Abstract
Amniotic fluid contains heterogeneous cell types and has become an interesting source for obtaining fetal stem cells. These stem cells have a high proliferative capacity and a good differentiation potential and may thus be suitable for regenerative medicine. As there is increasing evidence, that these stem cells are also able to be directed into the neural lineage, in our study we investigated the neuronal and glial differentiation potential of these cells, so that they may also be applied to cure degenerative diseases of the retina. Mesenchymal stem cells were isolated from routine prenatal amniocentesis at 15 to 18 weeks of pregnancy of human amniotic fluid and expanded in the cell culture. Cells were cultivated according to standard procedures for mesenchymal stem cells and were differentiated along the neural lineage using various protocols. Furthermore, it was also tried to direct them into cell types of the retina as well as into endothelial cells. Cells of more than 72 amniotic fluid samples were collected and characterized. While after induction neural-like phenotypes could actually be detected, which was confirmed using neural marker proteins such as GFAP and ßIII tubulina further differentiation into retinal like cells could not reliably be shown. These data suggest that amniotic fluid derived cells are an interesting cell source, which may also give rise to neural-like cells. However, a more specific differentiation into neuronal and glial cells could not unequivocally be shown, so that further investigations have to becarried out.
Keywords: Amniotic fluid, stem cells, pluripotency, retina, migratory potential
Introduction
Fetal stem cells derived from the amniotic fluid have in recent years attracted an increasing attention as they have recommended themselves as an interesting cell source for cellular therapies and tissue engineering approaches. They show a remarkable ability to self-renewal and differentiate into cell types of all three germ lines, sharing some overlapping properties with pluripotent ES-cells [1] The main characteristics of amniotic fluid stem cells are their fetal origin, the high number of isolated cells, their wide differentiation properties and their rapid expansion in vitro revealing a higher therapeutic potential than adult stem cells [2]. These fetal stem cells can be isolated from the amniotic fluid during routine prenatal diagnostic procedures in order to carry out cytogenetic investigations. The combination of being extraembryonic and providing relatively easy access for cell harvesting makes these cells very attractive candidates to further study their differentiation potential as well as their suitability for their application in therapeutic transplantations [1,3,4]. In the meantime it has become obvious that the amniotic fluid contains rather heterogenous cell populations, which have been classified according to mainly morphological criteria. There are epithelial-like cells, amniotic fluid specific cells and fibroblast like cells [5]. However, after the identification of Oct-4 positive stem cells in amniotic fluid, increasing effort was made to trace the possible differentiation lineages of these cells. Thus, the differentiation of amniotic fluid derived mesenchymal stem cells (AF-MSCs) into astrocytes, oligodendrocytes and neurons has been reported in vitro and in vivo [6,7]. In order to have a closer look at this neural differentiation potential of AF-MSCs and to elaborate a broader therapeutic applicability, in this work we thoroughly investigated the expression of neural and glial markers in amniotic fluid derived cells. Beforehand, the common differentiation potential of mesenchymal stem cells into adipocytes-, chondrocytes- and osteoblast-like cells was determined. For a possible application of AF-MSCs in degenerative disease of the retina such as retinitis pigmentosa or retinopathy of prematurity we also had a look for the expression of retinal markers like rhodopsin as well as for endothelial markers like PECAM and ofvon Willebrand factor after an appropriate stimulation.
Our data suggest, while the expression of cell markers of the commonly investigated cell lineages could be detected, a further differentiation potential into retinal-specific cells, i.e. photoreceptor cells could not be carved out.
Material and methods
Cell isolation and culture
Amniotic fluid was harvested from women undergoing amniocentesis, which is a clinical routine procedure performed during week 15-18 of gestation for prenatal diagnostics. All patients gave written informed consent and were informed in detail about our procedures for diagnostic fulfillment according to the protocol of this study. The investigation was approved by the local hospital ethics committee of the university hospital Giessen and Marburg and are in compliance with the Helsinki Declaration of 1975.
The volume of each fluid sample was 0.5 to 5 ml. Cells of 72 amniotic fluid samples were used for the study. Cell samples were only used when no major abnormalitiessuch as trisomy 21 were revealed by the cytogenetic analysis.
AF-MSCs were routinely cultured in Minimum Essential Medium (α-MEM; Invitrogen, Karlsruhe, Germany) supplemented with 20% fetal bovine serum (FBS, PAA, Cölbe, Germany) in a 100 mm culture dish and incubated at 37°C with 5% humidified CO2. Alternatively cell culture was carried out in Dulbeccos Modified Eagles medium (DMEM, Invitrogen) supplemented with 15% FBS + 20% Chang Medium (Irvine Scientific, CA) + L-Glutamin, Amniomax (Invitrogen) The first medium change was performed after 5 days in order to avoid any mechanical stress. After 10 to 14 days, when the cultures had reached 80% confluence, cells were harvested using Accutase (PAA, Cölbe, Germany), and were replated at 2000 cells/cm². Adherent cells consisted of cells of epithelial and mesenchymal morphology. In order to exclusively isolate cells with mesenchymal morphology for further experiments cloning cylinders were used. Obtained cells were kept in culture for up to 6 passages. For the experiments cells were exclusively taken from passage 3. Experiments were carried out in triplicates.
