of nitroxide radicals in human skin. J Magn Reson. 2001;148:155–164. doi: 10.1006/jmre.2000.2226. [DOI] [PubMed] [Google Scholar]
- 68.He G, Samouilov A, Kuppusamy P, Zweier JL. In vivo imaging of free radicals: applications from mouse to man. Mol Cell Biochem. 2002;234–235:359–367. [PubMed] [Google Scholar]
- 69.Ueda A, Yokoyama H, Nagase S, Hirayama A, Koyama A, Ohya H, et al. In vivo temporal EPR imaging for estimating the kinetics of a nitroxide radical in the renal parenchyma and pelvis in rats. Magn Reson Imaging. 2002;20:77–82. doi: 10.1016/s0730-725x(02)00467-8. [DOI] [PubMed] [Google Scholar]
- 70.Sano H, Matsumoto K, Utsumi H. Synthesis and imaging of blood-brain-barrier permeable nitroxyl-probes for free radical reactions in brain of living mice. Biochem Mol Biol Int. 1997;42:641–647. doi: 10.1080/15216549700203051. [DOI] [PubMed] [Google Scholar]
- 71.Sano H, Naruse M, Matsumoto K, Oi T, Utsumi H. A new nitroxyl-probe with high retention in the brain and its application for brain imaging. Free Radic Biol Med. 2000;28:959–969. doi: 10.1016/s0891-5849(00)00184-2. [DOI] [PubMed] [Google Scholar]
- 72.Itoh O, Obara H, Aoyama M, Ohya H, Kamada H. Development of a functional spin probe for in vivo ESR measurement. Anal Sci. 2001;17:1515–1517. ICAS2001. [Google Scholar]
- 73.Rosen GM, Burks SR, Kohr MJ, Kao JP. Synthesis and biological testing of aminoxyls designed for long-term retention by living cells. Org Biomol Chem. 2005;3:645–648. doi: 10.1039/b415586f. [DOI] [PubMed] [Google Scholar]
- 74.Kuppusamy P, Afeworki M, Shankar RA, Coffin D, Krishna MC, Hahn SM, et al. In vivo electron paramagnetic resonance imaging of tumor heterogeneity and oxygenation in a murine model. Cancer Res. 1998;58:1562–1568. [PubMed] [Google Scholar]
- 75.Kuppusamy P, Wang P, Shankar RA, Ma L, Trimble CE, Hsia CJ, et al. In vivo topical EPR spectroscopy and imaging of nitroxide free radicals and polynitroxyl-albumin. Magn Reson Med. 1998;40:806–811. doi: 10.1002/mrm.1910400604. [DOI] [PubMed] [Google Scholar]
- 76.Kuppusamy P, Krishna CM. EPR imaging of tissue redox status. Curr Top Biophys. 2002;26:29–32. [Google Scholar]
- 77.Ilangovan G, Li HQ, Zweier JL, Krishna MC, Mitchell JB, Kuppusamy P. In vivo measurement of regional oxygenation and Imaging of redox status in RIF-1 murine tumor: Effect of carbogen-breathing. Magn Reson Med. 2002;48:723–730. doi: 10.1002/mrm.10254. [DOI] [PubMed] [Google Scholar]
- 78.Yamada KI, Kuppusamy P, English S, Yoo J, Irie A, Subramanian S, et al. Feasibility and assessment of non-invasive in vivo redox status using electron paramagnetic resonance imaging. Acta Radiol. 2002;43:433–440. doi: 10.1080/j.1600-0455.2002.430418.x. [DOI] [PubMed] [Google Scholar]
- 79.Hyodo F, Matsumoto S, Devasahayam N, Dharmaraj C, Subramanian S, Mitchell JB, et al. Pulsed EPR imaging of nitroxides in mice. J Magn Reson. 2009;197:181–185. doi: 10.1016/j.jmr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Togashi H, Shinzawa H, Ogata T, Matsuo T, Ohno S, Saito K, et al. Spatiotemporal measurement of free radical elimination in the abdomen using an in vivo ESR-CT imaging system. Free Radic Biol Med. 1998;25:1–8. doi: 10.1016/s0891-5849(97)00385-7. [DOI] [PubMed] [Google Scholar]
- 81.He G, Kumar Kutala V, Kuppusamy P, Zweier JL. In vivo measurement and mapping of skin redox stress induced by ultraviolet light exposure. Free Radic Biol Med. 2004;36:665–672. doi: 10.1016/j.freeradbiomed.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 82.Sonta T, Inoguchi T, Matsumoto S, Yasukawa K, Inuo M, Tsubouchi H, et al. In vivo imaging of oxidative stress in the kidney of diabetic mice and its normalization by angiotensin II type 1 receptor blocker. Biochem Biophys Res Commun. 2005;330:415–422. doi: 10.1016/j.bbrc.2005.02.174. [DOI] [PubMed] [Google Scholar]
