Abstract
Context
Genetic factors play an important role in the etiology of both autism spectrum disorders (ASD) and autistic traits. However, little is known about the etiologic consistency of autistic traits across levels of severity.
Objective
We compared the etiology of typical variation in autistic traits with extreme scoring groups (including top 1%) which mimicked the prevalence of diagnosed ASD in the largest twin study of autistic traits to date.
Design
Twin study employing phenotypic analysis and genetic model-fitting in the total sample and extreme scoring groups (top 5%, 2.5%, 1%).
Setting
A nationally-representative general population twin sample from the United Kingdom.
Participants
The families of 5,988 12-year-old pairs in the Twin Early Development Study.
Main Outcome Measure
Autistic traits as assessed by the Childhood Autism Spectrum Test.
Results
Moderate to high heritability was found for autistic traits in the general population (52% for females; 76% for males). High heritability was found in extreme scoring groups. There were no differences in heritability among extreme groups or between the extreme groups and the general population. A continuous liability shift towards autistic trait affectedness was seen in the cotwins of individuals scoring in the top 1%, suggesting shared etiology between extreme scores and normal variation.
Conclusions
This evidence of similar etiology across normal variation and the extremes has implications for molecular genetic models of ASD and for conceptualizing ASD as the quantitative extreme of a neurodevelopmental continuum.
Keywords: Autism, twins, genetics, autistic traits
Autism spectrum disorders (ASDs) are a set of phenotypically heterogeneous neurodevelopmental syndromes of primarily genetic etiology. Monozygotic twins display between 60 and 90% concordance for ASD; the concordance in dizygotic twins has been estimated between 0% and 30%. This evidence suggests that ASD are one of the most highly heritable behavioral disorders 1-7.
Modest to high heritability has been reported for autistic traits assessed quantitatively in the general population 8-13, though assessments have varied in their estimates of genetic and environmental contributions 14-21. Reported values of heritability vary from 36%-87%.
One hypothesis regarding the causes of ASD or extreme autistic traits is that the same variants that influence risk for extreme behavioral profiles also influence mild or sub-threshold autism-like behavior 16, 22, 23. Under this hypothesis it is predicted that the etiologic structure of extreme autistic traits would be consistent across the range of impairment 24. Further, if extreme traits are genetically linked to sub-threshold variation, one would expect to see a shift towards affectedness in the continuous trait distribution of extreme-scoring individuals’ family members, a shift that is dependent upon the family members’ coefficient of genetic relatedness 25, 26. In other words, extreme scores should not simply predispose family members to equally severe levels of impairment, but predict an increased liability towards mild or moderate autism-like behavior as well 17, 26-29.
The etiology of extreme autistic traits (e.g. >95th percentile) was examined in the present sample at age 8 23. Those findings suggested that extreme autistic traits appeared to show similar etiology as diagnosed ASD. That study, however, was not large enough to examine an extreme group (top 1%) that shows a similar prevalence and average symptom burden to individuals with an ASD. In the present study, we employ a sample that is 75% larger (n=11,936) in order to examine, for the first time, the etiologic consistency of autistic traits from the general population across a clinically comparable threshold.
To test whether the etiology of extreme autistic traits was consistent across the range of impairment, heritability estimates were reported for the full sample, as well as individuals scoring in the top 5% (n=615), 2.5% (n=342), or 1% (n=120) of the general population. Leveraging the size and clinical comparability of the top 1% group, this study also presents the first direct examination of whether a quantitative shift in sibling liability to less extreme impairment is associated with extremely severe affectation in a representative twin sample, a phenomenon that would be indicative of etiologic overlap between very extreme scores and regular variation in autism-like behavior.
Methods
Sample
Participants were recruited from the Twins Early Development Study (TEDS) 30. TEDS is a cohort of twins in the United Kingdom born between 1994 and 1996. The original registry was established through birth records; zygosity of the twins was confirmed in over 75% of cases based on DNA markers. The remaining zygosity assessments were conducted using a validated scale 31. TEDS was approved by the King’s College London ethics committee and all parents completed informed consent.
At age 12, the Childhood Autism Spectrum Test (CAST 32) was completed and returned by the parents of 11970 eligible children. This represents 60.6% of the original TEDS sample who actively participate in TEDS. The response among individuals with scores above the 95th percentile on the CAST at age 8 was approximately 10% lower (54.8%). Twin pairs were ineligible if one or more of the twins had a noted non-ASD syndromic condition (e.g. Down Syndrome, chromosomal abnormalities), substantial pregnancy or perinatal complications, or if zygosity was unclear. Twin pairs were included if their parent completed at least half of the CAST items (15 or more) for both twins (final n =5,968 pairs). The scores of those missing less than half of the items (n=1,142 individuals) were adjusted such that their final value reflected the proportion of questions answered. Compared to participants included in the analysis, the eligible TEDS participants without valid CAST data were more likely to contain male twins (51.4% vs. 47.6%, χ2 =7.9, p=0.01) and have lower socioeconomic status (t=19.5, df=11028, p<0.0001), assessed using a combined measure including family income, maternal education and maternal occupation. There was no difference between responders and non-responders with regard to race or ethnicity (93.5% v. 93.0% white, χ2 =0.6, p=0.44).
The final sample included 1936 male MZ twins (MZM, 16.2%), 2316 female MZ twins (MZF, 19.4%), 1862 male DZ twins (DZM, 15.6%), 2042 female DZ twins (DZF, 17.1%), and 3760 opposite-sex DZ twins (DZOS, 31.7%). In total, the 11936 twins (5,968 pairs) were 47.7% male and 93.6% white. Nearly half (42.2%) of mothers worked and 32.4% had completed at least one A-level (school examinations taken at age 18). This sample is comparable to the UK population as a whole. Employing results from the General Household Survey (Office for National Statistics, 2005), 92% of the population is white, 50% of children are male, and 32% of mothers have completed one or more A-levels.
