Abstract
Objective
Macrophage Activation Syndrome (MAS) is a devastating cytokine storm syndrome complicating many inflammatory diseases and characterized by fever, pancytopenia, and systemic inflammation. It is clinically similar to Hemophagocytic Lymphohistiocytosis (HLH), which is caused by viral infection of a host with impaired cellular cytotoxicity. Murine models of MAS and HLH illustrate Interferon-γ (IFN-γ) as the driving stimulus for hemophagocytosis and immunopathology. We sought to understand the inflammatory contributors to a murine model of Toll-like Receptor 9 (TLR9)-induced fulminant MAS.
Methods
Wild-type (WT), transgenic, and cytokine-inhibited mice were treated with an IL-10 receptor blocking antibody and TLR9 agonist, and parameters of MAS were evaluated.
Results
Fulminant MAS was characterized by dramatic elevations in IFN-γ, IL-12, and IL-6. Serum IFN-γ correlated with enhanced IFN-γ production within some hepatic populations, but fewer IFN-γ+ cells. Surprisingly, IFN-γKO mice developed immunopathology and hemophagocytosis comparably to WT mice. However, IFN-γKO mice did not become anemic and had greater numbers of splenic erythroid precursors. IL-12 neutralization phenocopied disease in IFN-γKO mice. Interestingly, Type I interferons contributed to the severity of hypercytokinemia and weight loss, but their absence did not otherwise affect MAS manifestations.
Conclusion
These data demonstrate that both fulminant MAS and hemophagocytosis can arise independently of IFN-γ, IL-12, or Type I interferons. They also suggest that IFN-γ-mediated dyserythropoiesis, not hemophagocytosis, is the dominant cause of anemia in fulminant TLR9-MAS. Thus, our data establish a novel mechanism for the acute anemia of inflammation, but suggest that a variety of triggers can result in hemophagocytic disease.
Macrophage Activation Syndrome (MAS) is a potentially lethal hemophagocytic syndrome that complicates numerous infectious, oncologic, and rheumatic diseases. It is characterized by fever, pancytopenia, coagulopathy and inflammation of various organs including the liver and central nervous system. It bears striking clinical similarity to familial Hemophagocytic Lymphohistiocytosis (HLH), a disease caused by mutations leading to impaired NK and CD8 T-cell cytotoxicity (1). The hemophagocytic syndromes MAS and HLH are so-named due to the characteristic finding of hemophagocytes (HPCs) on examination of bone marrow, livers and/or spleens. HPCs are activated macrophages identified histologically to have engulfed other hematopoietic elements. While commonly identified with HLH and MAS, they have been observed in a number of other inflammatory diseases (2).
The mechanisms resulting in human hemophagocytic syndromes remain largely unknown. Serum cytokines in active MAS demonstrated elevation of a number of inflammatory pathways with heterogeneity likely reflecting the triggering effect of the disease of origin (3, 4). Other groups have shown that some patients with systemic juvenile idiopathic arthritis (sJIA), a disease frequently associated with MAS, had defects in cytotoxicity akin to those seen in HLH (5–7). Serum cytokines in HLH patients similarly showed polycytokinemia with emphasis on IFN-γ and related cytokines (8, 9). Interestingly, PBMC microarray data in HLH showed upregulation of certain pro- and anti-inflammatory factors, and downregulation of genes associated with NK & CD8 T-cell activation, TLR signaling, and apoptosis (10). Notably, IFN-γ responsive genes were not differentially regulated in HLH (10), and the role of IFN-γ in sJIA is unclear (11).
Murine models of hemophagocytic syndromes, however, have largely implicated IFN-γ as the principal causative cytokine. Several models of HLH utilize lymphocytic choriomeningitis virus (LCMV) infection in the context of genetic cytotoxic defects and rely on IFN-γ to drive immunopathology (12–14). Indeed, IFN-γ alone, by infusion pump, was sufficient to drive immunopathology (15). However, Krebs et al demonstrated in an HLH model that immunopathology was dependent on the presence of MyD88, an adaptor critical for IL-1 and TLR signaling (14). The IL-1 receptor was dispensable in this model, indicating TLR signaling as important in the pathogenesis of HLH. Concordant with a role for TLR signaling as a driver of hemophagocytic disease, repeated TLR9 agonism created an MAS-like phenotype and was similarly IFN-γ dependent (16). Overall, these models suggest that TLRs and IFN-γ are critical mediators hemophagocytic disease.
Despite the abundance of clinical and animal data supporting the ability of IFN-γ to drive hemophagocytosis and cytokine storm, investigations into the contributions of other cytokines to hemophagocytic pathology are lacking. Mice constitutively expressing IL-6 develop an MAS-like reaction to TLR stimulation (17). We have previously reported that the anti-inflammatory cytokine IL-10 regulated TLR9-mediated MAS, since disruption of IL-10 signaling resulted in far more severe disease including hemophagocytosis (a collection of symptoms we here refer to as “fulminant TLR9-MAS”) (16).
Investigating fulminant TLR9-MAS, we found that fulminant MAS was accompanied by dramatically enhanced inflammatory cytokinemia, including IFN-γ. Surprisingly, mice deficient in IFN-γ experienced fulminant MAS similarly to WT mice, complete with hemophagocytosis. However, fulminant MAS in IFN-γ deficient mice occurred without anemia, correlating with greater splenic erythropoiesis. Further evaluation demonstrated that IL-12 drove IFN-γ production, and that Type I IFNs contributed to fulminant MAS immunopathology. These data demonstrate that most manifestations of hemophagocytic disease, including hemophagocytosis itself, can occur independently of IFN-γ. They also suggest that IFN-γ-mediated dyserythropoiesis, not hemophagocytosis, is the dominant mechanism of MAS-related anemia.
Materials and Methods
Mice
WT and IFN-γKO mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Yeti mice were a gift from M. Mohrs (Trudeau Institute, Saranac Lake, NY) (18). IFNAR-KO mice were a gift from J. M. Bergelson (CHOP Research Institute, Philadelphia, PA) (19). All mice were bred on a C57BL/6 background and housed in an Association for Assessment and Accreditation of Laboratory Animal Care–certified animal facility. All experiments were performed with approval of the University of Pennsylvania and The Children’s Hospital of Philadelphia IACUC.
Induction of MAS
WT and transgenic mice were injected i.p. with PBS or 37.5 µg CpG ODN 1826 (Integrated DNA Technologies) with or without 200 µg IgG2b control antibody (clone SFR8) or IL-10R1 blocking antibody (referred to as IL10RB, clone 1B1.3A, both Bio X-cell) on days 0, 2, 4, and 6, and mice were sacrificed on day 7. 500 µg control or IL-12p40 neutralizing antibody (C17.8, Bio X-cell) was injected on days 0 and 4. 1.5 mg control or TNFα neutralizing antibody (XT3.11, Bio X-cell) was injected on days 0 and 4.