Characterisation of stem cell properties and differentiation potential of AF-MSCs
To determine the pureness of the acquired cell population according to their surface marker expression and their identification as stem cells 2 x 105 cells of random patient material were incubated with the primary mouse anti-human CD 29, CD 44, CD 105 (monoclonal antibodies, DSHB, University of Iowa, USA, 1:500) and the CD 90 antibody (BD Biosciences Pharmingen, Belgium, 1:800) and labelled with the secondary antibody anti-mouse IgG1-FITC (Southern Biotech, USA, 1:300) carrying the fluorochrome fluorescein isothiocyanate (FITC). For CD 105 an IgG 2a secondary antibody (Cy3-conjugated affinity-purified goat anti-mouse IgG (Rockland, Gilbertsville, PA, USA) 1:1000) was used. The fluorescence emitted by FITC was induced by a laser (Argon, 488 nm) and measured by the flow cytometer (FACSCalibur™, Becton Dickinson, USA). As negative control 2 x 105 cells diluted in a solution of PBS containing 1% BSA, 0, 1% sodium acid (Sigma-Aldrich, Deisenhofen, Germany) and 0,5% goat serum (Sigma-Aldrich) were used. The antibody mouse IgG1 (AbDSerotec, UK, 1:800) combined with the secondary IgG 2a antibody was used for isotype control. The measured results were analysed with the software FACS Express, Version 2 (De Novo, Canada).
In vitro differentiation was carried out as described previously for mesenchymal stem cells [8]. Cells were analysed for their capacity to differentiate into the osteogenic and chondrogenic lineage.
Osteogenic differentiation was performed in a monolayer. Cells were plated at a density of 3 × 103 cells/cm², and cultured at 37°C in a humidified atmosphere (5% CO2) for various periods. The medium was changed three times a week. Osteogenic differentiation was induced by culturing AF-cells for 3 weeks in osteogenic medium (OM). OM consisted of DMEM (Invitrogen) containing 10% FCS, 0.05 mM ascorbic acid-2-phosphate (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), and 0.1 μM dexamethasone (Sigma-Aldrich). Osteogenic differentiation of ASCs was assessed by evaluating morphological changes and calcified extracellular matrix (ECM) deposition.
Induction of chondrogenic differentiation was carried out in a three-dimensional (3D) pellet culture. For 3D pellet cultures AF-cells were resuspended at a density of 5 × 105 cells/ml. The chondrogenic medium was supplemented with insulin-transferrin-selenium (ITS) 1:100 v/v (1.0 mg/ml insulin from bovine pancreas, 0.55 mg/ml human transferrin, and 0.5 μg/ml sodium selenite) (Sigma-Aldrich), 0.1 μM dexamethasone, 0.05 mM ascorbic acid, 50 μM l-proline (Sigma-Aldrich), 1 mM sodium pyruvate (Sigma-Aldrich). Cell suspension (500 μl) was aliquoted into 15 ml polypropylene centrifuge tubes (Eppendorf, Hamburg, Germany), and centrifugated at 500 g for 5 min. as described previously [8]. The chondrogenic medium was supplemented with 5 ng/ml of TGF-β1 (Sigma-Aldrich). Fresh medium was added every third day. The medium without TGF-ß1 was used as the control medium for control 3D cultures. The tubes were placed in anincubator at 37°C in a humidified atmosphere with 5% CO2 for up to 3 weeks. The caps of the tubes were loosened to allow air exchange.
Investigation of the migratory potential of AF-MSCs
For the assessment of the migration potential a wound and healing assay was conducted [9]. In order to study the migration capacity of the cells we used the ibidi culture insert (ibidi, Planegg, Germany). Cryopreserved cells of passage 3 were seeded at a density of 35 x 103 cells in each well of the insert, which had been placed in a 3.5 cm² cell culture dish (Greiner Bio-one, Frickenhausen, Germany). After 24 hours the cells were appropriately attached and the culture insert was carefully removed from the culture dish with sterile tweezers. The consequent cell-free gap is about 500 μm wide.
The assay was carried out using the Axio ObserverZ1 microscope based life-cell imaging system by Zeiss (Oberkochen, Germany) at 37°C and 5% CO2. Pictures were taken every 10 minutes over at least 36 hours.
The microphotographs were analyzed using the adobe Photoshop cs5 software (Adobe, San Jose, CA), looking at the cell uncovered area in % over the same time period.
Neural induction of AF-MSCs
For the enhancement of neural differentiation characteristics AF-MSCs were transferred to a specific neural induction medium NPMM (Lonza, Basel, Switzerland) [10]. This neural basal medium was supplemented with the basic Fibroblast growth factor (bFGF, 20 ng/ml, Lonza) as well as the Epithelial growth factor (EGF, 20 ng/ml, Lonza). Cells were cultivated for 3 weeks in these induction media prior processing for immunocytochemical investigation.
