Abstract
BACKGROUND
In patients with ischemic stroke, endovascular treatment results in a higher rate of recanalization of the affected cerebral artery than systemic intravenous thrombolytic therapy. However, comparison of the clinical efficacy of the two approaches is needed.
METHODS
We randomly assigned 362 patients with acute ischemic stroke, within 4.5 hours after onset, to endovascular therapy (intraarterial thrombolysis with recombinant tissue plasminogen activator [t-PA], mechanical clot disruption or retrieval, or a combination of these approaches) or intravenous t-PA. Treatments were to be given as soon as possible after randomization. The primary outcome was survival free of disability (defined as a modified Rankin score of 0 or 1 on a scale of 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability despite symptoms, and 6 death) at 3 months.
RESULTS
A total of 181 patients were assigned to receive endovascular therapy, and 181 intravenous t-PA. The median time from stroke onset to the start of treatment was 3.75 hours for endovascular therapy and 2.75 hours for intravenous t-PA (P<0.001). At 3 months, 55 patients in the endovascular-therapy group (30.4%) and 63 in the intravenous t-PA group (34.8%) were alive without disability (odds ratio adjusted for age, sex, stroke severity, and atrial fibrillation status at baseline, 0.71; 95% confidence interval, 0.44 to 1.14; P = 0.16). Fatal or nonfatal symptomatic intracranial hemorrhage within 7 days occurred in 6% of the patients in each group, and there were no significant differences between groups in the rates of other serious adverse events or the case fatality rate.
CONCLUSIONS
The results of this trial in patients with acute ischemic stroke indicate that endovascular therapy is not superior to standard treatment with intravenous t-PA. (Funded by the Italian Medicines Agency, ClinicalTrials.gov number, NCT00640367.)
Intravenous recombinant tissue plasminogen activator (t-PA) is the standard treatment for acute ischemic stroke, but more than half the treated patients do not recover completely or die.1 Alternative treatments, such as endovascular treatment, have been used for many years. As compared with endovascular treatment, intravenous thrombolysis is associated with a lower probability of recanalization2–9 (46% of cases with intravenous t-PA vs. >80% with endovascular treatment10–15). Nevertheless, the two approaches have not been directly compared, re-canalization is not invariably associated with a favorable clinical outcome,16 and it is not known whether clinical outcomes are superior with endovascular therapy as compared with intravenous t-PA.
Although previous randomized, controlled clinical trials of endovascular treatment yielded promising results,8,15,17 the generalizability of these results remains questionable, because the trials involved highly selected patients, did not compare endovascular treatment with intravenous t-PA, and did not assess endovascular treatment as a multimodal procedure. Many case series and observational cohort studies of endovascular treatment have shown encouraging clinical results, but there have been concerns about selection and publication biases.18
To investigate whether endovascular treatment, including the options of a mechanical device and intraarterial t-PA, is more effective than the currently available treatment with intravenous t-PA, we randomly assigned a total of 362 patients to the two treatment options, after a pilot study showed that prompt initiation of endovascular treatment is a safe and feasible alternative to intravenous t-PA.19
METHODS
STUDY DESIGN
This was a pragmatic, multicenter, open-treatment clinical trial with a blinded end point20 (see Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org), designed to test whether outcomes were better with endovascular treatment than with intravenous t-PA. The study protocol was approved by the institutional review board at each participating center and is available at NEJM.org. The authors vouch for the completeness and accuracy of the data and data analysis and for the fidelity of this report to the study protocol. The study was funded by the Italian Medicines Agency (AIFA). The AIFA reimbursed participating hospitals for the catheters and devices used in the trial and purchased t-PA from Boehringer Ingelheim Italia for use in the endovascular-treatment group. There was no industry support for or industry involvement in this trial.