Peripheral blood was obtained immediately prior to sacrifice and complete blood counts performed on a hemavet analyzer. IL-10, IFN-γ, IL-6, IL-12p70, IL-1β, IL-4 (BD OptEIA), IL-18 (MBL International), and IFNβ (PBL interferonsource) were measured in sera using ELISA according to manufacturers’ specifications.
Flow Cytometry
Organs were harvested, homogenized, and passed through a 70µm filter to generate single cell suspensions. Spleens were injected with media containing 1 mg/mL collagenase and 50 µg/mL DNAse and incubated at 37°C for 15 minutes prior to homogenization and filtering. Hepatic leukocytes were isolated from liver single-cell suspensions by means of percoll gradient centrifugation. Cell concentrations were assessed using a hemocytometer or a Countess Automated Cell Counter (Life Technologies). Cells were stained with B220, CD4, CD8α, CD11b, CD11c, CD19, NK1.1, TCRβ, CD71 (BD Pharmingen), and/or Ter119 (Biolegend) fluorochrome-tagged antibodies and analyzed using a BD LSR II Flow Cytometer and FlowJo software version 9.3 (Tree Star, Inc).
Gating Strategies
All gates are drawn on doublet-excluded, live cells. CD4 & CD8 T-cells: TCRβ+NK1.1-CD4+ or CD8+, respectively NKT cells: TCRβ+NK1.1+CD8- NK cells: TCRβ-B220-NK1.1+CD11b+ Plasmacytoid Dendritic Cells: TCRβ-CD11clo B220intermediate to high, CD8- CD8+ Conventional Dendritic Cells: TCRβ-CD11chiB220-CD8+ Erythroid precursors: TCRβ-CD19-CD71+Ter119+
Evaluation of hemophagocytosis
Touch preps were made by lightly touching spleens to a glass slide, air drying, and Wright-Giemsa staining. Slides were evaluated by a blinded pediatric hematopathologist (MP) and qualitatively scored as excessive hemophagocytosis present, absent, or indeterminant.
Evaluation of hepatitis
Unperfused livers were fixed overnight at 4°C in 10% formalin in PBS and embedded in paraffin. H&E stained slides were prepared and evaluated by a blinded pediatric pathologist (PK) who assigned a lobular hepatitis score based on the number of foci of lobular inflammation per 20X high power field in the most inflamed area of the specimen.
Statistics
Data were plotted and analyzed using Prism 5.0 (GraphPad Software). Statistical significance was tested using one-way ANOVA with Tukey’s post test, 2-tailed unpaired Student’s t-test or repeated measures ANOVA, as indicated. Error bars in all figures represent SEM, with the midline representing the mean value. All comparisons reaching statistical significance (less than 5% type I error) are indicated.
Results
Massive elevations in inflammatory serum cytokines characterize fulminant MAS
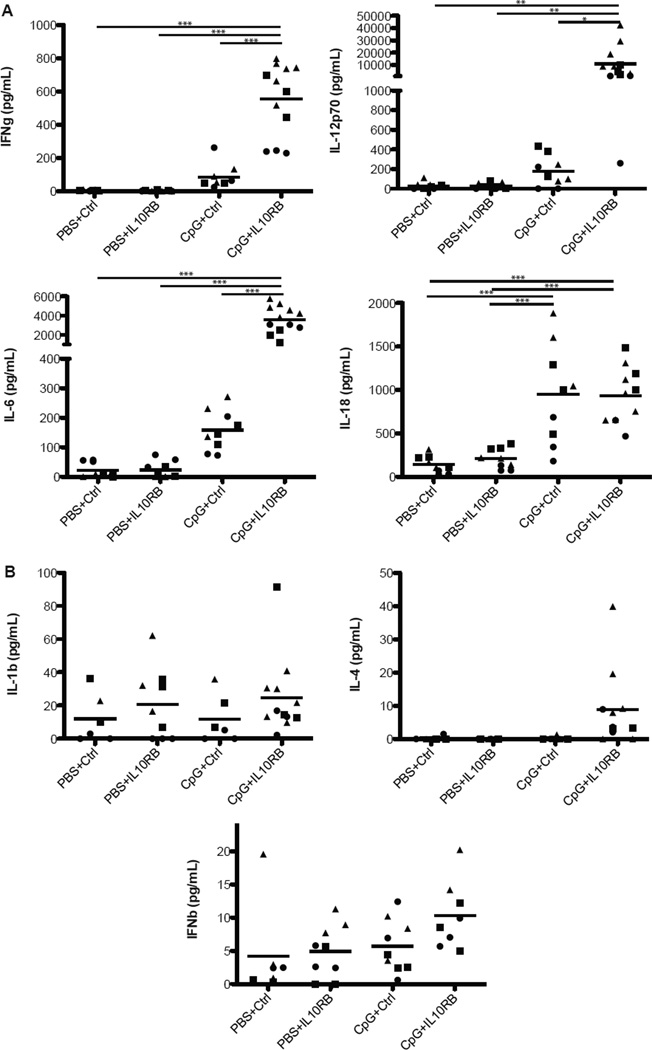
We have previously shown that coadministration of repeated CpG with an IL-10R1 blocking antibody results in fulminant MAS complete with more severe cytopenias, hyperferritinemia, and the development of HPCs (16). Fulminant MAS was also characterized by dramatic elevations in the pro-inflammatory cytokines IFN-γ, IL-12p70, and IL-6 (Figure 1A). Other macrophage-stimulatory cytokines such as IL-18, IL-1β, IL-4, and IFN-β were less dramatically elevated (Figure 1A & B). We detected no substantial serum IFN-α or TNF-α (data not shown). These data are consistent with fulminant MAS as a cytokine storm syndrome associated with a Th1-predominant inflammatory polycytokinemia.
Figure 1. Massive elevations in various serum cytokines characterize fulminant MAS.
Sera were collected from control or fulminant MAS-treated mice 24 hours after the final injection of CpG. (A) Serum IFN-γ, IL-12p70, IL-6, and IL-18 were assayed by ELISA. (B) Serum IL-1β, IL-4, and IFN-β were assayed by ELISA. Graphs represent pooled data from up to 4 individual experiments: each point is an individual mouse, each shape represents an individual experiment. *p<0.05, **p<0.01, ***p<0.001, using one-way ANOVA followed by Tukey’s Post-test.