Measure
The CAST is a thirty item, dichotomous (yes, no) response scale. Items address all three core domains of symptoms that currently characterize autism spectrum disorders in DSM-IV-TR (social impairments, communication impairments and restrictive and repetitive behaviors and interests) 33. The CAST is designed for parents to complete. The sensitivity of the CAST as a screening tool for ASD with a designated cut-point of 15 has been shown, in a sample of children clinically assessed using the ADI-R and ADOS, to be 100 percent, the specificity 97 percent, and the positive predictive value 50 percent 34. In the TEDS sample, the CAST displayed adequate overall internal consistency (Kuder-Richardson-20=0.74) and strong within-individual correlations across a 4-year period from ages 8 to 12 (r = .64) 35. The parents of 80 children with a suspected ASD diagnosis based on the Development and Well Being Assessment (DAWBA) telephone parent interview 36 completed the CAST at age 12, including both twins in a pair. As reported previously 37, a rate of likely ASD cases in the sample has been estimated at 1.10%, which is comparable with the rate of 1.16% reported for all ASDs in a UK epidemiological study 38. The DAWBA-identified suspected ASD group’s mean score was 18.26 (sd=4.99), between the 98th and 99th percentile of the population distribution. Those within the 99th percentile of the general population accordingly had parent-reported traits equal to or greater than that of the children meeting DAWBA criteria for an ASD diagnosis. As such, the top 5%, 2.5%, and 1% extreme scoring groups range from subthreshold to suspected diagnostic group-level severity.
Continuous Shift in Liability Across the Trait Distribution
The continuous liability shift analysis was designed to investigate the relationship between very extreme scores (>99%) in one twin (proband) and autistic traits in their sibling (cotwin). The effects of the sex of both twins and the age of the twin pair were regressed out of the raw data prior to analysis. Across the sample, one twin from each pair was selected at random to be the proband (twin 1). Probands were placed into three groups: 1) Those scoring below the 99th percentile, 2) DZ probands scoring at or above the 99th percentile, 3) MZ probands scoring at or above the 99th percentile. Mann-Whitney U tests were used to examine the difference in average cotwin trait scores between each of the groups; p-values were corrected for multiple comparisons (n=3) 39.
The empirical distributions of the three groups were plotted as kernel density estimates (smoothed histograms) to examine the nature of the cotwin shift in liability to autistic traits. There are conditions under which an increase in the cotwin mean given extreme scores in probands may not indicate an etiologic relationship between the extremes and normal variation. For example, an increase in cotwin liability to extreme scores only may appear in the form of a bimodality in the cotwin distribution: cotwins are either affected at the severity of the proband or quantitatively unaffected by the risk. This would result in an increase in cotwin mean scores but reflect distinct etiologies between extreme and normal range traits. A continuous shift in liability, however, in which cotwins of extreme scoring probands are at increased risk for higher scores across the range of impairment, would suggest an etiologic relationship between the extreme scores of the proband and normal variation in their siblings. A model that considers only the average scores of cotwins, like DeFries-Fulker extremes models, cannot distinguish between these potential sources of mean change. The plot of the cotwin distributions in Figure 1, however, is designed to consider whether these data indicate a true continuous shift in liability across the range of scores. A greater continuous shift in MZ than DZ twins would suggest a genetic relationship between very extreme scores and variation in cotwins.
Figure 1.
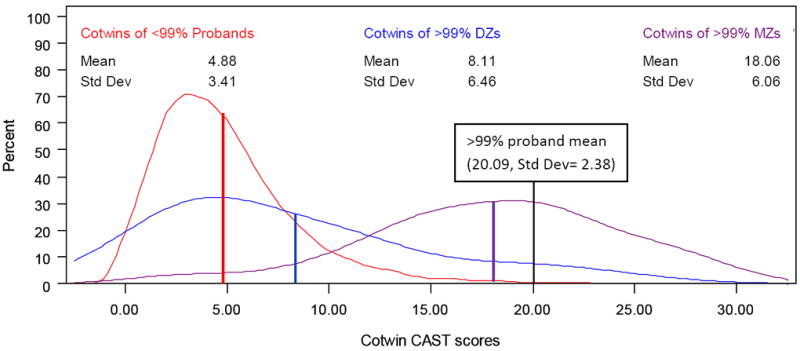
Shift in cotwin trait distribution associated with proband scores above the 99th percentile
Note: One twin from each pair selected at random to act as the proband; CAST= Childhood Autism Spectrum Test; curves denote kernel density estimates (smoothed histograms). Red: all cotwins of probands with scores <99th percentile. Blue: DZ cotwins of probands with scores ≥ 99th percentile. Purple: MZ cotwins of probands with scores ≥ 99th percentile (purple). Colored lines indicate mean values for each group; all mean differences significant (purple>blue>red, p<0.001 for all comparisons, see text for analytic details regarding comparison of means).
Categorical shift in liability to lesser extreme scores
Etiologic overlap between different levels of affectation can also be investigated using a categorical approach. Reich et al. (1979) demonstrated that etiologic independence between severe (narrow) and milder (broad) forms of a disorder is demonstrated through the absence of an association between the narrow form in probands and the broad form in their family members. In the case of a quantitative trait distribution, narrow and broad forms correspond to varying levels of affectation (e.g. the narrow, most severe form is indicated by scores above the 99th percentile; the broad, milder form is indicated by scores above the 95th and 90th percentiles). In the context of twin analyses, the null hypothesis of no etiologic relationship between the narrow and broad forms can be tested by estimating tetrachoric correlations between the narrow form (1=present, 0=absent) in probands (twin 1) and broad form (1=present, 0=absent) in their cotwins (twin 2) 27, 28. As above, one twin from each pair is selected at random to act as the proband. Correlations between narrow and broad forms that are significantly different from zero suggest shared familial etiology between severe (>99%) and sub-threshold impairment 26. Given varying coefficients of genetic relationship, correlations for MZ and DZ twins are estimated separately. We assumed no qualitative sex effects and included DZOS twins. MZ cross-twin, cross-level correlations greater than DZ cross-twin, cross-level correlations suggest shared genetic influence between the narrow and broad forms. Narrow form was defined as >99%. Two broad forms were considered: >95% and >90%.
