Abstract
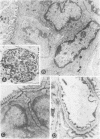
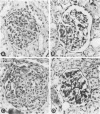
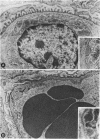
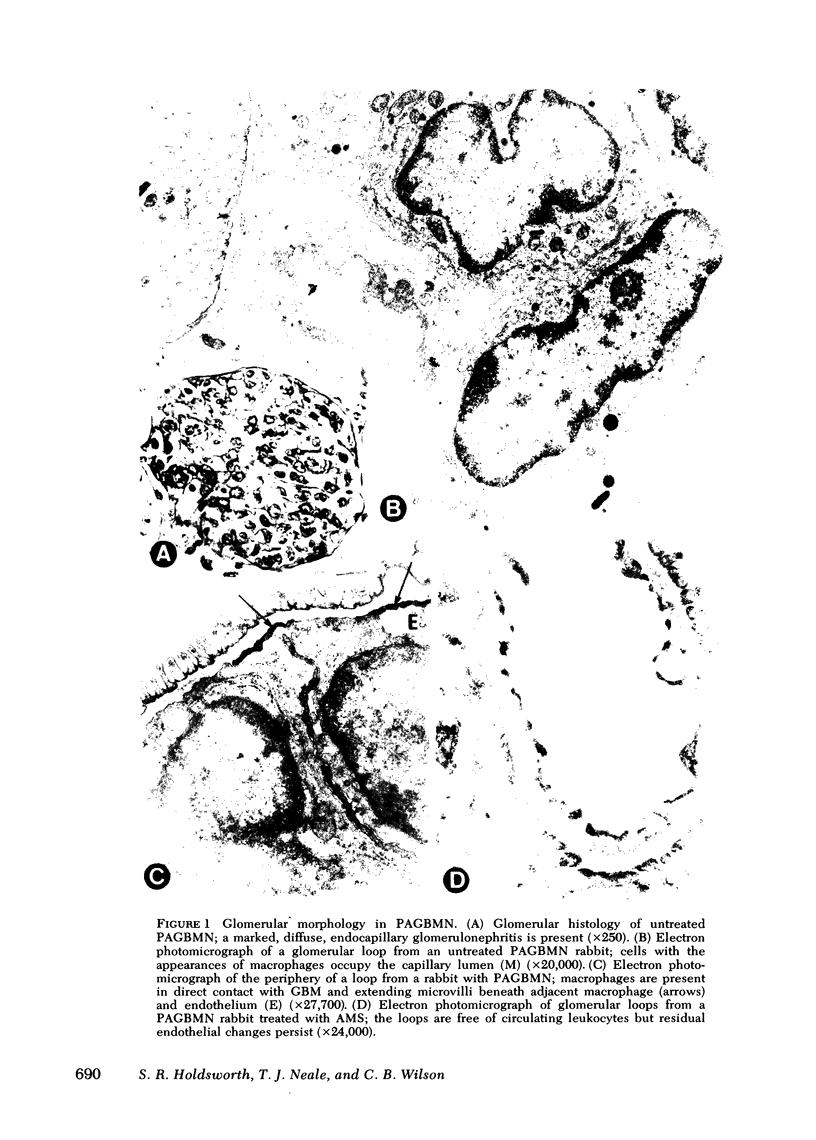
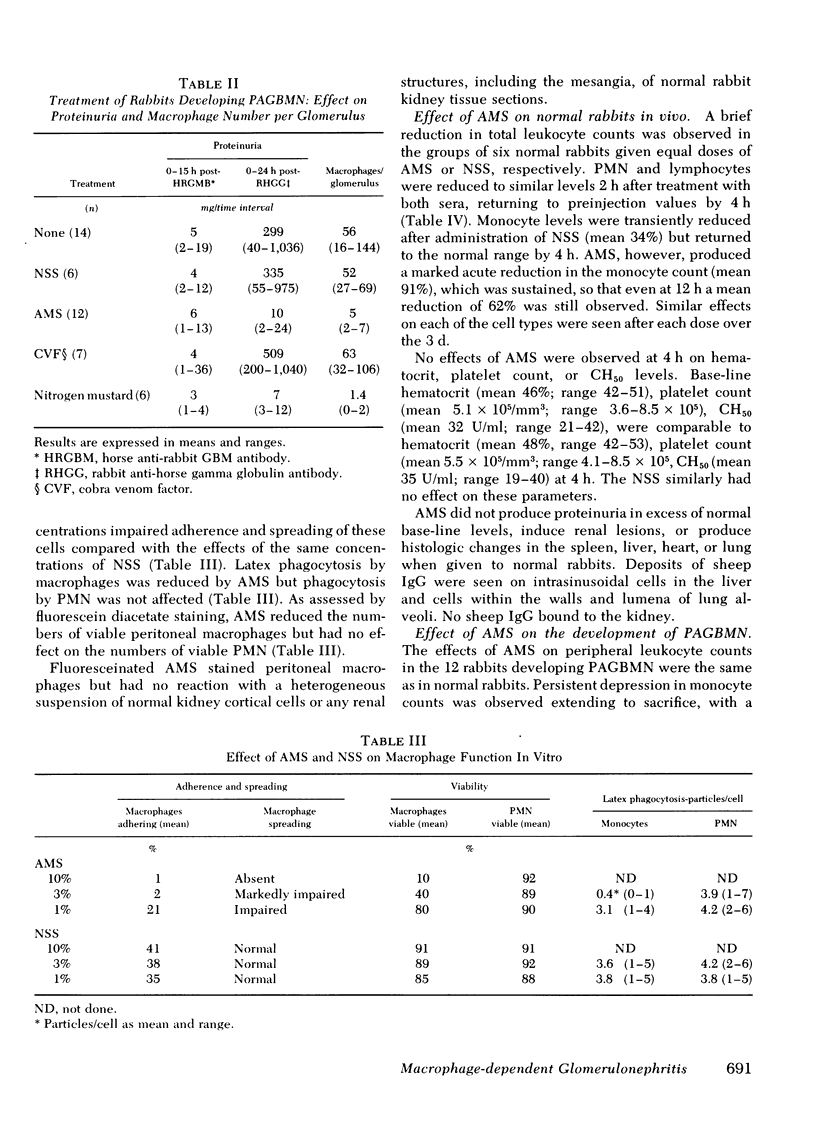
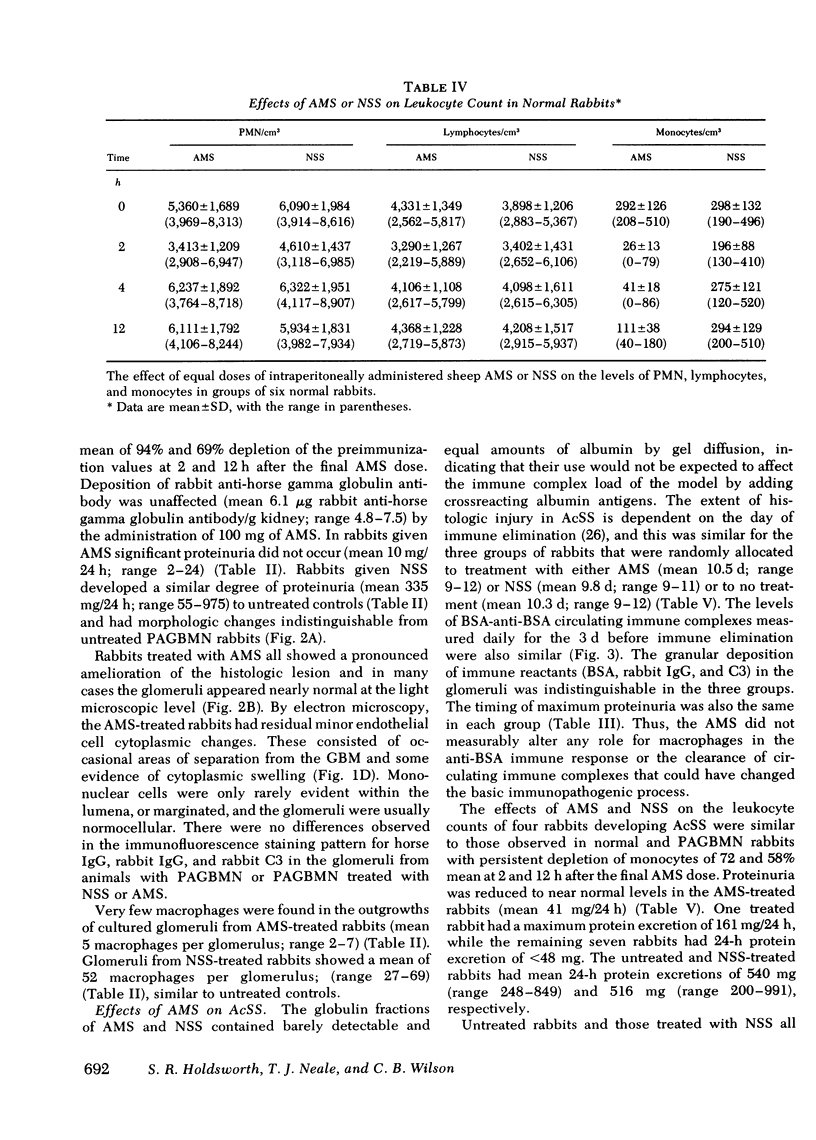
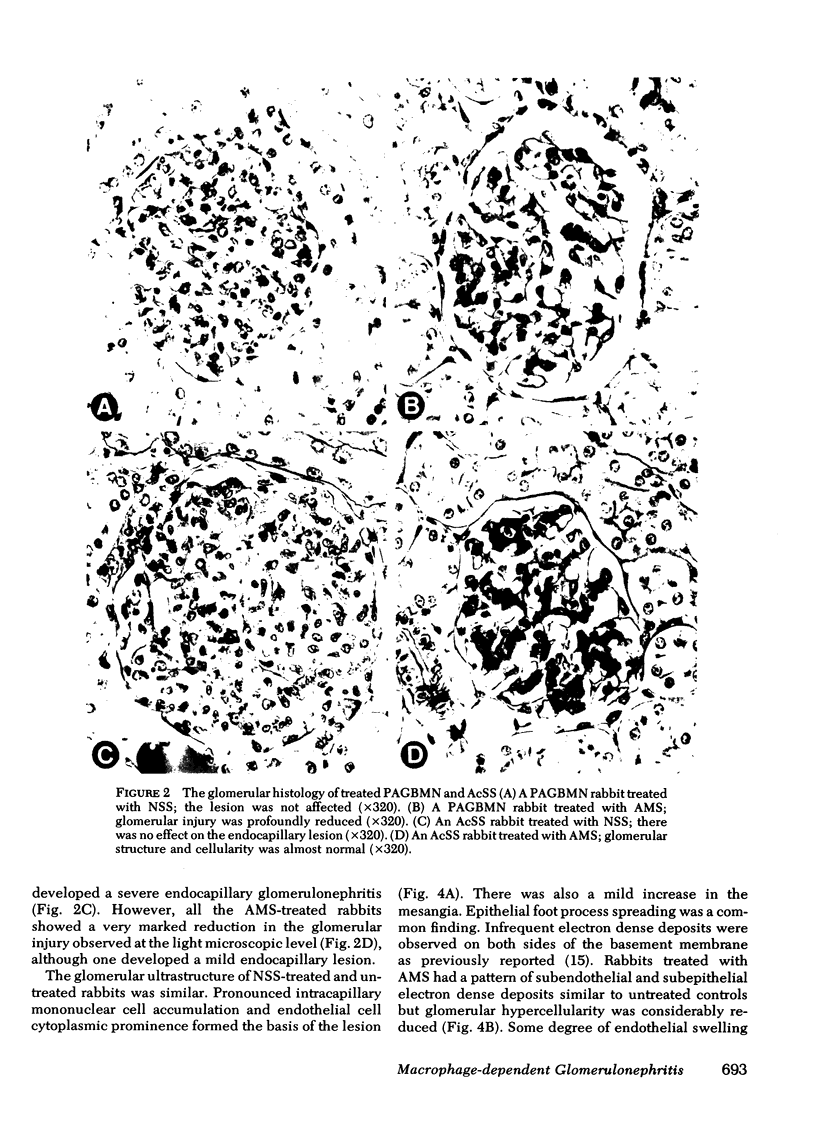
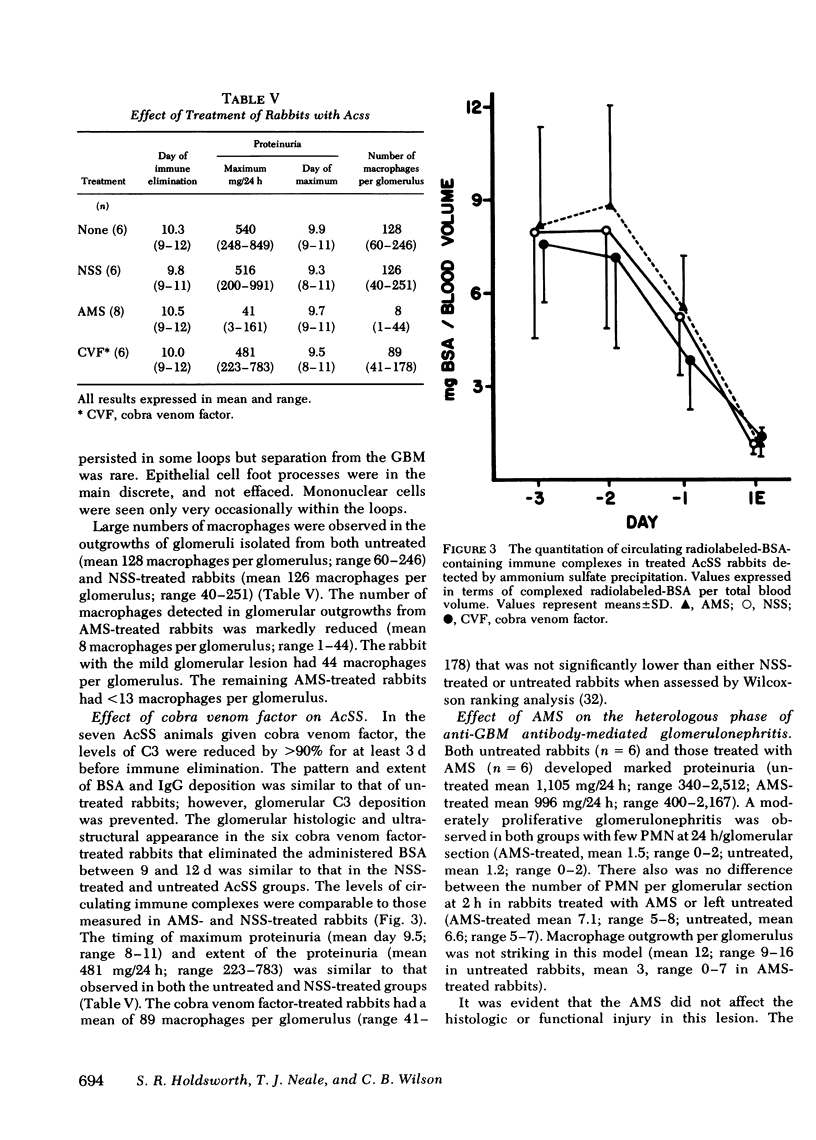
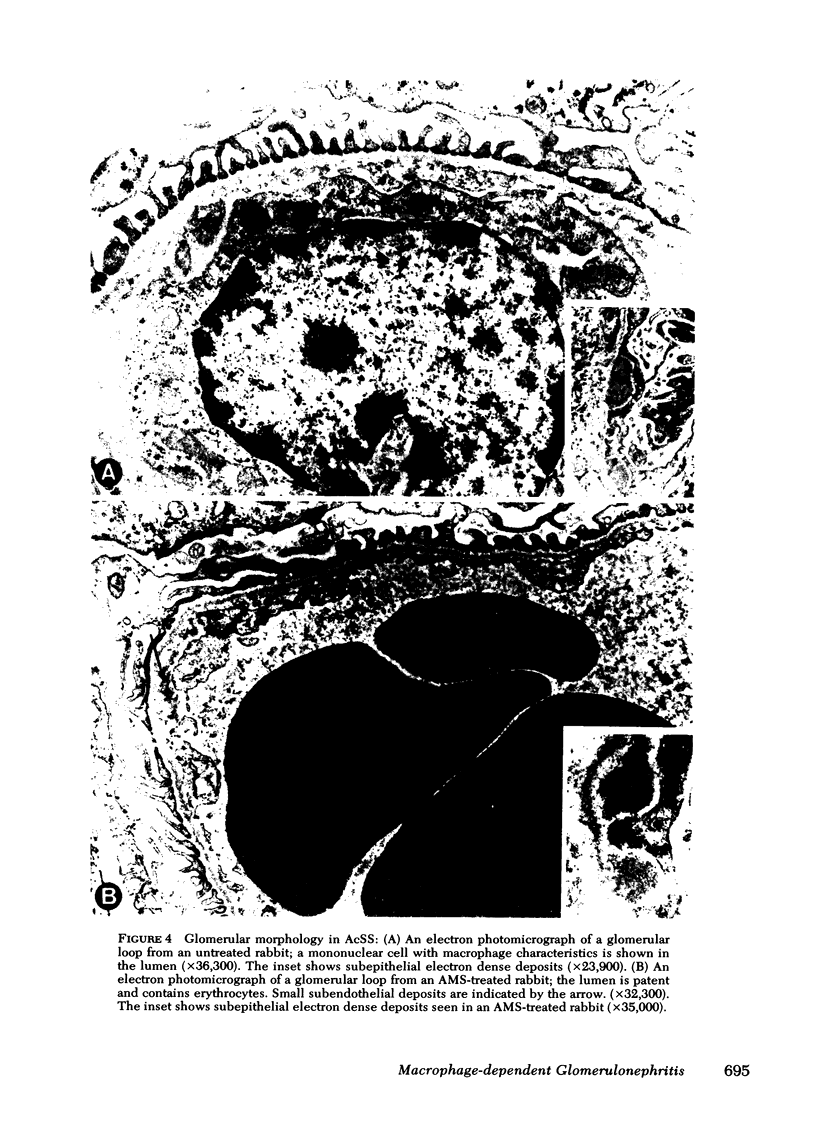
Macrophages were shown by the use of glomerular cell culture and morphologic techniques to be present in large numbers within the glomeruli of rabbits with acute serum sickness (AcSS) and in a passive model of the autologous phase of antiglomerular basement membrane (GBM) antibody-induced glomerulonephritis (PAGBMN). To determine the part played by these cells in the glomerular injury, animals were treated with a sheep anti-rabbit macrophage serum (AMS) or normal sheep serum (NSS). NSS administration had no effect on the development of either model of glomerulonephritis. The use of AMS reduced the number of circulating monocytes and prevented the accumulation of macrophages within glomeruli in both models (AcSS/NSS, mean 126/glomerulus, range 40-251; AcSS/AMS, mean 8, range 1-44; PAGBMN/NSS, mean 52, range 27-69; PAGBMN/AMS, mean 5, range 2-7). The AMS-treated rabbits had