Stimulation of differentiation into retina-typical neurons and endothelial cells
Following differentiation of AF-cells into neural cells a further differentiation into retina specific cells and endothelial cells was anticipated. For the differentiation induction into retina-specific neurons AF-MSCs were confronted with a medium containing the factors activin A (100 ng/ml) or alternatively taurine (50 μM) [11]. Besides photo-receptor cell differentiation also differentiation into endothelial cells was investigated. For endothelial cell differentiation AF-MSCs were cultivated in a medium containing 2% FBS and vascular endothelial growth factor (VEFG, 50 ng/ml, Lonza).
RT-PCR and RT-qPCR analysis
Total RNA was extracted from a minimum of 5 x 105 cells using Tri Reagent (Sigma-Aldrich) according to manufacturer’s protocol. Specimens were adjusted to 200 ng/μl RNA, treated with a recombinant DNAse I (Roche) and subsequently reverse transcribed using GeneAmp® Gold RNA PCR Core Kit (Applied Biosystems) according to manufacturer’s protocol. Minus RT samples for each specimen were included. PCR was conducted using 10 μl cDNA, 2 μl MgCl2, 4 μl 10x PCR Gold Buffer, 32.75 μl nuclease-free water, 0.25 μl AmpliTaq Gold® (Applied Biosystems) and 1 μl of a 10 pmol forward and reverse primer mix. All primers were purchased from Eurofins MWG Operon. Cycling conditions were as follows: 95°C for 10 min, following 39 cycles of 95°C for 1 min , 60°C for 1 min, 72°C for 1.30 min and finally at 72°C for 10 min. PCR products were separated using a 2% agarose gel electrophoresis and visualized by SYBR Green (Sigma-Aldrich).
RT-qPCR for β-III-Tubulin, BDNF (Brain derived nerve factor) und NGF (nerve growth factor) was carried out on a CF X 96 Realtime Cycler (Bio-Rad, München, Germany) using IQ SybrGreenSupermix (Bio-Rad) with the following protocol: 3 min 95°C, following 40 cycles of 15 sec 95°C and 1 min 60°C, with a subsequent melting curve. Data was analyzed using the CFX Manager software 1.6 (Bio-Rad) applying the ΔΔCT-method for relative gene expression relative to GAPDH as housekeeping gene.
Immunocytochemical staining of cells
For the investigation of neural marker expression by amniotic fluid derived cells, cells were fixed after rinsing in 0.1 M PBS in 4% PFA. Immunocytochemistry was performed at room temperature. Cells and tissue sections were treated for 30 min with a blocking solution composed of 2% goat serum and 5% FBS. In experiments requiring the labelling of intracellular epitopes, the cells and tissue sections were further treated with 0.025% Triton X-100 (Sigma-Aldrich) for 30 min and rinsed in PBS.
The primary antibodies used were β-III-Tubulin (Covance, München, Germany 1:8000), Nestin (BD Biosciences, Heidelberg, Germany 1:500), α-internexin (Abcam, Cambridge, UK, rabbit polyclonal AB, 1:1000) GABA (Sigma-Aldrich, Steinheim, Germany, rabbit polyclonal AB, 1:1000) Glutamate (Sigma-Aldrich, rabbit polyclonal AB, 1:1500) and GFAP (polyclonal antibody, Sigma-Aldrich, 1:1000), PECAM (CD31 Dianova, Hamburg, Germany, 1:100), and von-Wille-Brand-Faktor (Dianova, 1:100).
Incubations were performed over night at 4°C. Secondary antibodies were used at the following dilutions:
Cy3-conjugated affinity-purified goat anti-mouse or anti-rabbit IgG (Rockland, Gilbertsville, PA, USA) 1:1000 or by a Biotin-conjugated goat anti-guinea pig (Sigma-Aldrich, Deisenhofen, Germany) at a dilution of 1:400 followed by FluoroLinkTMCyTM3 labelled streptavidin (Amersham Pharmacia Biotech, Freiburg, Germany) at a dilution of 1:1000.
After antibody incubations nuclear DNA was stained with Hoechst Dye 33342 (Sigma-Aldrich). Preparations were coverslipped with Entellan (Merck, Darmstadt, Germany). Analysis of mounted specimens was carried out using an Axiophot Fluorescence microscope (Zeiss, Oberkochen, Germany).
Negative controls were performed by omitting the primary antibodies. Specificity of neural markers was tested using appropriate cell populations such as neural stem cells and neural stem cell derived neurons and glial cells.
Statistical analysis of data
A two sided t-test was performed for comparison of groups. Subsequently, the groups of treated and untreated cells were compared pairwise. P values of < 0.05 were taken as significant. For all tests the statistical software program, SPSS 19.0, was used (IBM, Germany).