The Twin Design and Estimates of Heritability
Twin analyses are designed to partition the variation of quantitative traits into genetic and environmental components. This is accomplished through comparison of monozygotic and dizygotic twin similarity. Monozygotic twins share more than 99% of their DNA code; dizygotic twins share on average half. Heritability is suggested when monozygotic twins display greater similarity than dizygotic twins on a measured trait.
The fraction of influence attributable to genetic factors includes additive and nonadditive genetic effects. Additive genetic effects (A) are independent genetic influences. Nonadditive genetic effects (D) are characterized by interactions either within (dominance) or between (epistasis) relevant loci. Environmental influence is divided into that which is shared and that which is nonshared (unique to the individual). Shared environmental effects (C) refer to environmental influences that make children growing up in the same family similar; nonshared environmental effects (E) refer to environmental influences that make children growing up in the same family different, and include measurement error in their estimation. Total phenotypic variance is calculated by summing the specific variance attributable to A,D, C, and E 40.
Full Sample Analyses
Twin Correlations
Twin correlations were estimated for each sex and zygosity group. Twin data suggest shared environmental effects when DZ twin correlations are more than half the MZ twin correlations. They suggest non-additive genetic effects when DZ correlations are less than half those of the MZ twins. Non-additive and shared environmental effects cannot be tested for simultaneously using a twin-only design. Accordingly, ACE and ADE models were run separately to investigate both possibilities.
Twin Model-fitting
Univariate ACE, ADE, CE, AE, and E structural equation models were employed to estimate the relative contribution of genetic and environmental influences on variation in autistic traits. CAST scores were log-transformed prior to the model-fitting to correct for skewness. Both qualitative and quantitative sex effects were examined. Quantitative sex effects indicate variation in the magnitude of genetic or environmental effects between males and females. Qualitative sex effects indicate that different genetic or environmental effects may be influencing males and females. Nested models were compared using the log likelihood criterion; non-nested models were compared using the Akaike Information Criterion (AIC). The most parsimonious model achieved without a significant reduction in fit was considered the best match to the data. Mean effects of sex and age were controlled for in all analyses.
Analyses of the Extremes
The sample (n=11936) was large enough to examine heritability in three very high scoring groups: the top 5%, 2.5%, and 1%. For each of the high scoring categories, probandwise concordances, extreme group correlations, and tetrachoric correlations were estimated to examine autistic trait heritability at the extremes of the general population. For each measure, genetic influences were implicated when MZ twins displayed more similarity than DZ twins.
Estimates of the Heritability of Extreme Scores
The heritability of extreme scores was investigated using two methods. The first, DeFries-Fulker (DF) extremes analysis, investigates the role of genes and environment in the difference between the mean scores of extreme groups and the population as a whole 41. In doing so, one employs the quantitative data available. The second method, using liability threshold (LT) models, investigates the fraction of variation in categorical status (e.g. high scoring or not) attributable to genetic and environmental factors 42, 43. The LT and DF analysis sets were designed to determine whether the heritability of extreme scores is consistent across varying levels of severity (>95%, >97.5%, >99%), using both categorical and continuous outcome definitions. Heritability, or familiality, that differs substantially between varying levels of severity suggests differences in etiology between the most severe and less severe forms of a phenotype. For example, differences in familial liability towards low IQ have been noted based on the level of intellectual disability in probands: the family members of individuals with mild intellectual disability are at increased risk for low IQ themselves, the family members of individuals with severe intellectual disability are not 44. In the context of twin analyses, such a pattern would be reflected in a reduction of heritability in the most extreme scoring groups, and indicate that severe impairment and mild impairment arise from distinct etiologic processes. In contrast, consistent heritability would be consistent with a singular distribution of liability across the range of extreme scores, as would be expected when etiologies are shared across levels of impairment 24.
Sex effects were not examined in any of the DF or liability threshold models presented in Table 3 as a result of the very limited number of female probands in the >99% (n=21) group. This afforded consistency in analytical technique across the high scoring categories. As sex effects were not estimated, DZOS twins were excluded from these analyses.
Table III.
Extremes Analyses – CAST at 12
| Cutoff Level | >95% | >97.5% | >99% |
|---|---|---|---|
|
Probandwise Concordances
| |||
| MZ | 0.65 | 0.63 | 0.55 |
| DZSS/DZOS | 0.17/0.17 | 0.13/0.13 | 0.12/0.16 |
|
| |||
|
Extreme group correlations (no. of probands)
| |||
| MZM | 0.51*(125) | 0.47* (76) | 0.60* (32) |
| MZF | 0.81* (62) | 0.84* (40) | 0.87* (13) |
| DZM | 0.08 (121) | -0.01 (71) | -0.34 (24) |
| DZF | -0.12 (75) | -0.12 (34) | -0.22 (8) |
| DZOS | 0.06 (232) | 0.00 (121) | -0.11 (43) |
|
| |||
|
Tetrachoric correlations (95% CIs)
| |||
| MZM | 0.91 (0.86, 0.96) | 0.92 (0.86, 0.98) | 0.86 (0.74, 0.99) |
| MZF | 0.82 (0.71, 0.93) | 0.86 (0.75, 0.97) | 0.94 (0.82, 1.00) |
| DZM | 0.26 (0.05, 0.46) | 0.41 (0.18, 0.64) | 0.36 (-0.08, 0.80) |
| DZF | 0.50 (0.30, 0.71) | 0.46 (0.12, 0.80) | 0.80 (0.45, 1.00) |
| DZOS | 0.35 (0.21, 0.48) | 0.36 (0.17, 0.55) | 0.51 (0.24, 0.78) |
|
| |||
|
DeFries Fulker Estimates (95%CI)
| |||
| hg 2 | 0.70 (0.64, 0.75) | 0.69 (0.61, 0.75) | 0.68 (0.52, 0.74) |
| residual | 0.30 (0.25, 0.36) | 0.31 (0.25, 0.39) | 0.32 (0.23, 0.44) |
|
| |||
|
LT Modeling (95% CI)
| |||
| h2 | 0.88 (0.83, 0.92) | 0.90 (0.84, 0.94) | 0.89 (0.78, 0.95) |
| e2 | 0.12 (0.08, 0.17) | 0.10 (0.06, 0.16) | 0.11 (0.05, 0.22) |
Note. MZM = monozygotic male twins; MZF = monozygotic female twins; DZM = dizygotic male twins; DZF = dizygotic female twins; DZOS = Opposite-sex DZ twins. hg 2 = group heritability; h2 = heritability estimate; e2 = nonshared environment estimate. NCP = No concordant pairs.