only minor histologic lesion and profound reduction in proteinuria (AcSS/NSS, mean 516 mg/24 h, range 200-991; AcSS/AMS, mean 41, range 3-161; PAGBMN/NSS, mean 335, range 55-975; PAGBMN/AMS, mean 10, range 2-24). Similar studies in the heterologous phase of glomerular injury induced by the same anti-GBM antibody revealed no effect of the AMS on this polymorphonuclear leukocyte-related phase of injury, demonstrating the selectivity of the antisera. Complement depletion, with cobra venom factor, did not affect the development of glomerulonephritis nor the accumulation of macrophages in either model. Inhibition of macrophage accumulation can largely prevent these forms of experimental glomerulonephritis, thereby implicating macrophages as mediators of glomerular injury and consequent proteinuria.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins R. C., Holdsworth S. R., Glasgow E. F., Matthews F. E. The macrophagen in human rapidly progressive glomerulonephritis. Lancet. 1976 Apr 17;1(7964):830–832. doi: 10.1016/s0140-6736(76)90480-3. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Collins A. B., Schneeberger E. E., McCluskey R. T. A cell-mediated reaction against glomerular-bound immune complexes. J Exp Med. 1979 Dec 1;150(6):1410–1420. doi: 10.1084/jem.150.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Collins A. B., McCluskey R. T. Evidence for a pathogenic role of a cell-mediated immune mechanism in experimental glomerulonephritis. J Exp Med. 1978 Jul 1;148(1):246–260. doi: 10.1084/jem.148.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCHRANE C. G., UNANUE E. R., DIXON F. J. A ROLE OF POLYMORPHONUCLEAR LEUKOCYTES AND COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1965 Jul 1;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Gallily R. In vitro and in vivo studies of the properties and effects of antimacrophage sers (AMS). Clin Exp Immunol. 1971 Sep;9(3):381–391. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Acute immune complex disease in rabbits. The role of complement and of a leukocyte-dependent release of vasoactive amines from platelets. J Exp Med. 1971 Mar 1;133(3):554–571. doi: 10.1084/jem.133.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Gary G. W., Jr, Murphy F. A. In vitro and in vivo properties of antimacrophage sera. J Immunol. 1969 Mar;102(3):656–661. [PubMed] [Google Scholar]