INCLUSION AND EXCLUSION CRITERIA
Patients with acute stroke and an age of 18 to 80 years, in whom intracranial hemorrhage had been ruled out, were eligible if there was a clearly defined time of stroke onset that allowed for immediate initiation of intravenous t-PA therapy (defined as within 4.5 hours after symptom onset) or for the administration of endovascular treatment as soon as possible (within 6 hours after symptom onset). Inclusion and exclusion criteria are listed in detail in the protocol.20 Competent patients gave written informed consent before enrollment; otherwise, a witnessed waiver of consent was possible.21
RANDOMIZATION
The study protocol provided for centralized, simple randomization online. A single randomization list was prepared with the use of a hardware system, available at www.random.org. All patients underwent randomization within 4.5 hours after symptom onset.
ENDOVASCULAR TREATMENT
Patients who were assigned to this treatment group did not receive intravenous t-PA while awaiting endovascular treatment. Angiography was targeted to acquire data essential for guiding endovascular therapy. Anticoagulant therapy was recommended with an initial bolus dose of 5000 IU of intravenous heparin, followed by an infusion of 500 IU per hour until the conclusion of the angiography. Once the diagnostic information had been acquired, the interventionist could consider pharmacologic or mechanical thrombolysis or both. For pharmacologic thrombosis, a micro-catheter was to be positioned close to (or within or beyond) the thrombus with the use of a micro-guide; the full t-PA dose infused did not exceed 0.9 mg per kilogram of body weight (maximum, 90 mg for patients with a body weight of ≥100 kg) and was to be delivered within 1 hour. If complete recanalization was achieved before the maximum dose was reached, the t-PA infusion was stopped. The option of mechanical thrombolysis was left to each interventionist’s discretion. Mechanical thrombosis could involve the use of a micro-guidewire to facilitate disintegration of the thrombus, systems to capture and extract the thrombus, or more complex systems to crush and aspirate it.
In patients with a neurologic deficit but no corresponding occlusion, the endovascular procedure involved injecting t-PA into the vascular area that was presumably affected. The amount of drug to be injected, which again did not exceed 0.9 mg per kilogram (maximum, 90 mg for patients weighing ≥100 kg), was at the operator’s discretion. If the patient had no neurologic deficit, t-PA was not given. The choice of general anesthesia or sedation to carry out a procedure was discretionary.
INTRAVENOUS THROMBOLYSIS
Systemic thrombolytic treatment was to be started immediately after randomization, within 4.5 hours after symptom onset. Intravenous t-PA was administered at a dose of 0.9 mg per kilogram (maximum, 90 mg), with 10% given as an initial bolus and the remaining 90% as a constant infusion over a period of 60 minutes.
ASSOCIATED THERAPIES
All patients in both treatment groups were given the most appropriate therapy related to the treatments that they were assigned to receive. Anti-platelet and anticoagulant agents were to be avoided during the first 24 hours after symptom onset, with the exception of heparin used during endovascular treatment and antiplatelet therapy if a stent was to be deployed during the procedure.
ASSESSMENT OF PATIENTS
Neurologic deficit was quantified with the use of the National Institutes of Health Stroke Scale (NIHSS), a 15-item scale that rates the level of neurologic impairment.22 Total NIHSS scores range from 0 to 42, with higher scores indicating more severe cerebral infarctions (scores of ≤6 indicate mild neurologic impairment, and scores of ≥25 indicate very severe impairment). Examiners were trained and certified in the use of the NIHSS. Patients were assessed with the scale at baseline and on day 7 or at discharge or transfer to another hospital, whichever occurred first.
Long-term clinical condition was assessed 90 days after randomization by means of a telephone interview by a single neurologist, who was not aware of treatment assignments and who had specific training in outcome assessment with the modified Rankin Scale (which ranges from 0 to 6, with 0 indicating no symptoms and 6 indicating death); the examiner used a checklist of daily activities as a guide in questioning the patient.23,24 If a patient was not available, a proxy was interviewed.
OUTCOMES AND SAFETY MEASURES
The primary outcome was disability-free survival at 90 days, with freedom from disability defined as a modified Rankin score of 0 (no symptoms) or 1 (no clinically significant disability despite symptoms). Secondary outcomes included the proportion of patients with a mild neurologic deficit or none (NIHSS score, ≤6) and the following safety measures, assessed at day 7 after thrombolysis: fatal and nonfatal symptomatic intracranial hemorrhage, fatal and nonfatal symptomatic edema from an original brain infarction, fatal and nonfatal recurrent ischemic stroke, death from any cause, neurologic deterioration (defined as a increase of ≥4 points in the NIHSS score), and fatal and nonfatal extracerebral events. Evaluations of secondary end points were performed by local neurologists, who were aware of treatment assignments.