Fulminant MAS is accompanied by enhanced IFN-γ production within select populations, but diminution in the absolute numbers of IFN-γ producing cells
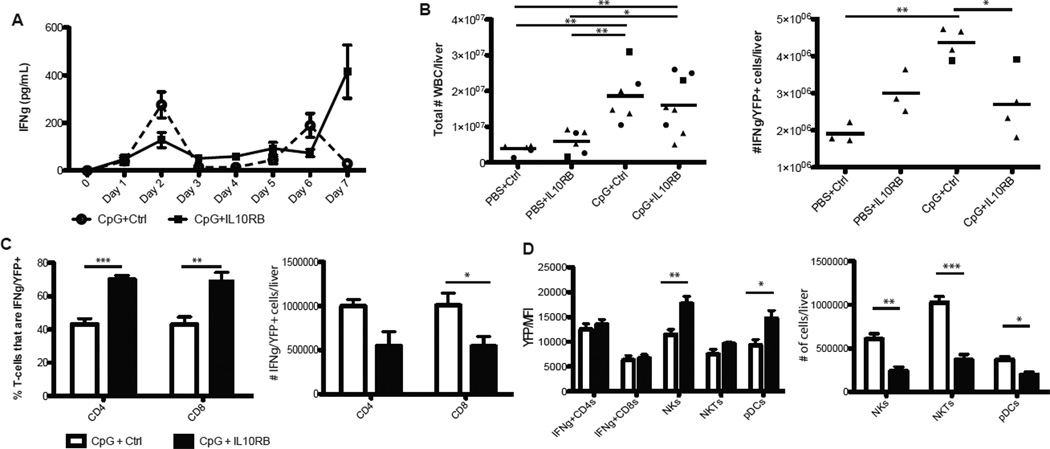
Given the IFN-γ dependence of most models of hemophagocytic diseases (12–16) and the enhanced IFN-γ production seen in fulminant MAS, we investigated the major cellular sources of IFN-γ in this model. To accomplish this and to avoid the necessity of different ex vivo stimuli, we made use of YETI mice in which YFP production marks transcription of IFN-γ (18). As some cells expressed IFN-γ mRNA at baseline (data not shown and (18)), IFN-γ induction was measured by increases in the proportion of YFP+ cells and/or by increases in the YFP median fluorescence intensity (MFI) of the population. We evaluated IFN-γ production at Day 7 because, in distinction to CpG-alone, serum IFN-γ in fulminant MAS was most dramatically elevated at this timepoint (Figure 2A). IFN-γ expression varied substantially by organ, but the liver showed by far the highest baseline and inducible expression as compared to spleen, peripheral blood, and mesenteric and inguinal lymph nodes (data not shown); thus we focused our investigations on hepatic IFN-γ production. The total number of liver-infiltrating cells increased with CpG-treatment and was comparable in both fulminant MAS and with CpG-alone (Figure 2B). However, despite the increased serum IFN-γ observed in fulminant MAS compared with CpG-alone, the absolute number of IFN-γ/YFP+ cells in fulminant MAS appeared to decrease as compared to CpG-alone (Figure 2B).
Figure 2. Increased serum IFN-γ in fulminant MAS is accompanied by enhanced production within some populatins, but diminution of absolute numbers of IFN-γ+ cells.
(A) Sera from mice treated with CpG alone or CpG+IL10RB were obtained daily by cheek bleed and measured for IFN-γ by ELISA. Livers of control or fulminant MAS-treated YETI mice were collected and analyzed as described in Materials and Methods, and prepared for flow cytometric analysis. Gating strategies are described in the Materials and Methods(B) The total number of hepatic leukocytes, and the subset of IFN-γ/YFP+ hepatic leukocytes, were calculated for each treatment group. (C) The proportion of hepatic CD4 and CD8 T-cells expressing IFN-γ (Left) was calculated. (Right) The absolute number of these cells per liver was calculated. (D) The median fluorescence intensity (MFI) of hepatic IFN-γ+ CD4 & CD8 Tcells, as well as all hepatic NK cells, NKT cells, and plasmacytoid dendritic cells (pDCs), left) were calculated. (Right) Absolute numbers of hepatic NK, NKT, and pDC populations were calculated. Graphs represent pooled data from up to three independent experiments with up to three mice per group. *p<0.05, **p<0.01, ***p<0.001, using one-way ANOVA with Tukey’s post-test for significance. For percentages, p-values represent the analysis of log10-transformed data.
Fulminant MAS was accompanied by an increase in the percentage of hepatic CD4 and CD8 T-cells that were IFN-γ/YFP+ without a change in the YFP-MFI (Figure 2C & D). However, consistent with the drop in total hepatic YFP+ cells, the total numbers of YFP+ hepatic CD4 and CD8 T-cells were lower in fulminant MAS than with CpG alone (Figure 2C). Accordingly, the YFP-MFI of hepatic NKT, NK, and plasmacytoid dendritic cells was substantially elevated in fulminant MAS versus mice treated with CpG alone (Figure 2D). However, the size of these populations was again lower in fulminant MAS than with CpG alone (Figure 2D). Only the above cell types and CD8+ conventional dendritic cells (cDCs) showed baseline or inducible IFN-γ/YFP production. cDCs did not appreciably change expression or cell number in any experimental condition (data not shown).
Collectively, these observations suggest that fulminant MAS was characterized by enhanced IFN-γ production within NK, NKT, & pDC populations, but that the total number of hepatic cells expressing IFN-γ was contracted versus mice treated with CpG alone. This is in contrast to models of familial HLH, where massive elevations in serum IFN-γ are attributable to large increases in the numbers of IFN-γ producing, antigen-specific CD8 T-cells (12, 20).