- Holdsworth S. R., Glasgow E. F., Thomson N. M., Atkins R. C. Tissue culture of isolated human glomeruli. Pathology. 1978 Jan;10(1):59–67. doi: 10.3109/00313027809063480. [DOI] [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. The participation of macrophages and monocytes in experimental immune complex glomerulonephritis. Clin Immunol Immunopathol. 1980 Mar;15(3):510–524. doi: 10.1016/0090-1229(80)90063-x. [DOI] [PubMed] [Google Scholar]
- Holdsworth S. R., Thomson N. M., Glasgow E. F., Dowling J. P., Atkins R. C. Tissue culture of isolated glomeruli in experimental crescentic glomerulonephritis. J Exp Med. 1978 Jan 1;147(1):98–109. doi: 10.1084/jem.147.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsicker L. G., Shearer T. P., Plattner S. B., Weisenburger D. The role of monocytes in serum sickness nephritis. J Exp Med. 1979 Sep 19;150(3):413–425. doi: 10.1084/jem.150.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIKER W. T., COCHRANE C. G. PATHOGENIC FACTORS IN VASULAR LESIONS OF EXPERIMENTAL SERUM SICKNESS. J Exp Med. 1965 Jul 1;122:83–98. doi: 10.1084/jem.122.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Kniker W. T., Cochrane C. G. The localization of circulating immune complexes in experimental serum sickness. The role of vasoactive amines and hydrodynamic forces. J Exp Med. 1968 Jan 1;127(1):119–136. doi: 10.1084/jem.127.