STATISTICAL ANALYSIS
The estimation of the sample size for the primary outcome was based on a standard test of two samples per difference in binomial proportions (two-tailed test) with an alpha level of 5% and a power of 80%. The study was designed to verify or refute an absolute difference of 15 percentage points between the proportions of patients with a favorable outcome in the two treatment groups. The rationale for this effect size was based on the results of the pilot phase of the trial,19 which showed a nonsignificant absolute difference of 20 percentage points in favor of endovascular treatment over intravenous t-PA; the favorable data on recanalization rates with endovascular treatment (a difference of 17 to 37 percentage points in indirect comparisons with intravenous t-PA10); and the need for a clinical effect sufficiently large to justify the switching from a well-established and simple procedure to one that is newer, more expensive, and more difficult to perform. We calculated that we would need to enroll at least 172 patients per study group, assuming that 40% of those treated with intravenous t-PA would have a favorable outcome.
Intention-to-treat analyses were used throughout the study. All analyses were performed by a statistician who was not aware of treatment assignments. The primary analysis compared the effects of endovascular treatment and intravenous t-PA on survival without disability (modified Rankin score of 0 or 1) at 90 days after enrollment; for this purpose, the modified Rankin scores were dichotomized as 0 or 1 versus 2 to 6 (including death). The binary score was then cross-tabulated against the type of treatment, and the results were evaluated with the use of a two-tailed Fisher’s exact test.
From the same tabulation, the Mantel–Haenszel odds ratio and its 95% confidence interval were obtained. A multivariate logistic-regression model was also used, with the binary end points as dependent variables, and including as independent regressors the type of treatment and some possible confounders together with other variables having possible clinical relevance (age, sex, severity of neurologic deficit, and status with respect to atrial fibrillation). The 90-day relative survivorship (the proportion of observed to expected number of survivors) was also included in the analysis, assessed with the use of the Kaplan–Meier product-limit method, followed by the log-rank test.
Secondary analyses were performed on safety measures, again with the use of Fisher’s exact test. Subgroup analysis was then planned according to the main prognostic variables. All analyses were performed with the use of the Stata/SE 12.1 statistical package (StataCorp); a P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
CHARACTERISTICS OF THE PATIENTS
Recruitment started on February 1, 2008, and ended on April 16, 2012. During this period, 362 patients with acute ischemic stroke underwent randomization (181 to endovascular treatment and 181 to intravenous t-PA). No patients were lost to follow-up, and no patients dropped out of the study (Fig. S1 in the Supplementary Appendix).
The two groups were generally well matched with regard to baseline characteristics (Table 1), except for atrial fibrillation, which was less frequent in the endovascular-therapy group than in the intravenous t-PA group (in 8% of patients vs. 16%, P = 0.02), and a diagnosis of dissection as the cause of the stroke, which was more frequent in the endovascular-therapy group (8% vs. 2%, P = 0.03).