Fulminant MAS and hemophagocytosis arise independently of IFN-γ, while MAS-related anemia and dyserythropoiesis are IFN-γ dependent
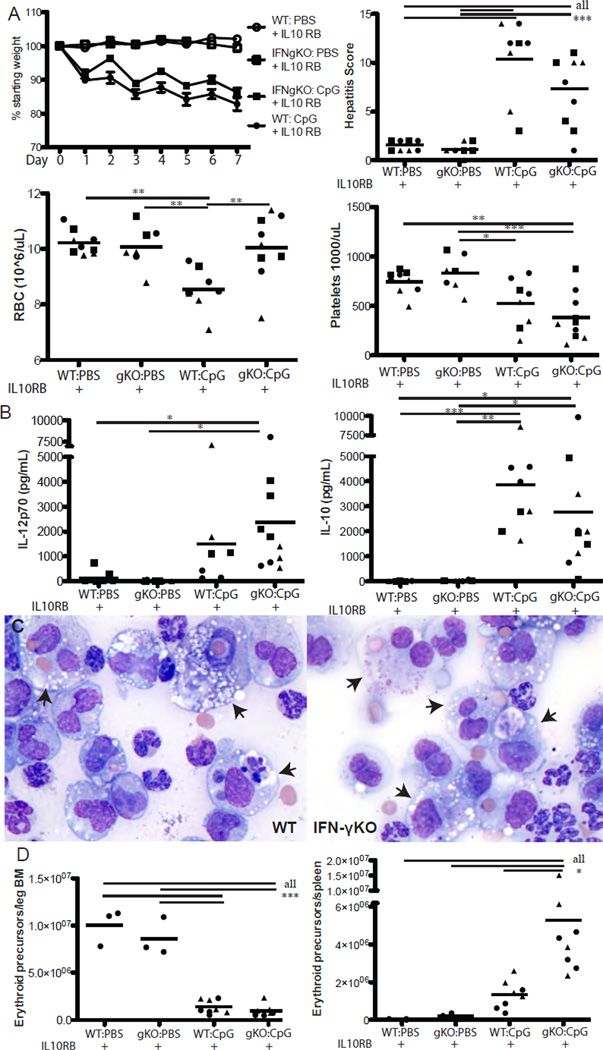
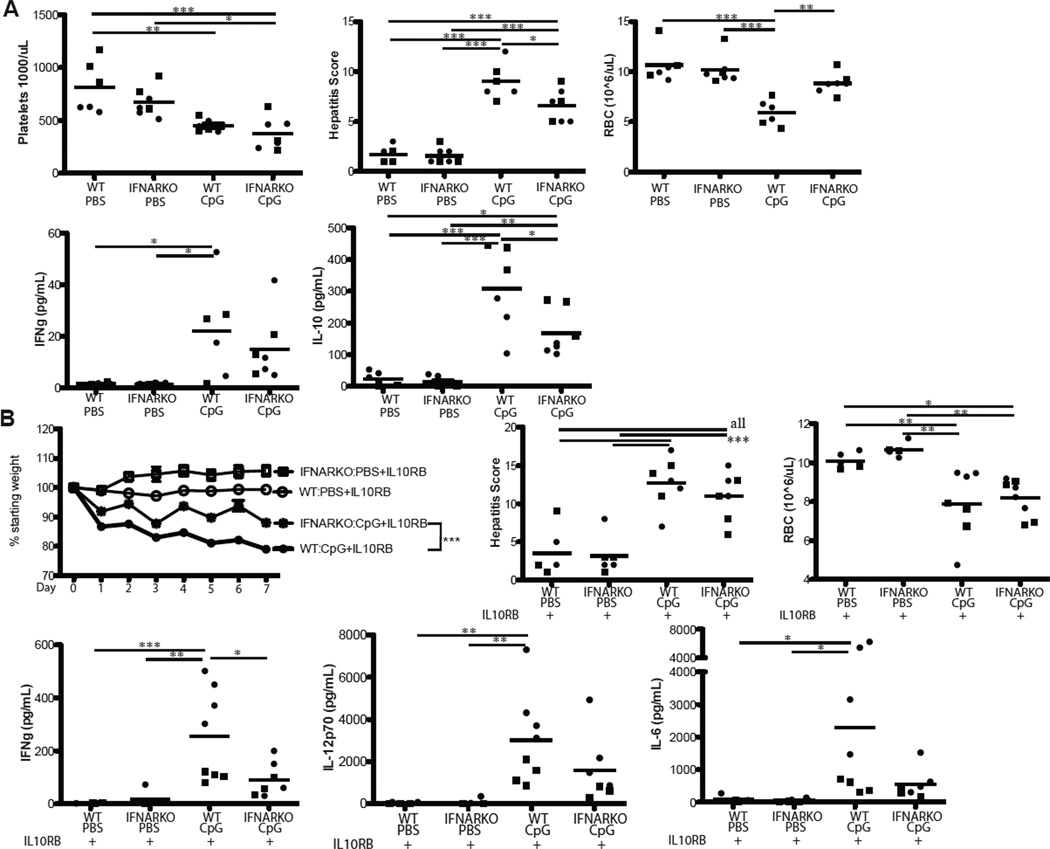
To directly test the role of IFN-γ in fulminant MAS, we administered CpG and IL-10R blocking antibody to mice incapable of producing IFN-γ. Surprisingly, IFN-γKO mice were not significantly protected from fulminant MAS-induced cachexia, thrombocytopenia, or hepatitis (Figure 3A). Additionally, elevations in serum IL-12, IL-6, and IL-10 in IFN-γKO mice were comparable to those seen in WT mice (Figure 3B and data not shown). IFN-γKO mice also developed HPCs at a rate comparable to WT mice (Table 1). These results suggest the importance of IL-10 in limiting the contributions of inflammatory mediators other than IFN-γ to MAS immunopathology.
Figure 3. Fulminant MAS and hemophagocytosis are IFN-γ independent, while anemia and dyserythropoiesis are IFN-γ dependent.
WT or IFN-γKO mice were treated with IL10RB and either PBS or CpG, then assessed for parameters of fulminant MAS as described in Materials and Methods. On day 7 of treatment, 24 hours after the final dose of CpG, mice were sacrificed and weight loss, thrombocytopenia, lobular hepatitis score, and anemia were evaluated (A). Sera were taken 24 hours after the final dose of CpG and the cytokines IL-12p70 and IL-10 assessed by ELISA (B). Representative 40X photomicrographs (C) of Wright-Giemsa stained splenic touch preps from WT and IFN-γKO mice treated with CpG & IL10RB. Arrowheads denote hemophagocytes. (D) Bone marrow and splenic erythroid precursors were evaluated by flow cytometry as described in Materials & Methods, and absolute numbers calculated. Graphs represent pooled data from up to 3 separate experiments: each point is an individual mouse, each shape represents an individual experiment. *p<0.05, **p<0.01, ***p<0.001 using one-way ANOVA with Tukey’s post-test for significance.
Table 1.
Hemophagocytosis is characteristic of fulminant MAS independent of a variety of pro-inflammatory cytokines
| Mice with excessive hemophagocytosis/total mice in group | |||||
|---|---|---|---|---|---|
| PBS + IL10RB |
KO/cytokine blocked PBS + IL10RB |
WT: CpG + IL10RB |
KO/cytokine blocked CpG + IL10RB |
||
| IFN-γKO | 0/9 | 0/9 | 7/8 | 7/8 | |
| antiIL-12p40 | 0/3 | 0/3 | 3/3 | 3/3 | |
| antiTNFα | 1/3 | 0/1 | 3/3 | 3/3 | |
| IFNARKO | 0/2 | 0/3 | 3/3 | 4/4 | |
Splenic touchpreps were prepared as described in Methods and a blinded pediatric hematopathologist (MP) designated each sample as excessive hemophagocytosis present or absent.
We were most surprised to see that while IFN-γKO mice were susceptible to most aspects of TLR9-induced immunopathology, they were protected from anemia (Figure 3A). We have previously shown that TLR9-induced anemia is IFN-γ dependent, but occurs in the absence of overt hemophagocytosis (16). We now show that IFN-γKO mice develop hemophagocytic macrophages under fulminant MAS conditions and yet these mice do not become anemic. Such IFN-γKO HPCs had engulfed both erythrocytes and leukocytes comparably to HPCs from WT mice (Figure 3C). To investigate the mechanism of this IFN-γ dependent anemia, we evaluated bone marrow, liver, and spleen CD71+Ter119+ erythroid precursors (21). We found that CpG was toxic to bone marrow hematopoiesis irrespective of IFN-γ (Figure 3D). However, IFN-γKO mice showed substantially higher numbers of splenic and to a lesser extent hepatic erythroid precursors (Figure 3D and data not shown). Thus, our data suggest that MAS instigates a demand for extramedullary hematopoiesis and that IFN-γ mediates anemia in part by inhibiting the ability to respond to this demand.