significant at p < 0.05
DeFries-Fulker (DF) extremes analyses
A model-based extension of classic DeFries-Fulker (DF) regression analysis was used to estimate the etiology of quantitatively-defined extreme scores in the general population, estimating group heritability, shared environmental effects, and unique environmental effects 41, 45. As DF models employ a continuous outcome, the heritability estimates derived from these models can be compared to those obtained in the full sample analyses. An etiologic continuum across the range of scores would be evidenced by similar heritability between the full sample and DF models. Age and sex were regressed out of raw scores; the residuals were then transformed prior to analysis. Transformed scores were calculated by dividing cotwin scores by the proband mean for each zygosity group. DF estimates of heritability, and the associated confidence intervals, were constrained to the monozygotic transformed cotwin mean 34, the empirically-derived upper limit of twin similarity.
Liability threshold models
Liability threshold (LT) models were used to estimate the etiology of categorically-defined extreme scores 43. LT models assume a bivariate normal liability distribution underlies risk for the categorical phenotype. ACE, ADE, CE, AE, and E structural equation models were examined.
Results
Descriptives
The sample overall mean was 4.96 (range 0-28, skewness 1.56). As the CAST response options were dichotomous (0, 1), this corresponds to an average of slightly under 5 endorsed autistic traits per child. Males scored 1.16 points higher on average than females and MZ twins scored 0.34 points lower on average than DZ twins. There was no birth order effect on the mean (no difference in CAST scores between first and second born twins, p=0.78). Sex and zygosity together explained 3% of total variation in parent-rated autistic traits. Mean CAST scores for each sex and zygosity group are presented in Table 1.
Table I.
Descriptive Statistics
| Analysis Group | Raw Score Cutoff | Z Score Cutoff | No. of Individuals | % Male | CAST Mean (SD) |
|---|---|---|---|---|---|
| 100% | - | - | 11936 | 47.65 | 4.96 (3.54) |
| >95% | 12.00 | 1.99 | 615 | 66.67 | 15.03 (3.25) |
| >97.5% | 14.00 | 2.55 | 342 | 69.30 | 17.07 (3.07) |
| >99% | 18.00 | 3.68 | 120 | 74.17 | 20.47 (2.64) |
| ASD | - | - | 80 | 78.75 | 18.26 (4.99) |
|
| |||||
| Zygosity Group | |||||
|
| |||||
| MZM | 1936 | 5.25 (3.78) | |||
| MZF | 2316 | 4.27 (3.16) | |||
| DZM | 1862 | 5.39 (3.74) | |||
| DZF | 2042 | 4.55 (3.18) | |||
| DZOS | 3780 | 5.25 (3.65) | |||
Note. MZM = monozygotic male twins; MZF = monozygotic female twins; DZM = dizygotic male twins; DZF = dizygotic female twins; DZOS = Opposite-sex DZ twins.
Continuous Shift in Liability Across the Trait Distribution
Figure 1 presents the distribution of cotwin CAST values, where cotwins are grouped by extreme scoring status and zygosity of the proband. Both MZ (n=22, mean=18.06) and DZ (n=36, mean=8.11) cotwins of >99% scorers displayed significantly greater autistic trait scores (corrected p<0.005 for both comparisons) than the cotwins of probands below the 99th percentile (n=5910, mean=4.88), suggesting an etiologic relationship between extreme scores and cotwin autistic trait variation. The shift in liability was continuous—cotwins of affected probands had higher scores across the distribution-- indicating a relationship between extreme scores and cotwin variation in the normal range. The MZ increase in cotwin mean was significantly greater than the DZ increase (corrected p<0.001), suggesting a genetic relationship between autistic trait scores across the distribution.
Categorical shift in liability to lesser extreme scores
Table 2 presents the results from the cross-twin, cross-affectation-level correlations. All MZ (r= 0.63-0.89) and DZ (r= 0.18-0.51) correlations were significantly different from zero, within and across the three extreme-scoring categories. As the cross-level correlations (e.g. >99% twin 1 and >90% twin 2) were non-zero, we reject the null hypothesis of no shared etiology between top 10%, 5% and 1% affectation 26. As MZ correlations were, on average, twice or more than twice the DZ correlations, these data are consistent with shared genetic influence on autistic traits above and below the top 1% threshold, a level consistent with ASD prevalence and severity.
Table II.