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Endovascular Treatment (N = 181) | Intravenous t-PA (N = 181) |
|---|---|---|
| Age — yr | 66±11 | 67±11 |
| Male sex — no. (%) | 106 (59) | 103 (57) |
| Weight — kg | 75±14 | 75±13 |
| Blood pressure — mm Hg | ||
| Systolic | 155±26 | 150±23 |
| Diastolic | 84±12 | 83±12 |
| NIHSS score† | ||
| Median (interquartile range) | 13 (9–17) | 13 (9–18) |
| Range | 2–26 | 3–24 |
| Atrial fibrillation — no. (%) | 14 (8)‡ | 29 (16)‡ |
| Antihypertensive therapy — no. (%) | 102 (56) | 105 (58) |
| Antidiabetic therapy — no. (%) | 20 (11) | 19 (10) |
| Antiplatelet therapy — no. (%) | 73 (40) | 59 (33) |
| Stroke cause on day 7 — no. (%) | ||
| Cardiogenic embolism | 58 (32) | 62 (34) |
| Dissection | 14 (8)§ | 4 (2)§ |
| Large-artery atherosclerosis | 55 (30) | 50 (28) |
| Small-vessel disease | 13 (7) | 12 (7) |
| Other or unknown | 39 (22) | 53 (29) |
| Condition mimicking stroke | 2 (1)¶ | 0 |
| Stroke territory on day 7 — no. (%) | ||
| Anterior circulation | 160 (88) | 170 (94) |
| Posterior circulation | 18 (10) | 11 (6) |
| Anterior and posterior circulation | 1 (1) | 0 |
| Time from stroke onset to randomization — hr:min | ||
| Median (interquartile range) | 2:28 (2:04–3:10) | 2:25 (1:59–2:59) |
| Range | 0:45–4:30 | 0:30–4:17 |
| Time from stroke onset to start of treatment — hr:min|| | ||
| Median (interquartile range) | 3:45 (3:14–4:20)** | 2:45 (2:20–3:20)** |
| Range | 1:30–5:55 | 0:55–4:30 |
Plus–minus values are means ±SD. There were no significant differences between groups unless otherwise indicated. The abbreviation t-PA denotes tissue plasminogen activator.
Scores on the National Institutes of Health Stroke Scale (NIHSS) range from 0 to 42, with higher scores indicating more severe neurologic impairment.
P = 0.02.
P = 0.03. The higher frequency of dissections in the endovascular-treatment group was probably due to the use of angiography as a diagnostic supplement.
Conditions mimicking stroke were seizure in one patient and somatoform disorder in one patient.
The time from stroke onset to the start of treatment in the endovascular-treatment group was calculated to the beginning of intraarterial pharmacologic or mechanical thrombolysis. Patients who did not receive the assigned treatment (endovascular treatment or intravenous t-PA) were not included.
P<0.001.
TREATMENT METHOD
Of the 181 patients assigned to endovascular treatment, 15 did not receive the treatment (6 because of clinical improvement, 3 because of a lack of evidence of occlusion, 3 because of dissection, 1 because of an unknown bleeding diathesis, 1 because of a groin hematoma, and 1 because of the delayed availability of the interventionist). Three procedures had to be interrupted, owing to equipment breakdown (in one procedure) and intraprocedural complications (in two procedures). Endovascular treatment was thus completed in 163 patients.
Among the 165 patients who received endovascular treatment without an equipment breakdown requiring interruption, locoregional infusion of t-PA and fragmentation of the thrombus with a micro-guidewire were achieved in 109 patients, and in 56 patients, a device was added. The median t-PA dose was 40 mg (interquartile range, 20 to 50). The most widely used devices were Solitaire (EV3/Covidien; in 18 patients), Penumbra (Penumbra; in 9 patients), Trevo (Concentric/Stryker; in 5 patients), and Merci (Concentric/Stryker; in 5 patients). During the procedure, intravenous heparin was infused in 57 patients, and 22 patients underwent general anesthesia.
In patients assigned to intravenous t-PA, the median dose of t-PA was 66 mg (interquartile range, 59 to 72). Three patients did not receive the treatment (one because of spontaneous improvement and two because they underwent thrombectomy).
EFFICACY
The primary outcome at 90 days is shown in Figure 1. A total of 55 of the 181 patients (30.4%) in the endovascular-treatment group survived with-out disability (modified Rankin score, 0 or 1), as compared with 63 of the 181 patients (34.8%) in the intravenous t-PA group (absolute difference, −4.4 percentage points; 95% confidence interval [CI], −14.1 to 5.2; crude odds ratio, 0.82; 95% CI, 0.53 to 1.27; P = 0.37). The odds ratio adjusted for the key variables (age, sex, initial stroke severity as measured by the NIHSS, and presence or absence of atrial fibrillation at baseline) was 0.71 (95% CI, 0.44 to 1.14; P=0.16). At 90 days, 26 patients in the endovascular-treatment group (14.4%) and 18 in the intravenous t-PA group (9.9%) had died (P=0.22 by the log-rank test). There were no significant differences between the groups with respect to the secondary outcome measures (Table 2).