Results
As reported before after the first passage plastic adherent AF-cells show marked morphological differences [11]. Mainly spindle shaped cells with elongated processes as well as cells with an epithelial-like morphology can be identified. All together heterogeneity of the cell population is apparent (Figure 1A and 1B). Some of the cells grow in colonies and reveal a markedly higher proliferation capacity then cells growing as single cells outside the colonies. As an average one to three colonies can be observed until the first passage, which is usually carried out on day 10. Using cloning cylinders from 12 out of 73 cell samples it was possible to isolate cells with a mesenchymal morphology, which showed a proliferation capacity typical for multipotent mesenchymal stem cells. Cells of the other 51 cell samples showed mainly epithelial-like morphologies with partially huge cell bodies.
Figure 1.
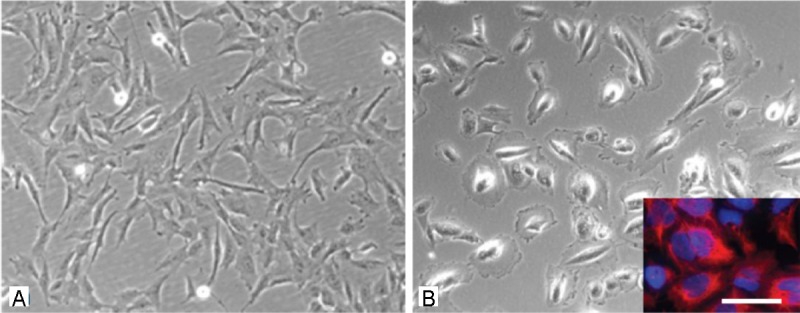
Morphologic analysis of cells isolated from the amniotic fluid. A: spindle shaped cells of the AF type. B: epithelial-like cells of the AE type, inset in B) immunostaining with the specific marker for epithelial cells cytokeratin (red), stining of the total cell population with the nuclear marker Hoechst dye (blue). Scale bar in A) and B) = 20 μm, in the inset = 15 μm.
After isolation and expansion of mesenchymal cells using cloning cylinders cells were characterized by flow cytometry (FACS) using the stem cell specific surface markers CD 29, CD 44, CD 105 und CD 90. Most of the cells (more than 98%) were positive for the markers CD 29, CD 44 und CD 90 (Figure 2). Only the percentage of cells expressing CD 105 varied among the different populations derived from different cell samples. A parallel expression of CD 105 and CD 90 could be detected in 69% of the cells (Figure 2). Because of the high percentage of cells expressing the stem cell marker CD 90 a further selection procedure using magnetic assisted cell sorting (MACS) was renounced.
Figure 2.
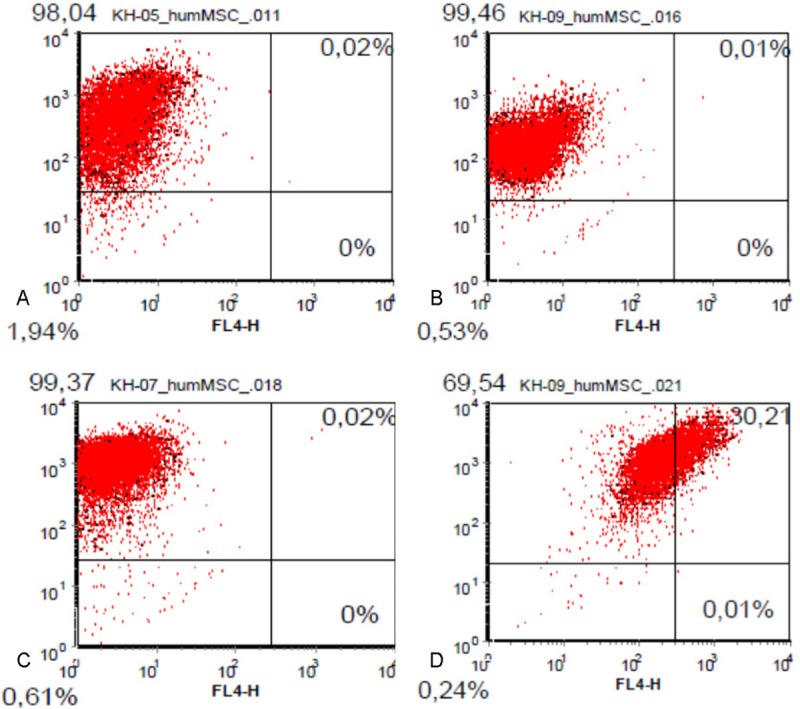
FACS analysis of AF derived MSC. More than 98% of analysed cells express the surface markers A) CD 90, B) CD 44 and C) CD 29. C) a simultaneous expression of CD 90 and CC 105 can be detected in 69.5 % of the total cell population.
In order to further detect stem cell characteristics of the selected AF derived cell populations, pluripotency was analyzed by induction of differentiation into the osteogenic, chondrogenic and adipogenic lineage. Using the appropriate histologic staining procedures differentiation into all three lineages could actually be detected (Figure 3).
Figure 3.