Cross-Twin Cross-Affectation-Level Correlations
| MZ Twins: Tetrachoric Correlations (95% CI)
| |||
|---|---|---|---|
| >99% T2 | >95% T2 | >90% T2 | |
| >99% T1 (n=22) | 0.89 (0.81-0.98) | 0.86 (0.78-0.95) | NE |
| >95% T1 (n=86) | 0.79 (0.68-0.90) | 0.89 (0.84-0.93) | 0.85 (0.80-0.90) |
| >90% T1 (n=225) | 0.63 (0.49-0.77) | 0.76 (0.70-0.83) | 0.84 (0.80-0.88) |
|
| |||
| DZ Twins: Tetrachoric Correlations (95% CI)
| |||
| >99% T2 | >95% T2 | >90% T2 | |
|
| |||
| >99% T1 (n=36) | 0.51 (0.31-0.71) | 0.37 (0.20-0.54) | 0.31 (0.16-0.46) |
| >95% T1 (n=230) | 0.25 (0.07-0.43) | 0.36 (0.26-0.46) | 0.36 (0.28-0.44) |
| >90% T1 (n=568) | 0.18 (0.02-0.34) | 0.32 (0.24-0.41) | 0.37 (0.30-0.43) |
Note: T1= Twin 1; T2= Twin 2; CI= confidence interval; NE= tetrachoric correlation could not be estimated as only one T2 scored below the 90th percentile (high scores too strongly associated, odds ratio=179.84).
Estimating the genetic and environmental influence across the behavioral range
Twin Correlations for Full Sample
MZ twins displayed significantly greater similarity than DZ twins, suggesting that autistic traits were heritable in the general population at age 12. The MZ correlations were more than twice the DZSS correlation for males (MZ: 0.78, 95% CI 0.75-0.80; DZ: 0.26, 0.20-0.32) but less than twice the DZSS correlation for females (MZ: 0.75, 95% CI 0.73-0.78; DZ: 0.42, 0.37-0.47). This suggests additive and possibly non-additive genetic effects in males, additive genetic and shared environmental effects in females, and quantitative sex effects in the general population. For both sexes, MZ correlations less than unity indicated unique environmental effects. The DZOS correlation (raw: 0.27, 95% CI 0.23 - 0.31) was not lower than the geometric mean of the DZSS correlations when sex effects on the means were accounted for (adjusted: 0.34, 95% CI 0.30-0.38), suggesting no influence of qualitative sex effects.
Twin Similarity in Extreme Groups
Table 3 presents the analyses of heritability at the extremes of the general population. The probandwise concordances, extreme group correlations, and tetrachoric correlations were strong for MZ twins across the extreme-scoring categories. The MZ concordances (0.55-0.65) were more than twice the DZ concordances (0.12-0.17), suggesting additive and possibly nonadditive genetic influences on extreme autistic traits. The small negative values seen in some of the DZ extreme group correlations arise from the ceiling effect imposed by group definition in the probands: when group definition becomes more restrictive, the range of possible proband CAST scores is limited. As the negative correlations are not significantly different from zero, they can be interpreted as null. Overall, the relationship between twins did not systematically increase or decrease across cutoff levels (top 5%, 2.5%, 1%) in any of the comparisons.
Extreme Group Heritabilities
The lower section of Table 3 presents the DeFries-Fulker estimates of group heritability. The DF analyses displayed high group heritability (0.68-0.70), no shared environmental effects, and modest unique environmental effects. Heritability estimates were stable with changes to the cutoff criterion, suggesting similar quantitative etiologic patterns across affectation levels. The LT models also indicated consistent and high heritability: estimated additive heritability ranged from 0.88 to 0.90. The LT models suggested neither dominance nor shared environmental effects. Unique environmental effects were estimated to influence between 10 and 12 percent of categorical variation.
Full Sample Heritability
Table 4 presents the full-sample heritability models. The best-fitting model suggests that, at age 12, autistic traits were moderately to highly heritable and a small portion of their variability was attributable to shared environmental effects. Unique environmental effects explained approximately 23% of phenotypic variation. The best-fitting model indicated quantitative sex differences in the etiology of general population autistic traits. Males displayed significantly greater additive heritability (0.72, 95% CI 0.68-0.76) than females (0.53, 95% CI 0.44-0.62). Females displayed significantly greater shared environmental effects (0.25, 95% CI 0.17-0.33) than males (0.04, 95% CI 0.02-0.08). There was no evidence of qualitative sex effects.
Table IV.
Full Sample Univariate Models
| -2LL | df | Par | LRT (df) | ΔAIC | A (95% CI) | C/D (95% CI) | E (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Saturated | 18776.51 | 11910 | 26 | 18828.51 | ||||
| ACE Fix DZOS covariance‡ | 18802.83 | 11925 | 11 | 26.32 (15) p=0.03 | -3.68 | 0.72 (0.68, 0.76)a | 0.04 (0.02, 0.08)a | 0.24 (0.22, 0.26)a |
| 0.53 (0.44, 0.62)b | 0.25 (0.17, 0.33)b | 0.22 (0.20, 0.24)b | ||||||
|
| ||||||||
|
ADE models
| ||||||||
| Sex-limited ADE | 18831.62 | 11875 | 13 | +29.11 | 0.73 (0.64, 0.79)a | 0.04 (0.00, 0.13)a | 0.23 (0.21, 0.26)a | |
| 0.79 (0.76, 0.80)b | 0.00 (0.00, 0.04)b | 0.21 (0.20, 0.23)b | ||||||
| ADE Fix DZOS covariance | 18331.62 | 11925 | 11 | 0.00 (1) p=1 | +25.11 | 0.73 (0.64, 0.79)a | 0.04 (0.00, 0.13)a | 0.23 (0.21, 0.26)a |
| 0.79 (0.76, 0.80)b | 0.00 (0.00, 0.02)b | 0.23 (0.21, 0.23)b | ||||||
| AE | 18832.37 | 11927 | 9 | 0.75 (4) p = 0.95 | +21.86 | 0.77 (0.74, 0.79) a | - | 0.23 (0.21, 0.26)a |
| 0.79 (0.77, 0.80) b | - | 0.21 (0.20, 0.25)b | ||||||
|
| ||||||||
|
ACE models
| ||||||||
| Sex-limited ACE | 18802.83 | 11924 | 12 | -1.68 | 0.72 (0.68, 0.76)a | 0.04 (0.02, 0.08)a | 0.24 (0.22, 0.26)a | |
| 0.53 (0.44, 0.62)b | 0.25 (0.17, 0.33)b | 0.22 (0.20, 0.24)b | ||||||
| ACE Fix DZOS covariance | 18802.83 | 11925 | 11 | - (1) p=1.0 | -3.68 | 0.72 (0.68, 0.76)a | 0.04 (0.02, 0.08)a | 0.24 (0.22, 0.26)a |
| 0.53 (0.44, 0.62)b | 0.25 (0.17, 0.33)b | 0.22 (0.20, 0.24)b | ||||||
| AE males; ACE females | 18822.11 | 11926 | 10 | 19.28 (2) P<0.001 | +13.60 | 0.77 (0.78, 0.79)a | - | 0.23 (0.21, 0.26)a |
| 0.65 (0.57, 0.73)b | 0.14 (0.051, 0.22)b | 0.22 (0.20, 0.23)b | ||||||
| AE | 18832.37 | 11927 | 9 | 29.54 (3) p<0.001 | +21.68 | 0.77 (0.74, 0.79)a | - | 0.23 (0.21, 0.26)a |