Figure 1. Modified Rankin Score at 3 Months According to Treatment Group.
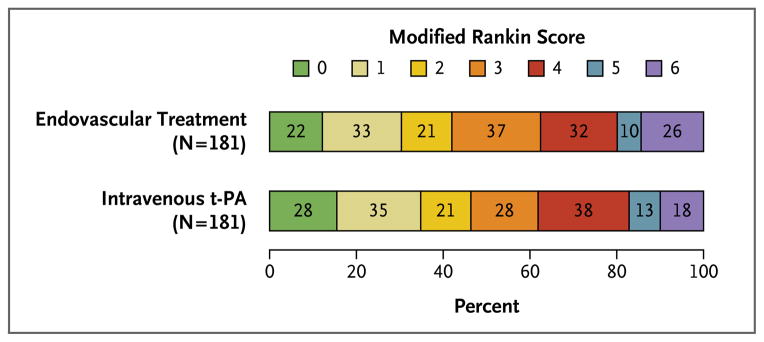
The primary outcome was disability-free survival at 3 months, with freedom from disability defined as a score of 0 or 1 on the modified Rankin scale (range, 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability despite symptoms, and 6 death). The number in each cell denotes the number of patients with that score. The statistical analysis plan specified dichotomized scores of 0 or 1 (good outcome) versus 2 to 6 (poor outcome). The proportion of patients with a modified Rankin score of 0 or 1 at 3 months was 30.4% with endovascular treatment and 34.8% with intravenous tissue plasminogen activator (t-PA). In the analysis adjusted for age, sex, stroke severity, and presence or absence of atrial fibrillation at baseline, the odds ratio with endovascular treatment was 0.71 (95% confidence interval, 0.44 to 1.14; P = 0.16).
Table 2.
Secondary Outcomes at Day 7.*
| Outcome | Endovascular Treatment (N = 181) | Intravenous t-PA (N = 181) | P Value |
|---|---|---|---|
| NIHSS score ≤6 — no. of patients (%)† | 97 (54) | 100 (55) | 0.89 |
| Neurologic deterioration — no. of patients (%)‡ | 16 (9) | 12 (7) | 0.39 |
| Death — no. of patients (%) | 14 (8) | 11 (6) | 0.53 |
| Symptomatic intracranial hemorrhage — no. of patients (%) | 10 (6) | 10 (6) | 0.99 |
| Nonfatal | 6 | 9 | |
| Fatal | 4 | 1 | |
| Symptomatic edema from original brain infarction — no. of patients (%) | 37 (20) | 32 (18) | 0.53 |
| Nonfatal | 30 | 23 | |
| Fatal | 7 | 9 | |
| Recurrent ischemic stroke — no. of patients (%) | 4 (2) | 4 (2) | 0.99 |
| Nonfatal | 4 | 4 | |
| Fatal | 0 | 0 | |
| Noncerebral event | |||
| Nonfatal or fatal — no. of patients (%) | 10 (6) | 5 (3) | 0.29 |
| Nonfatal — no. of patients (%) | 7 | 4 | |
| Fatal — no. of patients (%) | 3 | 1 | |
| Type of event — no. of events§ | |||
| Severe extracranial bleeding | 2¶ | 2 | |
| Pulmonary embolism | 1 | 0 | |
| Myocardial Infarction | 4 | 2 | |
| Sepsis | 1 | 0 | |
| Deep venous thrombosis | 1 | 0 | |
| Pulmonary edema | 2 | 2 | |
All outcomes were assessed on day 7±2.
An NIHSS score of 6 or less indicates mild neurologic impairment.
Neurologic deterioration was defined as an increase of 4 or more points in the NIHSS score.
Types of nonfatal events are not exclusive because a given patient could have more than one type.
Both patients had a nonfatal hematoma at the point of angiographic access that required evacuation and blood replacement.