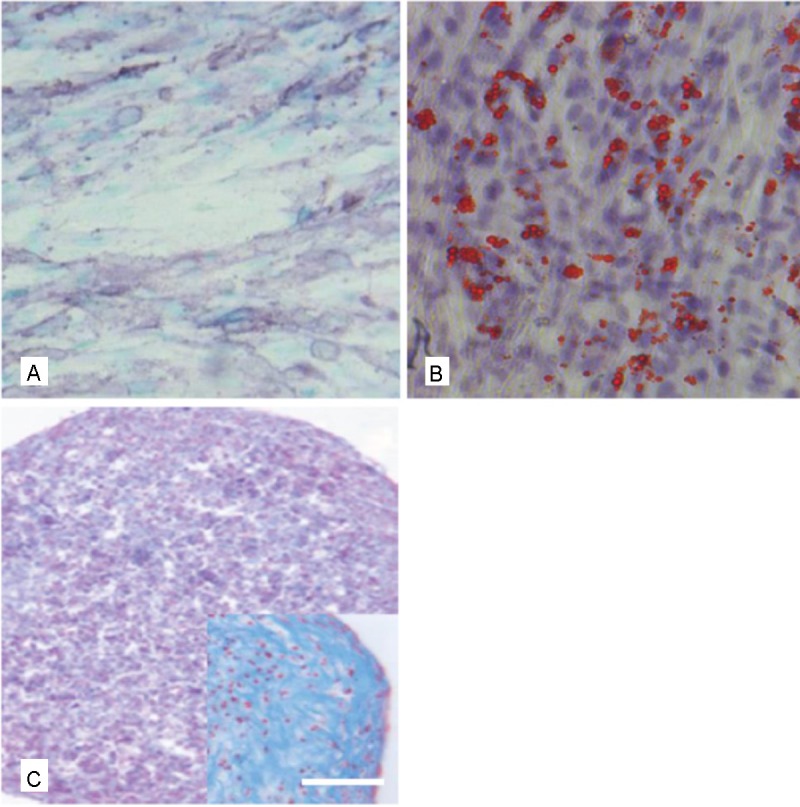
Functional characterization of AF derived MSC by stimulation of differentiation into the osteogenic, adipogenic and chondrogenic lineage. A: osteogenic differentiation as shown by the ALP staining, B) adipogenic differentiation shown by the Red oil O staining and C) chondrogenic differentiation as shown by the Alcian blue staining, Inset in C) higher magnification of a chondrogenic pellet. Scale bar for A-C = 30 μm, for the inset = 25 μm.
To further characterize the general neural differentiation capacity, after treatment with a neural induction medium in the presence of EGF, bFGF and NGF there was no morphological alteration of cells to a more neuronal phenotype detectable, not even after a cultivation period of 21 days. However, the expression of common neural markers such as βIII-tubulin nestin, α-internexin, GABA, glutamate and GFAP could be shown (Figure 4). The analysis of neural marker expression using PCR the expression of β-III Tubulin, brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) could be analyzed after mRNA isolation in all cell populations, differentiated and non-differentiated (Figure 5). However, data were not as uniform as expected. The quantitative analysis revealed that while transcripts for NGF could be detected in undifferentiated as well as in differentiated cells, in some cell populations after the three week induction period a markedly higher expression of ß-III Tubulin and BDNF could be shown in the cells treated with the induction medium (Figure 6).
Figure 4.
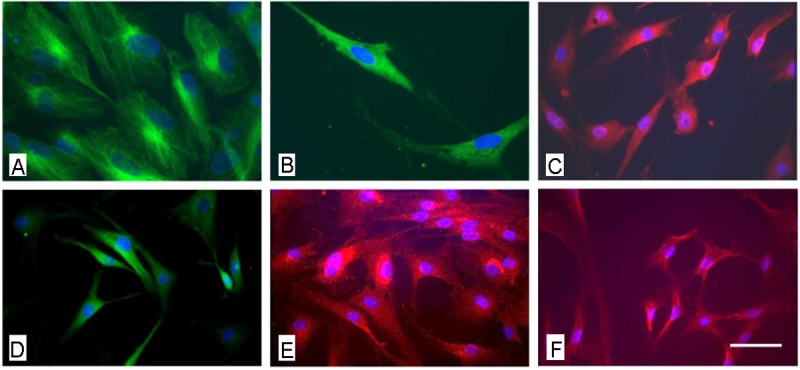
Immunocytochemical analysis of AF derived MSC after neuronal induction using the NPMM Lonza medium. A: fluorescence microscopical analysis of the expression of nestin, B) ßIII tubulin and B) GFAP. Scale bar for a-c = 12 μm.
Figure 5.