| 0.79 (0.77, 0.80)b | - | 0.21 (0.20, 0.23)b | ||||||
| AE; equate males and females | 18837.93 | 11929 | 7 | 35.11 (5) p<0.001 | +23.42 | 0.78 (0.76, 0.79) | - | 0.22 (0.21, 0.24) |
| - | ||||||||
| E | 21586.95 | 11930 | 6 | 2784.12 (6) p<0.001 | +2770.44 | - | - | 1.00 (1.00, 1.00) |
| - | - | 1.00 (1.00, 1.00) | ||||||
Note. –2LL = log likelihood fit; df = degrees of freedom; par = parameters; LRT(df) = likelihood ratio χ2 test with Δ df comparing model to first model listed within group (e.g. LRT for the ACE submodels is calculated through comparison with the Scalar ACE). AIC = Akaike’s Information Criterion. A = Additive genetic influences, D = Nonadditive genetic influences, C = Shared environmental influences, E = Nonshared environmental influences.
= male estimates;
=female estimates
= Best-fitting model.
Sex-limited ACE/ADE= ACE/ADE model permitting both qualitative and quantitative sex effects; ACE/ADE Fix DZOS Covariance= ACE/ADE model permitting only quantitative sex effects (no qualitative sex effects); AE males, ACE females= model in which C term has been dropped for males only, quantitative sex effects estimated, no qualitative sex effects; AE= model with A and E terms only, quantitative sex effects estimated, no qualitative sex effects.
The best-fitting variance components model did not fit as well as the saturated (LRT=26.32, df=15, p=0.03). This occurs frequently in studies with very large sample sizes as minimal variance differences between groups can be highly statistically significant. In this case, there was a small but significant sex effect on variance (p= 0.003) which the saturated model accounts for but the variance components model assumes is equal.
The heritabilities predicted by the best-fitting, full sample model were similar to those derived for the extreme groups. The 95% confidence interval of the male full sample heritability estimate (0.68-0.76) overlapped with the group heritability estimates of each DeFries-Fulker model; the female full sample heritability estimate overlapped with the group heritability estimates of the >97.5% and >99% DF models. While not a statistical assessment of equivalency, this suggests consistent etiologic structure between the general population and the extremes when employing the same (continuous) outcome definition 24. One would anticipate lesser agreement between the female-specific full sample values and overall estimates at the extremes since most high scorers were male and a sex difference in heritability was noted in the general population. To test whether this was the source of the modest deviation between females in the general population and at the extremes, we estimated an additional DF model at the >95% level in which male and female values were estimated separately. As expected, female heritability values at the extremes (estimated group heritability= 0.67, 95% CI 0.62-0.67) were less deviant from those in the general population when specified independently, and the confidence intervals overlapped.
Discussion
We compared the etiology of typical variation in autistic traits with extreme scoring groups (including 1%) which mimicked the prevalence of diagnosed autism spectrum disorders (ASD) in the largest twin study of autistic traits to date. Though individuals in the most extreme group (top 1%) had parent-rated CAST scores as high as those with DAWBA interview-identified ASD, the estimated heritability of autistic traits did not differ among the extreme groups (top 1%, 2.5% and 5%). Employing a continuous outcome definition, the heritability estimates at the extremes were highly similar to those derived from the general population for both males and females. This study therefore presents the strongest evidence to date that genetic and environmental influence is stable in the population with increasing levels of autistic traits.
Phenotypic analyses showed there was an etiologic relationship between extreme scores in probands and subthreshold trait variation in their cotwins. Very extreme (>99%) scores were associated with both continuous shifts in cotwin autistic trait liability and increases in the categorical probability of lesser higher scoring values, suggesting shared etiology between scores above and below the top 1 % threshold. Given both a) the liability shifts were much greater for MZ than DZ twins and b) variation in autistic traits across the range of impairment was predominantly genetic, these data are consistent with shared genetic influence on autism-like behavior across a clinically significant threshold. Analyses of the molecular structure of genetic risk for ASD will be the ultimate test of consistent genetic etiology: We predict that some genes associated with ASD will also be associated with autistic traits across the distribution, a hypothesis that has now begun to be tested 37, 46-48.
Evidence for etiologic continuity across the clinical threshold carries substantial implications for gene-finding studies. For common disorders with polygenic liability, statistical power for genome-wide association studies can be greatly improved by examining the entirety of a trait distribution. Dichotomized approaches becomes less powerful as the control group includes more individuals who nearly meet case status 29. Control group contamination is likely a problem in most case-control studies of common, complex neuropsychiatric phenomena. However, empirical evidence for the risk continuum that underlies the contamination is very rare. This study is unique in that its size allowed for direct examination of etiologic consistency up to a clinically-relevant extreme.
The primary limitation of this study was its reliance on parent report. Though the highest scoring group in this analysis had a symptom count similar to that of children with ASD, the degree to which their symptom clustering or severity is comparable is unknown. The analyses of parent response were additionally limited by both individual item missingness (less than 2% per item) and the yes-no response structure which reduced the degree of symptom variability that could be measured.