IL-12 acts upstream to induce IFN-γ in fulminant MAS
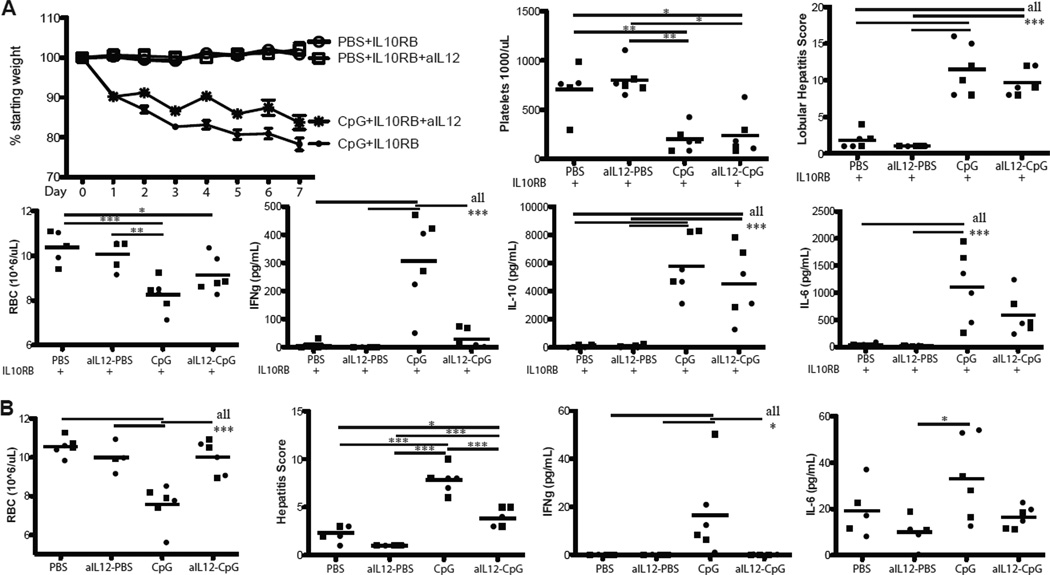
Since IFN-γ was dispensable for the majority of features of fulminant MAS, we sought to determine the contributions of other cytokines to the observed immunopathology. IL-12p70 is most notable for its induction of TH1 differentiation and stimulatory effects on NK cells and CD8 T-cells (22). Serum IL-12 was also markedly elevated in fulminant MAS even in the absence of IFN-γ (Figure 3B). Induction of fulminant MAS in mice concomitantly treated with an antibody blocking IL-12p40 resulted in a phenotype similar to that seen in IFN-γKO mice (Figure 4). Specifically, IL-12 blocked mice experienced weight loss, thrombocytopenia, and hepatitis comparably to WT mice (Figure 4A). However, IL-12 blocked mice were largely spared from anemia (Figure 4A), suggesting that IL-12 acts upstream to induce IFN-γ production. Hemophagocytosis was comparable between WT mice and IL-12 blocked mice (Table 1) and similar to that seen with IFN-γKO mice. Concordant with the interpretation that IL-12 functions to stimulate IFN-γ production, serum IFN-γ levels were substantially reduced in IL-12 blocked mice compared to WT (Figure 4A). Serum levels of IL-10 and IL-6 were not substantially different in IL-12 blocked mice exposed to fulminant MAS conditions (Figure 4A). Additionally, mice treated with CpG and IL-12 blockade with intact IL-10 signaling were substantially protected from most aspects of MAS as compared to mice treated with CpG without IL-12 neutralization (Figure 4B & data not shown), completely phenocopying the effects of CpG-alone on IFN-γKO mice (16). These experiments elucidate an important arm in the induction of pathogenic IFN-γ in the fulminant MAS model and support the dependence of anemia on the IL-12/IFN-γ axis. They also further confirm that hemophagocytosis can occur without accompanying anemia.
Figure 4. IL-12 acts upstream of IFN-γ in the pathogenesis of fulminant MAS.
(A) WT mice were all treated with IL10RB, PBS or CpG, and antibody neutralizing IL-12p40 (C17.8) or IgG control antibody, then assessed for parameters of fulminant MAS as in Materials and Methods. Weight loss, thrombocytopenia, hepatitis, anemia, and serum IFN-γ, IL-10, and IL-6 were assessed 24 hours after the final dose of CpG. (B) WT mice were treated with PBS or CpG and anti-IL-12p40 or IgG control and assessed for anemia, hepatitis, serum IFN-γ, and serum IL-6 as in Materials and Methods. Graphs represent pooled data from 2 separate experiments: each point is an individual mouse, each shape represents an individual experiment. *p<0.05, **p<0.01, ***p<0.001 using one-way ANOVA with Tukey’s post-test for significance.
Disruption of Type I Interferon signaling partially abrogates disease in fulminant MAS
Since most aspects of inflammatory pathology, save anemia, were independent of the IL-12/IFN-γ axis, we endeavored to determine the roles of other cytokines induced by TLR9 stimulation in the fulminant MAS model. We evaluated the contribution of TNFα, and consistent with the finding of minimal serum TNFα, we found that TNFα neutralization had no significant effect on TLR9-MAS or hemophagocytosis irrespective of IL-10 inhibition (data not shown and Table I).
Since TLR9 stimulation is known to induce a robust Type I interferon (IFNα/β) response in a MyD88-dependent fashion (23), we sought to evaluate the effects of disruption of IFNα/β signaling on MAS. When IL-10 signaling was intact, administration of CpG to mice lacking an intact IFN α/β receptor (IFNAR-KO mice) resulted in the development of MAS that was slightly less severe than that seen in WT mice. Specifically, hepatitis and serum IL-10 were marginally less severe in IFNAR-KO mice than WT counterparts, while thrombocytopenia and serum IFN-γ were comparable (Figure 5A). Interestingly, despite IFN-γ production comparable to WT mice, IFNAR-KO mice were almost entirely spared from anemia (Figure 5A) suggesting that Type I Interferons may help mediate anemia in the presence of IL-10 and low-level IFN-γ. Like WT mice, CpG-treated IFNAR-KO mice did not develop hemophagocytosis (data not shown).
Figure 5. Disruption of Type I interferon signaling partially abrogates cachexia and cytokinemia in fulminant MAS.