- 83.He G. Electron paramagnetic resonance oximetry and redoximetry. Methods Mol Biol. 2010;594:85–105. doi: 10.1007/978-1-60761-411-1_6. [DOI] [PubMed] [Google Scholar]
- 84.Utsumi H, Yamada K. In vivo electron spin resonance-computed tomography/nitroxyl probe technique for non-invasive analysis of oxidative injuries. Arch Biochem Biophys. 2003;416:1–8. doi: 10.1016/s0003-9861(03)00285-6. [DOI] [PubMed] [Google Scholar]
- 85.Han JY, Takeshita K, Utsumi H. Noninvasive detection of hydroxyl radical generation in lung by diesel exhaust particles. Free Radic Biol Med. 2001;30:516–525. doi: 10.1016/s0891-5849(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 86.Kasazaki K, Yasukawa K, Sano H, Utsumi H. Non-invasive analysis of reactive oxygen species generated in NH4OH-induced gastric lesions of rats using a 300 MHz in vivo ESR technique. Free Radic Res. 2003;37:757–766. doi: 10.1080/1071576031000103069. [DOI] [PubMed] [Google Scholar]
- 87.Lurie DJ, Foster MA, Yeung D, Hutchison JMS. Design, construction and use of a large-sample field-cycled PEDRI imager. Phys Med Biol. 1998;43:1877–1886. doi: 10.1088/0031-9155/43/7/008. [DOI] [PubMed] [Google Scholar]
- 88.Yokoyama H, Itoh O, Ogata T, Obara H, Ohya-Nishiguchi H, Kamada H. Temporal brain imaging by a rapid scan ESR-CT system in rats receiving intraperitoneal injection of a methyl ester nitroxide radical. Magn Reson Imaging. 1997;15:1079–1084. doi: 10.1016/s0730-725x(97)00136-7. [DOI] [PubMed] [Google Scholar]
- 89.Yokoyama H, Lin Y, Itoh O, Ueda Y, Nakajima A, Ogata T, et al. EPR imaging for in vivo analysis of the half-life of a nitroxide radical in the hippocampus and cerebral cortex of rats after epileptic seizures. Free Radic Biol Med. 1999;27(3–4):442–448. doi: 10.1016/s0891-5849(99)00093-3. [DOI] [PubMed] [Google Scholar]
- 90.Yokoyama H, Sato T, Ohya-Nishiguchi H, Kamada H. In vivo 300 MHz longitudinally detected ESR-CT imaging in the head of a rat treated with a nitroxide radical. MAGMA. 1998;7:63–68. doi: 10.1007/BF02592230. [DOI] [PubMed] [Google Scholar]
- 91.Ueda Y, Yokoyama H, Ohya-Nishiguchi H, Kamada H. ESR imaging of the rat brain with a nitroxide radical perfused by in vivo microdialysis. Magn Reson Imaging. 1997;15:355–360. doi: 10.1016/s0730-725x(96)00141-5. [DOI] [PubMed] [Google Scholar]
- 92.Yokoyama H, Itoh O, Aoyama M, Obara H, Ohya H, Kamada H. In vivo temporal EPR imaging of the brain of rats by using two types of blood-brain barrier-permeable nitroxide radicals. Magn Reson Imaging. 2002;20:277–284. doi: 10.1016/s0730-725x(02)00491-5. [DOI] [PubMed] [Google Scholar]
- 93.Yokoyama H, Itoh O, Aoyama M, Obara H, Ohya H, Kamada H. In vivo EPR imaging by using an acyl-protected hydroxylamine to analyze intracerebral oxidative stress in rats after epileptic seizures. Magn Reson Imaging. 2000;18:875–879. doi: 10.1016/s0730-725x(00)00183-1. [DOI] [PubMed] [Google Scholar]
- 94.Yokoyama H, Itoh O, Ohya-Nishiguchi H, Kamada H. Reducing ability of the striatum and cerebral cortex in rats following acute administration of risperidone or haloperidol: an estimation by in vivo electron paramagnetic resonance imaging. Neurochem Res. 2002;27:243–248. doi: 10.1023/a:1014840722626. [DOI] [PubMed] [Google Scholar]