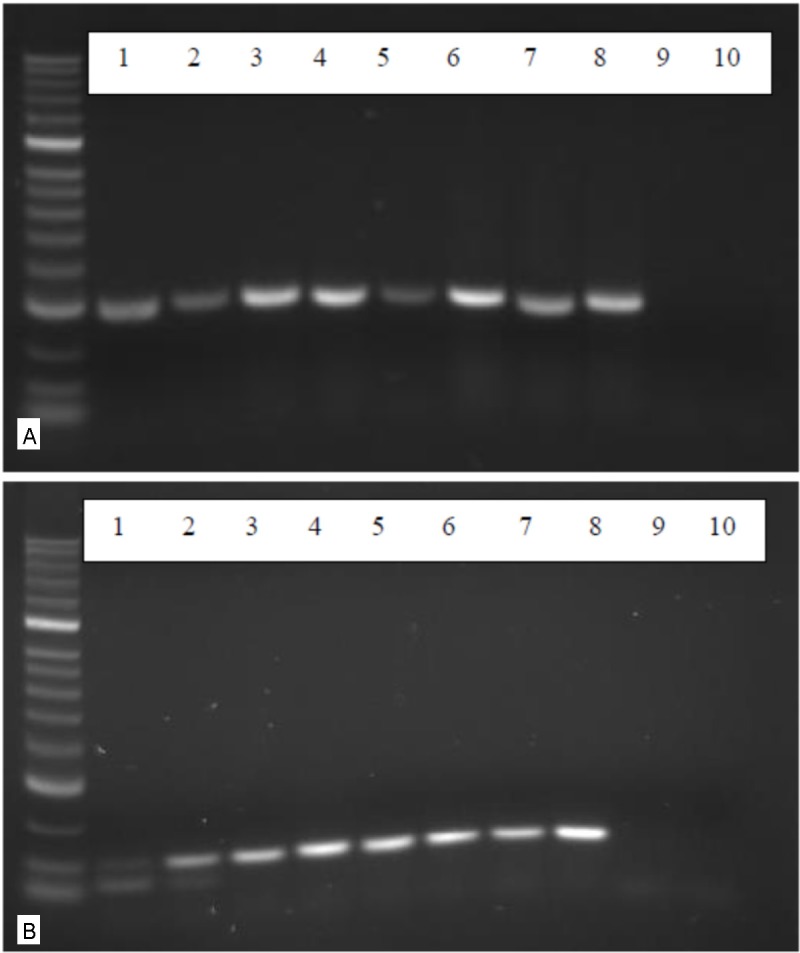
RT-PCR analysis of the expression of neurotrophic factors. A: using primers for a 302 bp fragment of the brain derived neurotrophic factor (BDNF) cDNAs of four different AF derived MSCs 3 weeks after incubation with the neuronal induction medium NPMM were analysed in lanes 1, 3, 5 and 7. In lanes 2, 4, 6, 8 the reults of cDNAs of cells cultivated in the standard medium without neuronal induction are shown. In lanes 9 and 10 the negative controls without c DNA are shown. B: using prmers for the neurotrophic factors NGF an NGF-expression can be equally detected in stimulated (lanes 1, 3 and 5) as well as in non-stimulated cells (lanes 2, 4, 6 and 8).
Figure 6.
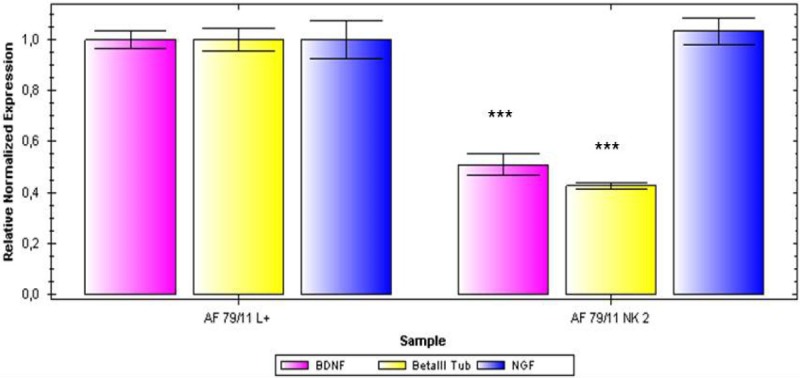
qPCR analysis of the expression of the neuronal markers BDNF, ßIII tubulin and NGF in AF derived MSCs in neuronally induced cells (left columns) and non-stimulated cells (right columns).
The migratory potential of AF-derived cells was analyzed using the scratch- or wound and healing- assay. After plating of 2 x 35.000 cells in culture inserts, cell migration was documented for 24 hours using a connected digital camera taking photos every 10 minutes. Migration of cells cultivated in the standard medium was compared with that of cells stimulated either by interleukin-1-beta (IL-1ß) or by stomal cell derived factor (SDF-1a). The time until complete closure of the cell free zone varied between the analyzed cell populations in the passages 2, 3 and 4 under standard culture conditions and it took between 17.5 and 40 hours with a mean culture time of 29.1 hours. Under the influence of the test substances IL-1-Beta and SDF-1-Alpha there was only a slight, non-significant, increase in migration velocity to be detected (Figure 7).
Figure 7.