(A) WT or IFNAR-KO mice were treated with either PBS or CpG and assessed for parameters of fulminant MAS as described in Materials and Methods. Thrombocytopenia, hepatitis, anemia, and serum IFN-γ and IL-10 were assessed 24 hours after the final dose of CpG. (B) WT or IFNAR-KO mice were all treated with IL10RB and either CpG or PBS and assessed as described in Materials and Methods. Weight loss, hepatitis, anemia and serum IFN-γ, IL12p70, and IL-6 were assessed 24 hours after the final dose of CpG. Graphs represent pooled data from 2 separate experiments: each point is an individual mouse, each shape represents an individual experiment. *p<0.05, **p<0.01, ***p<0.001. The comparison of weight loss between genotypes was performed on Log10 transformed percentages by use of the repeated measures ANOVA. Otherwise, comparisons were made using one-way ANOVA with Tukey’s Post-test for significance.
In fulminant MAS (where IL-10 signaling is blocked) IFNAR-KO mice were partially protected from immunopathology. Specifically, IFNAR-KO mice had less weight loss than WT counterparts but comparable thrombocytopenia and hepatitis (Figure 5B & data not shown). Contrary to the disease seen when IL-10 signaling was intact, IFNAR-KO mice treated with fulminant MAS conditions experienced anemia comparably to WT controls (Figure 5B). Inflammatory cytokinemia was similarly partially abrogated in IFNAR-KO mice (Figure 5B). Importantly, splenic hemophagocytosis in IFNAR-KO mice was again comparable to that seen in WT mice (Table 1). Together, these results suggest a complex and partially redundant interplay of Type I and Type II Interferons and other inflammatory cytokines in the pathogenesis of TLR9-mediated MAS.
Discussion
Cytokine-induced immunopathology is complex and results from the interactions of pathogen (if present) and host pro- and anti-inflammatory factors. Many of the existing animal models of hemophagocytic diseases rely on infection and/or genetic manipulation (12, 13, 24–26), making it difficult to dissect the contributions of pathogen and host response to disease manifestations. We have recently shown that repeated TLR9 stimulation of WT mice in the absence of pathogen recapitulates many of the findings seen in human hemophagocytic diseases (16). Like other models, TLR9-MAS relies on the activity of IFN-γ to drive disease. However, a few key differences further separate TLR9-MAS from other models of hemophagocytic disease: 1) TLR9-MAS results in the development of profound anemia in the absence of HPCs, and 2) inhibition of IL-10 severely aggravates disease (16). In the current study, we investigated the pro-inflammatory drivers of TLR9-mediated disease in the absence of IL-10 signaling (so-called “fulminant TLR9-MAS”).
The finding of enhanced cytokinemia in fulminant MAS was not surprising given the known suppressive effects of IL-10 on inflammatory cytokine production (27, 28). The elevations in IFN-γ and IL-12 were consistent with cytokinemia along a TH1 spectrum, which is consistent with observations in humans and mouse models of HLH (8, 9, 29). Additionally, IL-6 production is known to be suppressed by IL-10 and can contribute to pathology similar to MAS (17, 27). IL-18, a cytokine associated with human sJIA (30), was upregulated equally in the presence and absence of IL-10 signaling (Figure 1). As an inducer of IFN-γ, it may account for the residual serum IFN-γ and anemia observed in IL-12 blocked mice (Figures 4A & B). Other inflammatory cytokines were less clearly upregulated by CpG, although serum type I IFN protein levels often do not reflect the biological contributions of these cytokines as detected by IFN-inducible transcription (31).
A massive expansion of IFN-γ producing, antigen-specific CD8 T-cells is critical for disease pathogenesis in models of familial HLH (12, 14, 20). The manifestations of fulminant MAS (cytopenias, cachexia, mortality, hepatitis) were comparable in severity to those seen in models of familial HLH (12, 16). Therefore, we hypothesized that there would be a dramatic increase in one or more IFN-γ-producing cell types in the fulminant MAS model. However, our search for the sources of enhanced serum IFN-γ in fulminant MAS revealed some surprising results. Consistent with higher serum IFN-γ, fulminant MAS increased the proportion of T-cells producing IFN-γ without affecting MFI, and significantly increased the MFI of NK, NKT, and pDCs, which were largely YFP+ at baseline (Figures 2C & D). However, absolute numbers of IFN-γ producing cells were diminished in fulminant MAS versus with CpG alone (Figures 2C & D). Thus, despite high serum IFN-γ levels, we did not identify a “smoking gun” of IFN-γ production as described in other models of hemophagocytic disease.
The lack of expansion in the number of IFN-γ-producing cells made us question the relationship of fulminant MAS to IFN-γ. Serum levels of IFN-γ, while higher in fulminant MAS than with CpG alone, did not approximate the levels seen in models of familial HLH (Figure 1A versus (13, 20)). Thus, the severity of fulminant hemophagocytic syndromes is not purely a product of the level of IFN-γ reached. When formally evaluating the effects of CpG and IL-10 receptor blockade in mice incapable of making IFN-γ, there was no alteration in the manifestations of disease (with the notable exception of anemia, Figure 3). Thus, in the absence of IL-10 signaling, TLR9-mediated MAS was largely IFN-γ independent.
We were particularly interested to see that TLR9-driven hemophagocytosis was IFN-γ independent. Zoller et al showed that hemophagocytosis and anemia in IFN-γ-infused mice were dependent on IFN-γ receptor function in monocytes (15). These data suggested that IFN-γ acted directly on macrophages to engulf hematopoietic elements and cause the “Consumptive Anemia of Inflammation.” However, it was not clear whether IFN-γ induced hemophagocytosis directly in a cell-intrinsic manner, or whether IFN-γ induced macrophages to produce a secondary factor that in turn induced hemophagocytosis. Ours is not the first report of IFN-γ independent hemophagocytosis: IL-4 delivered by infusion pump was capable of inducing IFN-γ independent hemophagocytosis (32). Our data offer another example of how hemophagocytosis may occur via a process that does not specifically require IFN-γ. It remains to be determined whether multiple pathways act directly on a macrophage to become an HPC, or whether various stimuli (including IFN-γ) converge to induce hemophagocytosis.
Unlike hemophagocytosis, we found that TLR9-induced anemia was uniquely dependent on IFN-γ (Figure 3A). Analysis of hematopoietic organs showed that IFN-γ inhibited sufficient extramedullary hematopoiesis in fulminant MAS (Figure 3D). Thus, TLR9-agonism increased RBC demand that was IFN-γ independent and may have occurred in part through bone marrow failure. However, IFN-γ then blocked the response to this demand by inhibiting extramedullary hematopoiesis. These data are consistent with reports demonstrating red pulp expansion (sometimes without hemophagocytosis) in HLH patients’ spleens (33), while their bone marrows may show abnormal erythropoiesis (34). We have previously shown profound anemia in the absence of detectable hemophagocytosis (16), suggesting that HPCs are not necessary for IFN-γ driven anemia. It seems unlikely that we simply failed to detect hemophagocytosis, since anemia in fulminant MAS is comparable to that seen with CpG-alone (16) and we can readily detect HPCs in fulminant MAS (Figure 3C, Table 1, and (16)). Furthermore, we have shown the presence of hemophagocytes in mice that do not become anemic (Table I, Figure 3), proving that hemophagocytosis is insufficient for TLR9-induced anemia. These findings support the unique connection between IFN-γ and anemia, but fundamentally dissociate hemophagocytosis as a necessary or sufficient mechanism for anemia.