- 95.Fujii H, Sato-Akaba H, Kawanishi K, Hirata H. Mapping of redox status in a brain-disease mouse model by three-dimensional EPR imaging. Magn Reson Med. 2011;65:295–303. doi: 10.1002/mrm.22598. [DOI] [PubMed] [Google Scholar]
- 96.Yokoyama H, Ueda Y, Itoh O, Ikeda T, Noor JI, Ikenoue T. EPR imaging to estimate the in vivo intracerebral reducing ability of mature rats after neonatal hypoxic-ischemic brain injury. Magn Reson Imaging. 2004;22:1305–1309. doi: 10.1016/j.mri.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Lurie DJ, Hutchison JMS, Bell LH, Nicholson I, Bussell DM, Mallard JR. Field-cycled proton electron double-resonance imaging of free-radicals in large aqueous samples. J Magn Reson. 1989;84:431–437. [Google Scholar]
- 98.Overhauser AW. Paramagnetic relaxation in metals. Phys Rev. 1953;89:689–700. [Google Scholar]
- 99.Guiberteau T, Grucker D. EPR spectroscopy by dynamic nuclear polarization in low magnetic field. J Magn Reson Ser B. 1996;110:47–54. [Google Scholar]
- 100.Benial AM, Ichikawa K, Murugesan R, Yamada KI, Utsumi H. Dynamic nuclear polarization properties of nitroxyl radicals used in Overhauser-enhanced MRI for simultaneous molecular imaging. J Magn Reson. 2006;182:273–282. doi: 10.1016/j.jmr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 101.Yamato M, Shiba T, Yamada K, Watanabe T, Utsumi H. Noninvasive assessment of the brain redox status after transient middle cerebral artery occlusion using Overhauser-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab. 2009;29:1655–1664. doi: 10.1038/jcbfm.2009.84. [DOI] [PubMed] [Google Scholar]
- 102.Li H, He G, Deng Y, Kuppusamy P, Zweier JL. In vivo proton electron double resonance imaging of the distribution and clearance of nitroxide radicals in mice. Magn Reson Med. 2006;55:669–675. doi: 10.1002/mrm.20804. [DOI] [PubMed] [Google Scholar]
- 103.Foster MA, Seimenis I, Lurie DJ. The application of PEDRI to the study of free radicals in vivo. Phys Med Biol. 1998;43:1893–1897. doi: 10.1088/0031-9155/43/7/010. [DOI] [PubMed] [Google Scholar]
- 104.Matsumoto K, Hyodo F, Matsumoto A, Koretsky AP, Sowers AL, Mitchell JB, et al. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redoxsensitive contrast agents. Clin Cancer Res. 2006;12:2455–2462. doi: 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- 105.Utsumi H, Yamada K, Ichikawa K, Sakai K, Kinoshita Y, Matsumoto S, et al. Simultaneous molecular imaging of redox reactions monitored by Overhauser-enhanced MRI with 14N- and 15N-labeled nitroxyl radicals. Proc Natl Acad Sci USA. 2006;103:1463–1468. doi: 10.1073/pnas.0510670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Halpern HJ, Pou S, Peric M, Yu C, Barth E, Rosen GM. Detection and imaging of oxygen-centered free radicals with lowfrequency electron paramagnetic resonance and signal-enhancing deuterium-containing spin traps. J Am Chem Soc. 1993;115:218–223. [Google Scholar]
- 107.Alecci M, Seimenis I, McCallum SJ, Lurie DJ, Foster MA. Nitroxide free radical clearance in the live rat monitored by radiofrequency CW-EPR and PEDRI. Phys Med Biol. 1998;43:1899–1905. doi: 10.1088/0031-9155/43/7/011. [DOI] [PubMed] [Google Scholar]
- 108.Williams BB, Pan XC, Halpern HJ. EPR imaging: The relationship between CW spectra acquired from an extended sample subjected to fixed stepped gradients and the Radon transform of the resonance density. J Magn Reson. 2005;174:88–96. doi: 10.1016/j.jmr.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bales BL, Blum RA, Mareno D, Peric M, Halpern HJ. Solvent and temperature dependence of the hyperfine coupling constants in CTPO. J Magn Reson. 1992;98:299–307. [Google Scholar]