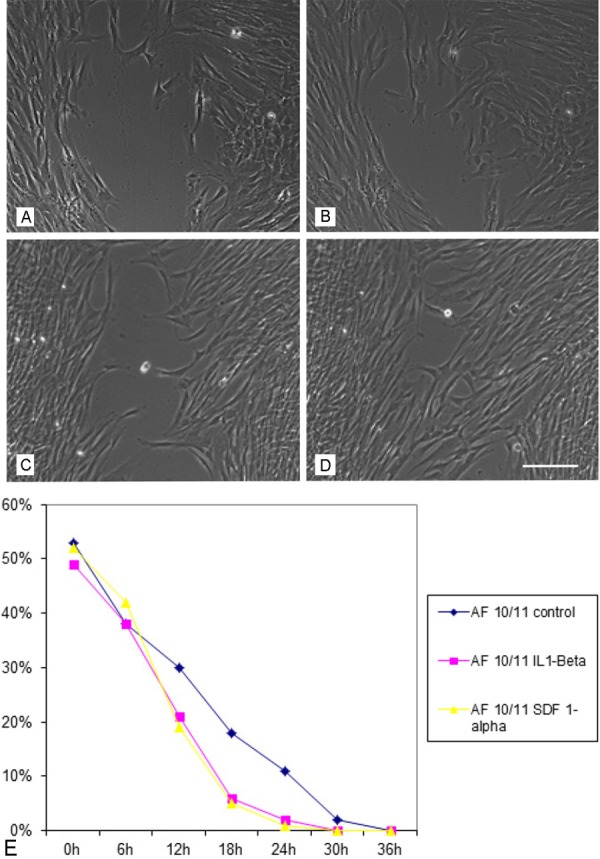
Functional analysis of the migratory potential of AF derived MSC under the influence of inflammatory stimuli. A: MSCs cultivated in the standard medium after 12 hours observation period, B) after 18 hours, C) MSCs cultivated in the presence of IL1- ßeta after 12 and D) after 18 hours, E) time course of the migratory potential of MSC under control conditions as well as under the stimulation of IL1-ßeta and SDF-1. Scale bar for A)-D) = 50 μm.
In order to achieve a differentiation into retina typical neurons using Taurin and Activin A no expression of RHOS a specific marker for retinal neurons could be detected in any of the cell populations studied. Similar negative data could also be obtained for the differentiation into endothelial cells. After induction of cells in the presence of VEGF a weak expression of the endothelial marker PECAM could be found in cells of the undifferentiated as well as in the differentiated cell fraction. In contrast, no immunopositivity using the antibody for the von Wille-Brand factor could be documented (data not shown).
Discussion
In this study we have investigated a progressive differentiation potential of AF cells, which have recommended themselves as a promising cell source of fetal stem cells for regenerative medicine. Amniotic fluid holds great promise as a stem cell source, especially in neonatal applications where autologous cells can be isolated and used. Because there are hardly any ethical concerns associated with this cell source, which can be obtained during routine prenatal diagnostics, the scientific and therapeutic use of these cells is worthwhile having a closer look on their differentiation capacity for various applications even in the central nervous system or in degenerative ophthalmologic disorders.
First of all with this study we can confirm previous data stating, that within the amniotic fluid there is a rather heterogenous cell population [12,13]. In contrast to earlier own findings about the possibility to select cells using the surface marker CD 117 [13] in this study we have refrained from carrying out a MACS based pre-selection procedure as the analysis by flow cytometry using antibodies against the surface markers CD 29, CD 44, CD 105 and CD 90 revealed a very high expression of these markers in AF- derived stem cells anyway (almost 98%) so that a further antibody associated purification of the cell population would not have guaranteed any further improvement of purity. Instead of using a MACs based selection procedure in the presented work, cells with a mesenchymal or fibroblastoid morphology were selected using cloning cylinders as described by Zhang and Chang 2010 [14]. After this morphology-based selection step a pure population of elongated cells with a mesenchymal morphology could be obtained and were used for all further experiments. Thus, the resulting cell population in part meets the criteria set up by the International Society of Cellular Therapy to define MSCs: (i) adhesion to plastic, (ii) expression of specific immunophenotypic marker combinations (CD 73, CD 90, and CD 105), accompanied by lack of expression of hematopoietic markers (CD 14, CD 34, and CD 45) and class II major histocompatibility complex (MHC) molecules; (iii) capability of differentiating into mesodermal lineages (adipocytes, osteoblasts, and chondrocytes) [15,16] The mesodermal differentiation capacity of the AF-cells has been shown in previous studies [13] and can also be confirmed by the data presented here as the osteogenic, chondrogenic and adipogenic differentiation potential could be shown. In order to make use of this cell population also for replacement strategies in neurodegenerative diseases the neuronal differentiation potential has been studied quite well in the past with rather heterogeneous data according to the induction factors used [10,17,13]. For a general neural induction in this study we used a protocol which involved the growth and differentiation factors bFGF, EGF, and NGF resulting in the expression of numerous neuronal and glial markers. In order to even look for a possible differentiation potential of AF-derived stem cells into retina specific neurons for a possible use in ophthalmologic disorders, induction was carried out using taurine and activin A as described for bone marrow derived MSCs [11]. The idea behind such a directed differentiation would be a possible application of AF-derived cells in the retinopathy of prematurity (ROP) in newborn children. In this disorder there is a degeneration of photo receptor cells and retinal glial cells due to a relative hypoxia. However, neither immunocytochemically nor using RT-PCR there was any hint for the expression of the photoreceptor specific marker RHOS, a finding which is in contrast to the work by Kicic et alusing bone marrow derived MSC [11]. As in ROP following hypoxia there is also an uncontrolled sprouting of blood vessels within the retina it was a further aim of this study to also look for a differentiation potential of AF-derived cells into endothelial cells, which might then also be used therapeutically in order to possibly induce a normal regulation of blood vessel growth for minimizing blood vessel related effects in that disorder.