The comparison between etiologic structure in the general population and at the extremes was impeded by lack of power to consider sex differences within the extreme scoring groups. The extremes analyses were underpowered to test the significance of either the modest shared environmental effects (25% for females; 4% for males) or quantitative sex difference in heritability (19% difference in additive heritability) noted in the general population models. This, however, is a limitation inherent to the goal of testing etiologic continuity between regular variation and extremely high trait scores. The problem of small extreme groups is amplified in this case by male preponderance among individuals with high autistic trait scores.
The analysis was additionally limited by the methodological challenges inherent to twin-only designs. Measured environmental variables and multi-generational designs, in conjunction with molecular genetic studies, would clarify the degree to which the aforementioned assumptions hold in the general population.
In conclusion, this study found that parent-rated autistic traits are moderately to highly heritable in the general population at age 12. There was evidence for equivalent heritability within normal variation and the extremes, suggesting a consistent etiology of strong genetic and modest nonshared environmental influences across different autistic trait concentrations. Phenotypic analyses suggested shared etiology between extremely severe autism-like impairment and both a) less extreme impairment and b) regular variation. These data accordingly provide support for a continuous risk hypothesis, which argues that inherited genetic risk sets are associated with both subthreshold autistic traits and the clinical ASD phenotype. Further, continuous genetic liability implies that clinical thresholds are etiologically arbitrary, as clinical disorders exist as the quantitative extreme of a continuum.
Acknowledgments
The Twin Early Development Study is funded by MRC grant G0500079 and has IRB approval. Dr. Robinson was supported by a National Institute of Mental Health / NIH Research Fellowship Mental Health and Developmental Disabilities (MH/DD) at The Children’s Hospital Boston, Harvard Medical School (MH71286) and the Training Program in Psychiatric Genetics and Translational Research at the Harvard School of Public Health (T32MH017119). The authors thank the participants of the Twins Early Development Study for making this research possible. The authors also thank Drs. Lauren McGrath, Benjamin M. Neale, and Shaun Purcell for the productive conversations that aided in the production of this manuscript.
Footnotes
Portions of this paper were presented at the International Meeting for Autism Research, May 20 2010, Philadelphia, PA.
References
- 1.Rutter M, Silberg J, O’Connor T, Simonoff E. Genetics and child psychiatry: II Empirical research findings. J Child Psychol Psychiatry. 1999 Jan;40(1):19–55. [PubMed] [Google Scholar]
- 2.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995 Jan;25(1):63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg RE, Daniels AM, Law JK, Law PA, Kaufmann WE. Trends in autism spectrum disorder diagnoses: 1994-2007. J Autism Dev Disord. 2009 Aug;39(8):1099–1111. doi: 10.1007/s10803-009-0723-6. [DOI] [PubMed] [Google Scholar]
- 4.Taniai H, Nishiyama T, Miyachi T, Imaeda M, Sumi S. Genetic influences on the broad spectrum of autism: study of proband-ascertained twins. Am J Med Genet B Neuropsychiatr Genet. 2008 Sep 5;147B(6):844–849. doi: 10.1002/ajmg.b.30740. [DOI] [PubMed] [Google Scholar]
- 5.Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989 May;30(3):405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. Nov;167(11):1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 7.Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. Jan 13; doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- 8.Skuse DH, Mandy WP, Scourfield J. Measuring autistic traits: heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 2005 Dec;187:568–572. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- 9.Stilp RL, Gernsbacher MA, Schweigert EK, Arneson CL, Goldsmith HH. Genetic variance for autism screening items in an unselected sample of toddler-age twins. J Am Acad Child Adolesc Psychiatry. 2010 Mar;49(3):267–276. doi: 10.1016/j.jaac.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelson LR, Saudino KJ. Genetic and environmental influences on autistic-like behaviors in 2-year-old twins. Behav Genet. 2009 May;39(3):255–264. doi: 10.1007/s10519-009-9270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantino JN, Todd RD. Genetic structure of reciprocal social behavior. Am J Psychiatry. 2000 Dec;157(12):2043–2045. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- 12.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005 Mar 15;57(6):655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Scourfield J, Martin N, Lewis G, McGuffin P. Heritability of social cognitive skills in children and adolescents. Br J Psychiatry. 1999 Dec;175:559–564. doi: 10.1192/bjp.175.6.559. [DOI] [PubMed] [Google Scholar]
- 14.Hoekstra RA, Bartels M, Verweij CJ, Boomsma DI. Heritability of autistic traits in the general population. Arch Pediatr Adolesc Med. 2007 Apr;161(4):372–377. doi: 10.1001/archpedi.161.4.372. [DOI] [PubMed] [Google Scholar]
- 15.Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. J Am Acad Child Adolesc Psychiatry. 2003 Apr;42(4):458–467. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- 16.Ronald A, Happe F, Bolton P, Butcher LM, Price TS, Wheelwright S, Baron-Cohen S, Plomin R. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006 Jun;45(6):691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- 17.Ronald A, Happe F, Plomin R. Genetic research into autism. Science. 2006 Feb 17;311(5763):952. doi: 10.1126/science.311.5763.952a. [DOI] [PubMed] [Google Scholar]
- 18.Ronald A, Happe F, Plomin R. A twin study investigating the genetic and environmental aetiologies of parent, teacher and child ratings of autistic-like traits and their overlap. Eur Child Adolesc Psychiatry. 2008 Dec;17(8):473–483. doi: 10.1007/s00787-008-0689-5. [DOI] [PubMed] [Google Scholar]