SAFETY
Complications in the 7 days after randomization in the endovascular-treatment group and the intravenous t-PA group included death, neurologic deterioration, fatal and nonfatal symptomatic intracranial hemorrhage, fatal and nonfatal symptomatic edema from an original brain infarction, nonfatal recurrent ischemic stroke, and extracerebral events. The incidence of events on day 7 was similar in the two groups, and none of the differences were significant (Table 2).
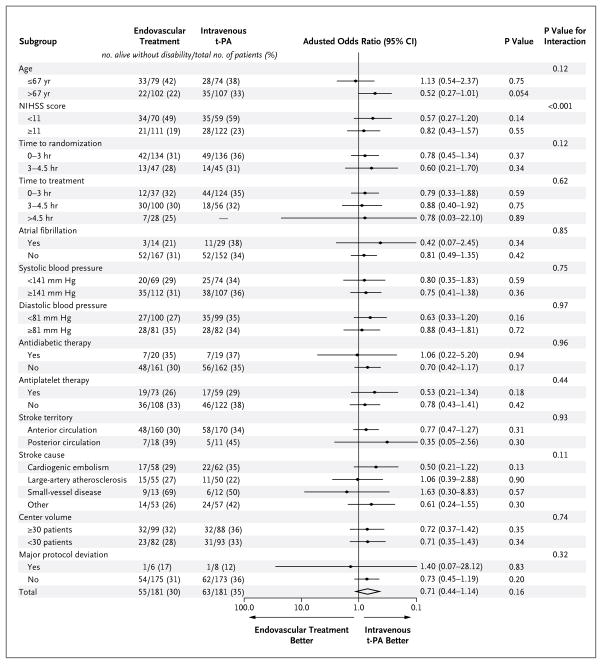
SUBGROUP ANALYSIS
We performed subgroup analyses to assess the effect of treatment on the primary outcome in subcategories, with adjustment for the main prognostic variables and taking into account that, for any specific prognostic factor, the distribution of other factors might differ between subcategories. Overall, there was little difference in the adjusted effects of treatment in the subgroups (Fig. 2).
Figure 2. Effect of Treatment on the Primary Outcome in Subgroups.
The odds ratio in each subgroup was adjusted for the effects of the key variables (age, sex, initial stroke severity, and presence or absence of atrial fibrillation at baseline). Initial stroke severity was measured by means of the National Institutes of Health Stroke Scale (NIHSS; range, 0 to 42, with higher scores indicating more severe neurologic impairment; scores of ≤6 indicate mild impairment, and scores of ≥25 indicate very severe impairment). Age and NIHSS score were used as continuous variables. The variables chosen for multivariate and subgroup analyses were prespecified. The cutoff points for age, NIHSS score, and blood pressure were determined with the use of the Youden method, after a receiver-operating-characteristic analysis tabulating sensitivity, specificity, and accuracy for each possible cutoff point. For time to treatment, patients who did not receive the assigned treatment were excluded, and an additional patient in the endovascular-treatment group was excluded because the procedure was interrupted owing to equipment breakdown. For stroke cause and territory, the 2 patients with conditions mimicking stroke were excluded. For stroke territory, a third patient was excluded because the stroke was in both the posterior and anterior circulation. The analysis of major protocol deviations, and all the other subgroup analyses, included the 12 patients at the center that was withdrawn from the study.
MAJOR PROTOCOL DEVIATIONS AND SENSITIVITY ANALYSES
One center was withdrawn from the study after it had enrolled 12 patients for failure to comply with the treatment assignments, because 5 patients in the intravenous t-PA group had been treated with intravenous t-PA followed by endovascular treatment (3 patients) or directly with endovascular therapy (2 patients), and 1 patient in the endovascular-treatment group had been given intravenous t-PA. To determine whether data from this center could have biased the final result, we did a post hoc sensitivity analysis on the primary outcome, excluding these 12 patients, all of whom had a poor outcome (modified Rankin score, 2 to 6), and found that the overall result was not affected (adjusted odds ratio, 0.71; 95% CI, 0.43 to 1.14; P = 0.16).
There were eight other patients (five assigned to endovascular treatment) with major protocol deviations at six centers. We ran a sensitivity analysis that excluded these patients, and the results, again, were not qualitatively different (adjusted odds ratio, 0.70; 95% CI, 0.43 to 1.14; P = 0.15).