After induction of AF-cells in the presence of VEGF an expression of the endothelial marker PECAM could be found in undifferentiated as well as in differentiated cells. Unfortunately, no immunopositivity using the antibody for the von Willebrand factor could be documented. These findings indicate that although there is the principal differentiation ability of AF-derived stem cells for endothelial cells, this is not as impressive as it has been shown in other investigations using AF-cells g [18]. This may be on one hand because of the highly heterogeneous starting population and on the other may the All together our findings indicate, that although amniotic fluid derived stem cells possess several advantages over embryonic and adult stem cells and are thus suitable especially for neonatal applications one has to be careful with too high expectations in these cells. Because of the heterogeneous cell population to be found in the amniotic fluid, cell cultures have to be thoroughly screened to obtain a similar parental cell population. Furthermore, due to the decrease of prenatal diagnostic procedures involving amniocenteses, the supply with sufficient material may also hamper continuous research with this cell population in the near future. Moreover, ethical concerns associated with obtaining more amniotic fluid than is needed for cytogenetic prenatal diagnostics will additionally prevent a thorough investigation of these cells and unless the donors do not see a direct benefit for themselves or their child will hardly support to give a higher volume of the fluid.
Acknowledgements
We thank Annika Goessl, Sigrid Kettner and Carina Luft for excellent technical assistance. We also thank the Else-Kröner-Fresenius Stiftung for the funding of the study.
References
- 1.De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 2.Trohatou O, Anagnou NP, Roubelakis MG. Human amniotic fluid stem cells as an attractive tool for clinical applications. Curr Stem Cell Res Ther. 2013;8:125–32. doi: 10.2174/1574888x11308020003. [DOI] [PubMed] [Google Scholar]
- 3.Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- 4.In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 5.Gosden CM. Amniotic fluid cell types and culture. Br Med Bull. 1983;39:348–354. doi: 10.1093/oxfordjournals.bmb.a071847. [DOI] [PubMed] [Google Scholar]
- 6.Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G, Hengstschlager M. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol. 2004;191:309–314. doi: 10.1016/j.ajog.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Arnhold S, Post C, Gluer S, Hoopmann M, Wenisch S, Volpers C, Addicks K. Neuronal characteristics of amniotic fluid derived cells after adenoviral transformation. Cell Biol Int. 2008;32:1559–1566. doi: 10.1016/j.cellbi.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 10.Mareschi K, Rustichelli D, Comunanza V, De Fazio R, Cravero C, Morterra G, Martinoglio B, Medico E, Carbone E, Benedetto C, Fagioli F. Multipotent mesenchymal stem cells from amniotic fluid originate neural precursors with functional voltage-gated sodium channels. Cytotherapy. 2009;11:534–547. doi: 10.1080/14653240902974024. [DOI] [PubMed] [Google Scholar]
- 11.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23:7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prusa AR, Hengstschlager M. Amniotic fluid cells and human stem cell research: a new connection. Med Sci Monit. 2002;8:RA253–RA257. [PubMed] [Google Scholar]
- 13.Arnhold S, Gluer S, Hartmann K, Raabe O, Addicks K, Wenisch S, Hoopmann M. Amniotic- Fluid Stem Cells: Growth Dynamics and Differentiation Potential after a CD-117-Based Selection Procedure. Stem Cells Int. 2011;2011:715341. doi: 10.4061/2011/715341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Chan C. Isolation and enrichment of rat mesenchymal stem cells (MSCs) and separation of single-colony derived MSCs. J Vis Exp. 2010 doi: 10.3791/1852. pii: 1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orbay H, Tobita M, Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int. 2012;2012:461718. doi: 10.1155/2012/461718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson ZR, Yang SH, Chan AW, Young LJ. Production of germline transgenic prairie voles (Microtus ochrogaster) using lentiviral vectors. Biol Reprod. 2009;81:1189–1195. doi: 10.1095/biolreprod.109.077529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P, Baxter J, Vinod K, Tulenko TN, Di Muzio PJ. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev. 2009;18:1299–1308. doi: 10.1089/scd.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]