- 19.Ronald A, Happe F, Plomin R. The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism. Dev Sci. 2005 Sep;8(5):444–458. doi: 10.1111/j.1467-7687.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- 20.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003 May;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 21.Ronald A, Larsson H, Anckarsater H, Lichtenstein P. A twin study of autism symptoms in Sweden. Mol Psychiatry. Jul 20; doi: 10.1038/mp.2010.82. [DOI] [PubMed] [Google Scholar]
- 22.Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, Singh D, Todd RD. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006 Feb;163(2):294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 23.Ronald A, Happe F, Price TS, Baron-Cohen S, Plomin R. Phenotypic and genetic overlap between autistic traits at the extremes of the general population. J Am Acad Child Adolesc Psychiatry. 2006 Oct;45(10):1206–1214. doi: 10.1097/01.chi.0000230165.54117.41. [DOI] [PubMed] [Google Scholar]
- 24.DeFries JC, Fulker DW. Multiple regression analysis of twin data: etiology of deviant scores versus individual differences. Acta Genet Med Gemellol (Roma) 1988;37(3-4):205–216. doi: 10.1017/s0001566000003810. [DOI] [PubMed] [Google Scholar]
- 25.Reich T, James JW, Morris CA. The use of multiple thresholds in determining the mode of transmission of semi-continuous traits. Ann Hum Genet. 1972 Nov;36(2):163–184. doi: 10.1111/j.1469-1809.1972.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 26.Reich T, Rice J, Cloninger CR, Wette R, James J. The use of multiple thresholds and segregation analysis in analyzing the phenotypic heterogeneity of multifactorial traits. Ann Hum Genet. 1979 Jan;42(3):371–390. doi: 10.1111/j.1469-1809.1979.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 27.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003 May;60(5):497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 28.Thapar A, Harrington R, McGuffin P. Examining the comorbidity of ADHD-related behaviours and conduct problems using a twin study design. Br J Psychiatry. 2001 Sep;179:224–229. doi: 10.1192/bjp.179.3.224. [DOI] [PubMed] [Google Scholar]
- 29.Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat Rev Genet. 2009 Dec;10(12):872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 30.Oliver BR, Plomin R. Twins’ Early Development Study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems from childhood through adolescence. Twin Res Hum Genet. 2007 Feb;10(1):96–105. doi: 10.1375/twin.10.1.96. [DOI] [PubMed] [Google Scholar]
- 31.Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Res. 2000 Sep;3(3):129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- 32.Scott FJ, Baron-Cohen S, Bolton P, Brayne C. The CAST (Childhood Asperger Syndrome Test): preliminary development of a UK screen for mainstream primary-school-age children. Autism. 2002 Mar;6(1):9–31. doi: 10.1177/1362361302006001003. [DOI] [PubMed] [Google Scholar]
- 33.APA. Diagnostic and statistic manual of mental disorders. 4. Washington DC: American Psychological Association; 2000. [Google Scholar]
- 34.Williams J, Scott F, Stott C, Allison C, Bolton P, Baron-Cohen S, Brayne C. The CAST (Childhood Asperger Syndrome Test): test accuracy. Autism. 2005 Feb;9(1):45–68. doi: 10.1177/1362361305049029. [DOI] [PubMed] [Google Scholar]
- 35.Hoekstra RA, Happe F, Baron-Cohen S, Ronald A. Limited genetic covariance between autistic traits and intelligence: findings from a longitudinal twin study. Am J Med Genet B Neuropsychiatr Genet. Jul;153B(5):994–1007. doi: 10.1002/ajmg.b.31066. [DOI] [PubMed] [Google Scholar]
- 36.Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000 Jul;41(5):645–655. [PubMed] [Google Scholar]
- 37.Ronald A, Butcher LM, Docherty S, Davis OS, Schalkwyk LC, Craig IW, Plomin R. A genome-wide association study of social and non-social autistic-like traits in the general population using pooled DNA, 500 K SNP microarrays and both community and diagnosed autism replication samples. Behav Genet. Jan;40(1):31–45. doi: 10.1007/s10519-009-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, Charman T. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP) Lancet. 2006 Jul 15;368(9531):210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- 39.Field A. Discovering Statistics Using SPSS for Windows. London: Sage; 2002. [Google Scholar]
- 40.Purcell S. Statistical Methods in Behavioral Genetics. In: Plomin R, DeFries J, McClearn G, McGuffin P, editors. Behavioral Genetics. 5. NY, NY: Worth; 2008. [Google Scholar]
- 41.DeFries JC, Fulker DW. Multiple regression analysis of twin data. Behav Genet. 1985 Sep;15(5):467–473. doi: 10.1007/BF01066239. [DOI] [PubMed] [Google Scholar]
- 42.Sham PC, Walters EE, Neale MC, Heath AC, MacLean CJ, Kendler KS. Logistic regression analysis of twin data: estimation of parameters of the multifactorial liability-threshold model. Behav Genet. 1994 May;24(3):229–238. doi: 10.1007/BF01067190. [DOI] [PubMed] [Google Scholar]
- 43.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002 Jun;3(2):119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- 44.Plomin R, DeFries J, McClearn G, McGuffin P, editors. Behavioral Genetics. NY,NY: Worth; 2008. [Google Scholar]
- 45.Purcell S, Sham PC. A model-fitting implementation of the DeFries-Fulker model for selected twin data. Behav Genet. 2003 May;33(3):271–278. doi: 10.1023/a:1023494408079. [DOI] [PubMed] [Google Scholar]
- 46.St Pourcain B, Wang K, Glessner JT, Golding J, Steer C, Ring SM, Skuse DH, Grant SF, Hakonarson H, Davey Smith G. Association between a high-risk autism locus on 5p14 and social communication spectrum phenotypes in the general population. Am J Psychiatry. Nov;167(11):1364–1372. doi: 10.1176/appi.ajp.2010.09121789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-Cawthorne G, Allison C, Banerjee-Basu S, Baron-Cohen S. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res. 2009 Jun;2(3):157–177. doi: 10.1002/aur.80. [DOI] [PubMed] [Google Scholar]
- 48.Steer CD, Golding J, Bolton PF. Traits contributing to the autistic spectrum. PLoS One. 5(9):e12633. doi: 10.1371/journal.pone.0012633. [DOI] [PMC free article] [PubMed] [Google Scholar]