Our data further suggest that anemia occurs by a mechanism distinct from other cytopenias. The presence of comparable lymphopenia (data not shown) and thrombocytopenia (Figure 3A) in IFN-γKO mice suggested that IFN-γ dependent effects on peripheral cellularity were unique to the erythroid line. We suggest that anemia arises due to an IFN-γ independent increase in RBC demand as well as an IFN-γ dependent inhibition of extramedullary hematopoiesis. While extramedullary hematopoiesis in humans is not well-described, the inhibitory effects of IFN-γ on erythropoiesis are well-documented (35, 36).
Our data also help to elucidate an important inflammatory axis downstream of TLR9 stimulation. We found that inhibition of IL-12 largely reproduced the phenotype seen in IFN-γKO mice, both in IL-10 sufficiency and blockade (Figure 4). The evidence for IL-12 acting upstream of IFN-γ in this model comes not only from a large body of literature (22), but by the finding that IL-12 neutralization nearly eradicated serum IFN-γ levels, even in the absence of IL-10 signaling (Figures 4A & B). The residual IFN-γ present in fulminant MAS with IL-12 neutralization is at a level comparable to that seen with CpG-alone (Figure 4A and (16)) and may explain the residual anemia seen with IL-12 blockade (Figure 4A). Thus, our data place IL-12 upstream of IFN-γ in fulminant MAS. By contrast, the effects of Type I IFN on TLR9-MAS were clearly complex, but suggested interplay with the IFN-γ system, and supported the role for Type I IFN’s in inducing IL-10 (28, 37, 38)
The independence of excessive hemophagocytosis from the actions of IFN-γ, IL-12, and Type I interferons (Table I) suggests that these cells may be part of a non-specific response to severe systemic inflammation. Indeed, excessive hemophagocytosis has been reported in a variety of cytokine storm syndromes not classically considered hemophagocytic (39, 40). However, the dependence of hemophagocytosis on IL-10 blockade in our model could represent a direct effect of IL-10 in restraining differentiation into hemophagocytic macrophages, or hemophagocytosis could result from some other factor(s) that correlates with more severe disease. Future studies are warranted to further characterize HPCs and how IL-10 may affect their differentiation and function.
In summary, we have shown that repeated TLR9-stimulation in the context of IL-10 receptor blockade resulted in an IFN-γ independent cytokine storm syndrome. These data alter the paradigm that IFN-γ is necessary for severe hemophagocytic disease, replacing it instead with a model wherein repeated TLR stimulation can drive polycytokinemia and result in a common inflammatory phenotype that includes hemophagocytosis but is largely independent of any one single cytokine. We have further shown that HPCs were neither necessary nor sufficient for acute anemia, but we found that IFN-γ crippled the ability to respond to anemic stress. We also demonstrated the presence of an IL-12/IFN-γ axis downstream of TLR9 stimulation. Finally, we found that fulminant MAS (including hemophagocytosis) occurred in the absence of IFN-γ, IL-12, TNFα, and Type I interferon signaling, although absence of Type I IFN signaling partially abrogated cachexia. Further study is clearly warranted to better understand the contributions of the various pro- and anti-inflammatory cytokines to hemophagocytic syndromes, and the development and function of the hemophagocyte itself. Nonetheless, our findings demonstrate that heterogeneous stimuli can result in the hemophagocytic phenotype, and suggest a varied approach to treatment of such diseases.
Acknowledgements
The authors would like to acknowledge Drs. Gary Koretzky, Taku Kambayashi, Kim Nichols, Sheila Rao and Lehn Weaver for critical review of the project and manuscript.
SC was supported by NIH F32-AI-98337 and a Rheumatology Research Foundation Rheumatology Scientist Development Award. EB was supported by NIH K08-AI-79396, an HHMI Early Career Physician Scientist Award, and an Arthritis Foundation Innovative Research Grant.
Abbreviations
- MAS
Macrophage Activation Syndrome
- HLH
Hemophagocytic Lymphohistiocytosis
- IL10RB
IL-10 Receptor blocking antibody
- HPC
Hemophagocyte
- pDC
Plasmacytoid Dendritic Cell
Footnotes
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version. Drs. Canna & Behrens had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Canna, Behrens.
Acquisition of data: Canna, Wrobel, Chu.