DISCUSSION
This trial, which was powered to detect an advantage of 15 percentage points with endovascular treatment for the primary outcome, failed to show the superiority of endovascular therapy as compared with intravenous t-PA. The disability-free survival rate was 4.4 percentage points lower after endovascular treatment than after intravenous t-PA, with a 95% confidence interval ranging from 14.1 percentage points lower to 5.2 percentage points higher.
The results for the secondary outcomes and the subgroup and sensitivity analyses were consistent with the result for the primary outcome. The subgroup analysis suggested that the lack of superiority of endovascular treatment did not depend on the time to endovascular treatment, the stroke subtype, or the type of center. However, a larger sample might have permitted better discrimination between effects in the subgroups of patients.
We did not detect any heterogeneity among centers, particularly between high-volume and low-volume centers — an important distinction, because a sufficiently large volume of neurointerventional procedures should ensure adequate operator experience.25 Operators at all centers had the opportunity to participate in training meetings organized during the study, in which cases or controversial issues could be discussed.
Some issues may affect the generalizability of our findings. As in large trials of intravenous t-PA,1 the demonstration of vessel occlusion was not a precondition for inclusion in our trial. There are several alternative approaches, some of which are already used in clinical practice, to select patients for endovascular treatment. For example, patients can be selected on the basis of the demonstration of vascular occlusion with noninvasive methods, such as computed tomographic angiography or magnetic resonance angiography. The use of these methods has pros and cons. Digital-subtraction angiography offers the possibility of treating patients right away, without any further loss of time, with the advantage of providing information on the dynamics of brain circulation and accurate information on the occluded vessel or vessels. We are not able to exclude the possibility that endovascular treatment is superior to standard intravenous t-PA in patients selected on the basis of the findings of computed tomographic angiography or magnetic resonance angiography.
Device technology is advancing rapidly, and it is conceivable that the latest-generation devices, called stentrievers,13,14 which were used infrequently in this trial, could provide greater benefit if used widely. This pragmatic trial assessed the technology available in the 4 years during which it took place. To avoid treatment delay, bridging is proposed with the start of intravenous thrombolysis while endovascular treatment is being organized.26 Our trial hypothesis was that the disadvantage of the endovascular treatment in terms of time spent, as compared with that required by intravenous t-PA, might be offset by more rapid and effective revascularization achieved with the endovascular approach.
Physicians’ belief that interventional approaches were superior to medical treatment was a serious obstacle in organizing randomized trials in the past decade.27 The high rate of recanalization with endovascular treatment might give the impression that this method is effective in most cases, although it may provide no clinical benefit in almost half the patients.28 This trial did not show that endovascular therapy achieves superior outcomes as compared with intravenous thrombolysis, and our findings do not provide support for the use of the more invasive and expensive endovascular therapy over intravenous treatment.
Supplementary Material
Acknowledgments
Supported by a grant from the Italian Medicines Agency (AIFA) (FARM6LN3KS). The trial received t-PA from Boehringer Ingelheim Italia, which was paid by the AIFA for use in the experimental group and by the individual participating hospitals for use in the control group. The catheters and devices used in the study were those present in the participating interventional radiologists’ apparatus and were refunded by Niguarda Ca’ Granda Hospital (Milan) with the AIFA funding.