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg J. I., Wayne D. B., Karnovsky M. J. Rapid and focal loss of negative charge associated with mononuclear cell infiltration early in nephrotoxic serum nephritis. Kidney Int. 1979 Sep;16(3):290–300. doi: 10.1038/ki.1979.131. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Monga G., Mazzucco G., di Belgiojoso G. B., Busnach G. The presence and possible role of monocyte infiltration in human chronic proliferative glomerulonephritides. Light microscopic, immunofluorescence, and histochemical correlations. Am J Pathol. 1979 Feb;94(2):271–284. [PMC free article] [PubMed] [Google Scholar]
- Naish P. F., Thomson N. M., Simpson I. J., Peters D. K. The role of polymorphonuclear leucocytes in the autologous phase of nephrotoxic nephritis. Clin Exp Immunol. 1975 Oct;22(1):102–111. [PMC free article] [PubMed] [Google Scholar]
- Naish P., Penn G. B., Evans D. J., Peters D. K. The effect of defibrination on nephrotoxic serum nephritis in rabbits. Clin Sci. 1972 May;42(5):643–646. doi: 10.1042/cs0420643. [DOI] [PubMed] [Google Scholar]
- Okumura K., Kondo Y., Tada T. Studies on passive serum sickness. I. The glomerular fine structure of serum sickness nephritis induced by preformed antigen-antibody complexes in the mouse. Lab Invest. 