Analysis and interpretation of data: Canna, Paessler, Kreiger, Behrens
References
- 1.Grom AA, Mellins ED. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr Opin Rheumatol. 2010;22(5):561–566. doi: 10.1097/01.bor.0000381996.69261.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canna SW, Behrens EM. Not all hemophagocytes are created equally: appreciating the heterogeneity of the hemophagocytic syndromes. Curr Opin Rheumatol. 2012;24(1):113–118. doi: 10.1097/BOR.0b013e32834dd37e. PMCID: 3285509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu M, Yokoyama T, Yamada K, Kaneda H, Wada H, Wada T, et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology. 2010;49(9):1645–1653. doi: 10.1093/rheumatology/keq133. [DOI] [PubMed] [Google Scholar]
- 4.Maruyama J, Inokuma S. Cytokine profiles of macrophage activation syndrome associated with rheumatic diseases. The Journal of rheumatology. 2010;37(5):967–973. doi: 10.3899/jrheum.090662. [DOI] [PubMed] [Google Scholar]
- 5.Grom AA, Villanueva J, Lee S, Goldmuntz EA, Passo MH, Filipovich A. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr. 2003;142(3):292–296. doi: 10.1067/mpd.2003.110. [DOI] [PubMed] [Google Scholar]
- 6.Vastert SJ, van Wijk R, D'Urbano LE, de Vooght KM, de Jager W, Ravelli A, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 2010;49(3):441–449. doi: 10.1093/rheumatology/kep418. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, Biroschak J, Glass DN, Thompson SD, Finkel T, Passo MH, et al. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis is associated with MUNC13-4 polymorphisms. Arthritis Rheum. 2008;58(9):2892–2896. doi: 10.1002/art.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janka G, Imashuku S, Elinder G, Schneider M, Henter JI. Infection- and malignancy-associated hemophagocytic syndromes. Secondary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am. 1998;12(2):435–444. doi: 10.1016/s0889-8588(05)70521-9. [DOI] [PubMed] [Google Scholar]
- 9.Takada H, Takahata Y, Nomura A, Ohga S, Mizuno Y, Hara T. Increased serum levels of interferon-gamma-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin Exp Immunol. 2003;133(3):448–453. doi: 10.1046/j.1365-2249.2003.02237.x. PMCID: 1808805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumegi J, Barnes MG, Nestheide SV, Molleran-Lee S, Villanueva J, Zhang K, et al. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood. 2011 doi: 10.1182/blood-2010-08-300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikora KA, Fall N, Thornton S, Grom AA. The limited role of interferon-gamma in systemic juvenile idiopathic arthritis cannot be explained by cellular hyporesponsiveness. Arthritis and rheumatism. 2012;64(11):3799–3808. doi: 10.1002/art.34604. PMCID: 3482423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 13.Pachlopnik Schmid J, Ho CH, Chretien F, Lefebvre JM, Pivert G, Kosco-Vilbois M, et al. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med. 2009;1(2):112–124. doi: 10.1002/emmm.200900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs P, Crozat K, Popkin D, Oldstone MB, Beutler B. Disruption of MyD88 signaling suppresses hemophagocytic lymphohistiocytosis in mice. Blood. 2011;117(24):6582–6588. doi: 10.1182/blood-2011-01-329607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoller EE, Lykens JE, Terrell CE, Aliberti J, Filipovich AH, Henson PM, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med. 2011;208(6):1203–1214. doi: 10.1084/jem.20102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121(6):2264–2277. doi: 10.1172/JCI43157. PMCID: 3104738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strippoli R, Carvello F, Scianaro R, De Pasquale L, Vivarelli M, Petrini S, et al. Amplification of the response to Toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis and rheumatism. 2012;64(5):1680–1688. doi: 10.1002/art.33496. [DOI] [PubMed] [Google Scholar]
- 18.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198(7):1069–1076. doi: 10.1084/jem.20030630. PMCID: 2194220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leitner WW, Bergmann-Leitner ES, Hwang LN, Restifo NP. Type I Interferons are essential for the efficacy of replicase-based DNA vaccines. Vaccine. 2006;24(24):5110–5118. doi: 10.1016/j.vaccine.2006.04.059. PMCID: 1484849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood. 2011 doi: 10.1182/blood-2010-12-324533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shearstone JR, Pop R, Bock C, Boyle P, Meissner A, Socolovsky M. Global DNA demethylation during mouse erythropoiesis in vivo. Science. 2011;334(6057):799–802. doi: 10.1126/science.1207306. PMCID: 3230325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy KP, Travers P, Walport M, Janeway C. Janeway's immunobiology. 7th ed. New York: Garland Science; 2008. Alumni and Friends Memorial Book Fund. [Google Scholar]
- 23.Zhou S, Cerny AM, Zacharia A, Fitzgerald KA, Kurt-Jones EA, Finberg RW. Induction and inhibition of type I interferon responses by distinct components of lymphocytic choriomeningitis virus. J Virol. 2010;84(18):9452–9462. doi: 10.1128/JVI.00155-10. PMCID: 2937596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crozat K, Hoebe K, Ugolini S, Hong NA, Janssen E, Rutschmann S, et al. Jinx, an MCMV susceptibility phenotype caused by disruption of Unc13d: a mouse model of type 3 familial hemophagocytic lymphohistiocytosis. J Exp Med. 2007;204(4):853–863. doi: 10.1084/jem.20062447. PMCID: 2118559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Misawa N, Nie C, Satou Y, Iwakiri D, Matsuoka M, et al. A novel animal model of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in humanized mice. Blood. 2011;117(21):5663–5673. doi: 10.1182/blood-2010-09-305979. [DOI] [PubMed] [Google Scholar]
- 26.Brown DE, McCoy MW, Pilonieta MC, Nix RN, Detweiler CS. Chronic murine typhoid fever is a natural model of secondary hemophagocytic lymphohistiocytosis. PLoS One. 2010;5(2):e9441. doi: 10.1371/journal.pone.0009441. PMCID: 2829187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 28.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunological reviews. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. PMCID: 2724982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pachlopnik Schmid J, Cote M, Menager MM, Burgess A, Nehme N, Menasche G, et al. Inherited defects in lymphocyte cytotoxic activity. Immunological reviews. 2010;235(1):10–23. doi: 10.1111/j.0105-2896.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu M, Yachie A. Compensated inflammation in systemic juvenile idiopathic arthritis: Role of alternatively activated macrophages. Cytokine. 2012;60(1):226–232. doi: 10.1016/j.cyto.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 31.von Wussow P, Jakschies D, Hochkeppel H, Horisberger M, Hartung K, Deicher H. MX homologous protein in mononuclear cells from patients with systemic lupus erythematosus. Arthritis and rheumatism. 1989;32(7):914–918. [PubMed] [Google Scholar]
- 32.Milner JD, Orekov T, Ward JM, Cheng L, Torres-Velez F, Junttila I, et al. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 2010;116(14):2476–2483. doi: 10.1182/blood-2009-11-255174. PMCID: 2953884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ost A, Nilsson-Ardnor S, Henter JI. Autopsy findings in 27 children with haemophagocytic lymphohistiocytosis. Histopathology. 1998;32(4):310–316. doi: 10.1046/j.1365-2559.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- 34.Macheta M, Will AM, Houghton JB, Wynn RF. Prominent dyserythropoiesis in four cases of haemophagocytic lymphohistiocytosis. J Clin Pathol. 2001;54(12):961–963. doi: 10.1136/jcp.54.12.961. PMCID: 1731338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Means RT, Jr, Krantz SB, Luna J, Marsters SA, Ashkenazi A. Inhibition of murine erythroid colony formation in vitro by interferon gamma and correction by interferon receptor immunoadhesin. Blood. 1994;83(4):911–915. [PubMed] [Google Scholar]
- 36.Zoumbos NC, Djeu JY, Young NS. Interferon is the suppressor of hematopoiesis generated by stimulated lymphocytes in vitro. J Immunol. 1984;133(2):769–774. [PubMed] [Google Scholar]
- 37.Ziegler-Heitbrock L, Lotzerich M, Schaefer A, Werner T, Frankenberger M, Benkhart E. IFN-alpha induces the human IL-10 gene by recruiting both IFN regulatory factor 1 and Stat3. J Immunol. 2003;171(1):285–290. doi: 10.4049/jimmunol.171.1.285. [DOI] [PubMed] [Google Scholar]
- 38.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34(5):1133–1138. [PubMed] [Google Scholar]
- 39.Schaer DJ, Schaer CA, Schoedon G, Imhof A, Kurrer MO. Hemophagocytic macrophages constitute a major compartment of heme oxygenase expression in sepsis. Eur J Haematol. 2006;77(5):432–436. doi: 10.1111/j.1600-0609.2006.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/ systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10(3):387–392. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]