We thank P. Sandercock (University of Edinburgh, Edinburgh), L. Candelise (University of Milan, Milan), and G. del Zoppo (University of Washington, Seattle), members of the data and safety monitoring committee who carried out the interim analyses and encouraged members of the study group.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–72. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 3.Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802–9. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 4.Endo S, Kuwayama N, Hirashima Y, Akai T, Nishijima M, Takaku A. Results of urgent thrombolysis in patients with major stroke and atherothrombotic occlusion of the cervical internal carotid artery. AJNR Am J Neuroradiol. 1998;19:1169–75. [PMC free article] [PubMed] [Google Scholar]
- 5.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–54. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 6.Rabinstein AA, Wijdicks EF, Nichols DA. Complete recovery after early intraarterial recombinant tissue plasminogen activator thrombolysis of carotid occlusion. AJNR Am J Neuroradiol. 2002;23:1596–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Tomsick T, Brott T, Barsan W, et al. Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. AJNR Am J Neuroradiol. 1996;17:79–85. [PMC free article] [PubMed] [Google Scholar]
- 8.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Mattle HP, Arnold M, Georgiadis D, et al. Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke. 2008;39:379–83. doi: 10.1161/STROKEAHA.107.492348. [DOI] [PubMed] [Google Scholar]
- 10.Rha JH, Saver JL. The impact of re-canalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–73. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–12. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 12.Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–8. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 13.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–40. doi: 10.1016/S0140-6736(12)61299-9. [Erratum, Lancet 2012;380:1230.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial pro-urokinase for acute ischemic stroke — the PROACT II study: a randomized controlled trial. JAMA. 1999;282:2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 16.Molina CA. Futile recanalization in mechanical embolectomy trials: a call to improve selection of patients for revascularization. Stroke. 2010;41:842–3. doi: 10.1161/STROKEAHA.110.580266. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa A, Mori E, Minematsu K, et al. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the Middle Cerebral Artery Embolism Local Fibrinolytic Intervention Trial (MELT) Japan. Stroke. 2007;38:2633–9. doi: 10.1161/STROKEAHA.107.488551. [DOI] [PubMed] [Google Scholar]
- 18.Mullen MT, Pisapia JM, Tilwa S, Messe SR, Stein SC. Systematic review of outcome after ischemic stroke due to anterior circulation occlusion treated with intravenous, intra-arterial, or combined intravenous+intra-arterial thrombolysis. Stroke. 2012;43:2350–5. doi: 10.1161/STROKEAHA.111.639211. [DOI] [PubMed] [Google Scholar]
- 19.Ciccone A, Valvassori L, Ponzio M, et al. Intra-arterial or intravenous thrombolysis for acute ischemic stroke? The SYNTHESIS pilot trial. J Neurointerv Surg. 2010;2:74–9. doi: 10.1136/jnis.2009.001388. [DOI] [PubMed] [Google Scholar]
- 20.Ciccone A, Nichelatti M, Valvassori L SYNTHESIS Expansion Investigators. SYNTHESIS Expansion: design of a nonprofit, pragmatic, randomized, controlled trial on the best fast-track endovascular treatment vs. standard intravenous alteplase for acute ischemic stroke. Int J Stroke. 2011;6:259–65. doi: 10.1111/j.1747-4949.2011.00587.x. [DOI] [PubMed] [Google Scholar]
- 21.Ciccone A. Consent to thrombolysis in acute ischaemic stroke: from trial to practice. Lancet Neurol. 2003;2:375–8. doi: 10.1016/s1474-4422(03)00412-5. [DOI] [PubMed] [Google Scholar]
- 22.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–70. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 23.Candelise L, Pinardi G, Aritzu E, Musicco M. Telephone interview for stroke outcome assessment. Cerebrovasc Dis. 1994;4:341–3. [Google Scholar]
- 24.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Inter-observer agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 25.Grigoryan M, Chaudhry SA, Hassan AE, Suri FK, Qureshi AI. Neurointerventional procedural volume per hospital in United States: implications for comprehensive stroke center designation. Stroke. 2012;43:1309–14. doi: 10.1161/STROKEAHA.111.636076. [DOI] [PubMed] [Google Scholar]
- 26.Khatri P, Hill MD, Palesch YY, et al. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke. 2008;3:130–7. doi: 10.1111/j.1747-4949.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccone A, Valvassori L, Gasparotti R, Scomazzoni F, Ballabio E, Sterzi R. Debunking 7 myths that hamper the realization of randomized controlled trials on intra-arterial thrombolysis for acute ischemic stroke. Stroke. 2007;38:2191–5. doi: 10.1161/STROKEAHA.106.465567. [DOI] [PubMed] [Google Scholar]
- 28.Hussein HM, Georgiadis AL, Vazquez G, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol. 2010;31:454–8. doi: 10.3174/ajnr.A2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.