1971 May;24(5):383–391. [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran R. S., Sholley M. M. Endothelial proliferation in the delayed hypersensitivity reaction: an autoradiographic study. J Immunol. 1977 Feb;118(2):529–532. [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M. G., Beller D. I., Unanue E. R. Demonstration of a soluble mediator that induces exudates rich in Ia-positive macrophages. J Exp Med. 1980 Dec 1;152(6):1684–1698. doi: 10.1084/jem.152.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M. S., Michael A. F. Renal cell turnover studied by Y chromosome (Y body) staining of the transplanted human kidney. J Lab Clin Med. 1978 Dec;92(6):841–848. [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindrey M., Marshall T. L., Naish P. Quantitative assessment of the effects of platelet depletion in the autologous phase of nephrotoxic serum nephritis. Clin Exp Immunol. 1979 Apr;36(1):90–96. [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Mannik M., Tung M. Y. Role of marrow-derived monocytes and mesangial cells in removal of immune complexes from renal glomeruli. J Exp Med. 1979 Jan 1;149(1):127–136. doi: 10.1084/jem.149.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson N. M., Holdsworth S. R., Glasgow E. F., Atkins R. C. The macrophage in the development of experimental crescentic glomerulonephritis. Studies using tissue culture and electron microscopy. Am J Pathol. 1979 Feb;94(2):223–240. [PMC free article] [PubMed] [Google Scholar]
- Thomson N. M., Naish P. F., Simpson I. J., Peters D. K. The role of C3 in the autologous phase of nephrotoxic nephritis. Clin Exp Immunol. 1976 Jun;24(3):464–473. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Calderon J., Kiely J. M., Stadecker M. J. Regulation of immunity and inflammation by mediators from macrophages. Am J Pathol. 1976 Nov;85(2):465–478. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Cooperation between mononuclear phagocytes and lymphocytes in immunity. N Engl J Med. 1980 Oct 23;303(17):977–985. doi: 10.1056/NEJM198010233031706. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Dixon F. J. Antigen quantitation in experimental immune complex glomerulonephritis. I. Acute serum sickness. J Immunol. 1970 Aug;105(2):279–290. [PubMed] [Google Scholar]
- Wilson C. B., Dixon F. J., Fortner J. G., Cerilli G. J. Glomerular basement membrane--reactive antibody in anti-lymphocyte globulin. J Clin Invest. 1971 Jul;50(7):1525–1535. doi: 10.1172/JCI106638. [DOI] [PMC free article] [PubMed] [Google Scholar]